Electrospinning of Ethylene Vinyl Acetate/Carbon Nanotube Nanocomposite Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Electrospun Fibers
2.3. Characterization of the Prepared Fibers
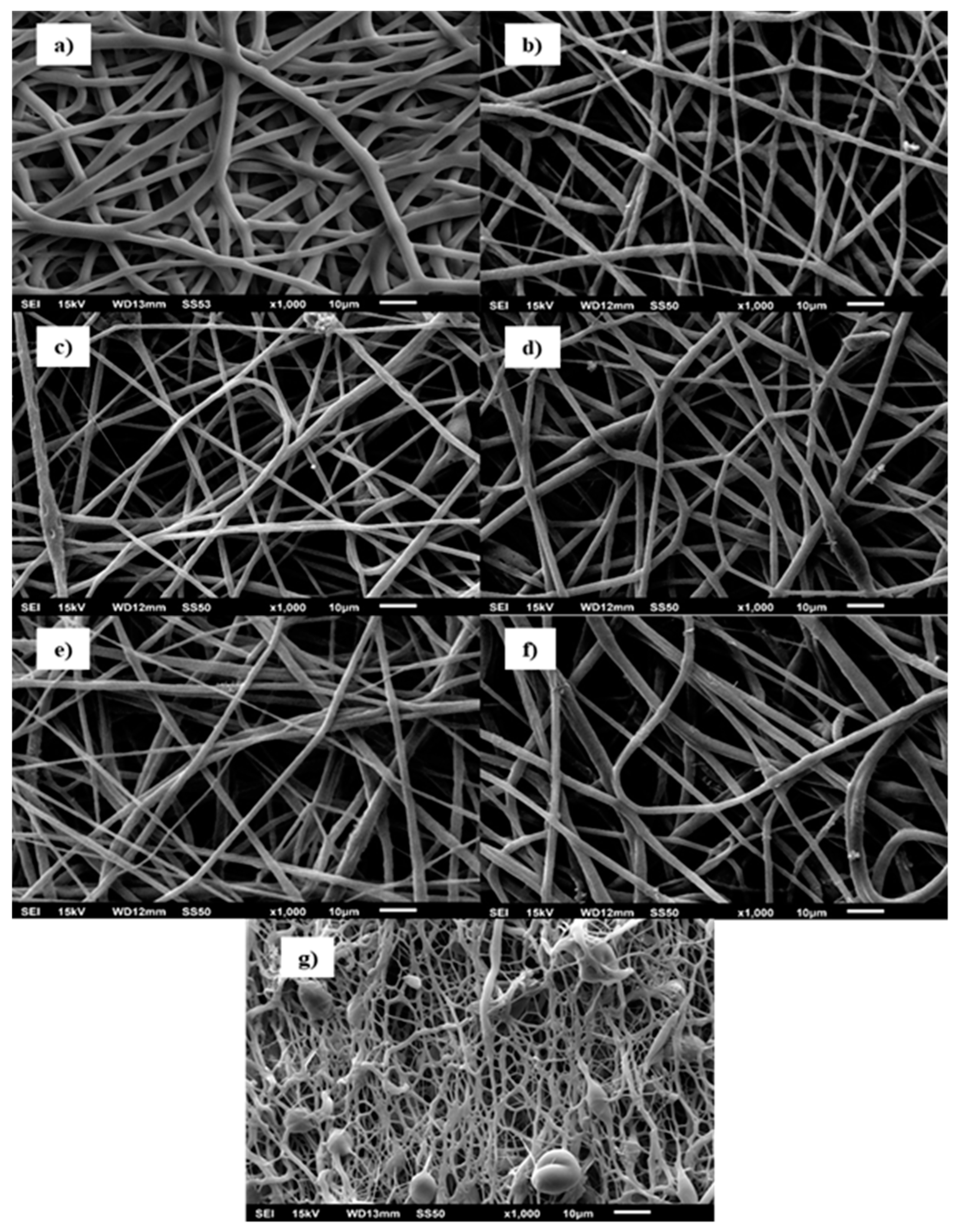
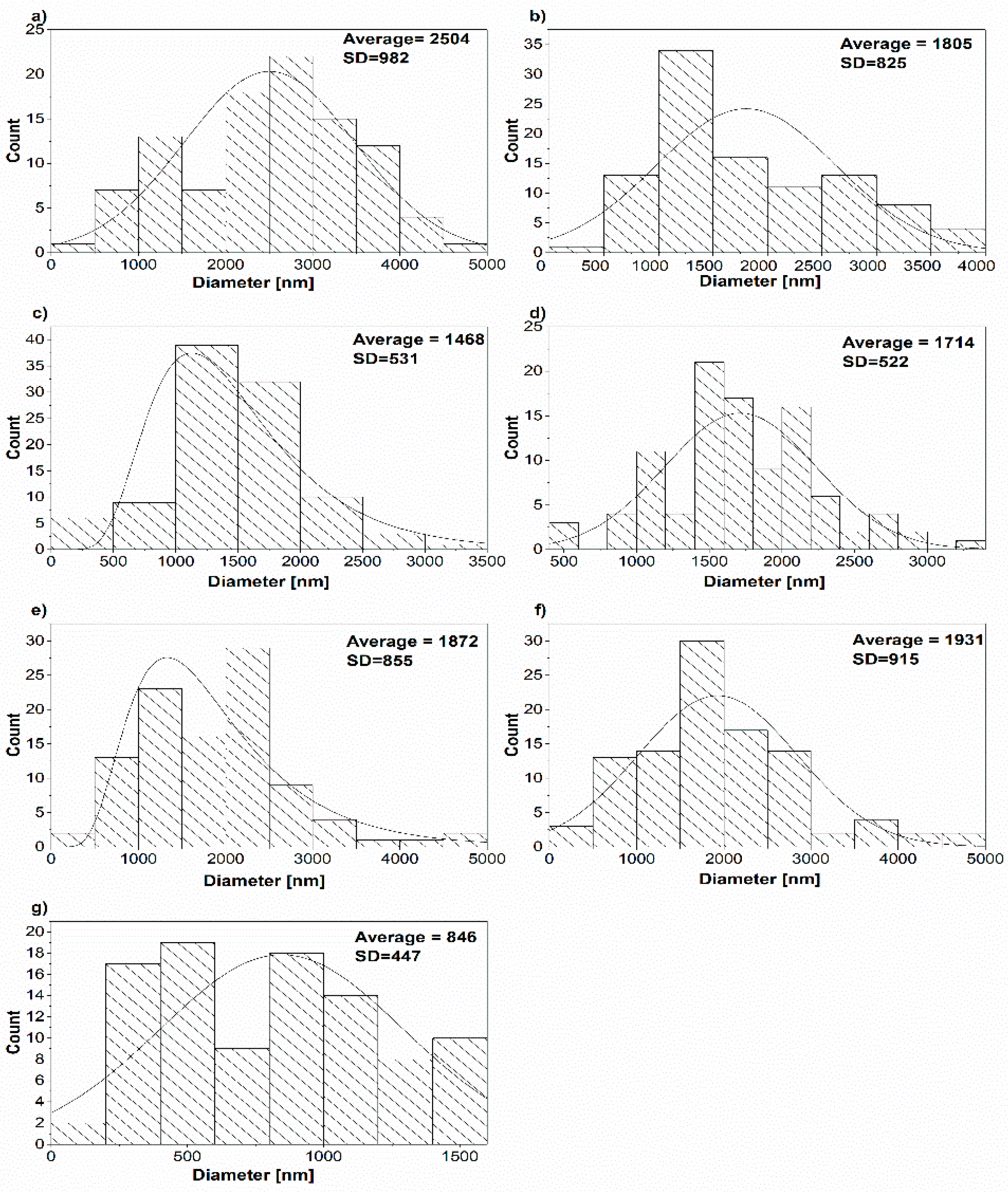
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henderson, A.M. Ethylene-vinyl acetate (EVA) copolymers: A general review. IEEE Electr. Insul. Mag. 1993, 9, 30–38. [Google Scholar] [CrossRef]
- Schneider, C.; Langer, R.; Loveday, D.; Hair, D. Applications of ethylene vinyl acetate copolymers (EVA) in drug delivery systems. J. Control. Release 2017, 262, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Ghose, S.; Watson, K.A.; Working, D.C.; Connell, J.W.; Smith, J.G.; Sun, Y.P. Thermal conductivity of ethylene vinyl acetate copolymer/nanofiller blends. Compos. Sci. Technol. 2008, 68, 1843–1853. [Google Scholar] [CrossRef]
- Czaniková, K.; Ilčíková, M.; Krupa, I.; Mičušík, M.; Kasák, P.; Pavlova, E.; Mosnáček, J.; Chorvát, D., Jr.; Omastová, M. Elastomeric photo-actuators and their investigation by confocal laser scanning microscopy. Smart Mater. Struct. 2013, 22, 104001. [Google Scholar] [CrossRef]
- Tian, D.; He, C.-H.; He, J.-H. Macromolecule orientation in nanofibers. Nanomaterials 2018, 8, 918. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, X.; Zhang, X.; Lai, Y.; Wang, X.; Li, J.; Wei, G.; Su, Z. Electrospun doping of carbon nanotubes and platinum nanoparticles into the β-phase polyvinylidene difluoride nanofibrous membrane for biosensor and catalysis applications. ACS Appl. Mater. Interfaces 2014, 6, 7563–7571. [Google Scholar] [CrossRef]
- Su, Z.; Ding, J.; Wei, G. Electrospinning: A facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications. RSC Adv. 2014, 4, 52598–52610. [Google Scholar] [CrossRef]
- Lee, J.K.Y.; Chen, N.; Peng, S.J.; Li, L.L.; Tian, L.L.; Thakor, N.; Ramakrishna, S. Polymer-based composites by electrospinning: Preparation & functionalization with nanocarbons. Prog. Polym. Sci. 2018, 86, 40–84. [Google Scholar] [CrossRef]
- Wu, S.; Peng, S.; Wang, C.H. Multifunctional polymer nanocomposites reinforced by aligned carbon nanomaterials. Polymers 2018, 10, 542. [Google Scholar] [CrossRef]
- Wang, G.; Tan, Z.; Liu, X.; Chawda, S.; Koo, J.S.; Samuilov, V.; Dudley, M. Conducting MWNT/poly(vinyl acetate) composite nanofibres by electrospinning. Nanotechnology 2006, 17, 5829–5835. [Google Scholar] [CrossRef]
- Su, Z.; Li, J.; Li, Q.; Ni, T.; Wei, G. Chain conformation, crystallization behavior, electrical and mechanical properties of electrospun polymer-carbon nanotube hybrid nanofibers with different orientations. Carbon 2012, 50, 560–5617. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Chen, S.; Wachtel, E.; Koga, T.; Sokolov, J.C.; Rafailovich, M.H. Electrospinning of poly(ethylene-co-vinyl acetate)/clay nanocomposite fibers. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 2501–2508. [Google Scholar] [CrossRef]
- Lewkowitz-Shpuntoff, H.M.; Wen, M.C.; Singh, A.; Brenner, N.; Gambino, R.; Pernodet, N.; Isseroff, R.; Rafailovich, M.; Sokolov, J. The effect of organo clay and adsorbed FeO3 nanoparticles on cells cultured on ethylene vinyl acetate substrates and fibers. Biomaterials 2009, 30, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Tijing, L.D.; Park, C.-H.; Choi, W.L.; Ruelo, M.T.G.; Amarjargal, A.; Pant, H.R.; Im, I.-T.; Kim, C.S. Characterization and mechanical performance comparison of multiwalled carbon nanotube/polyurethane composites fabricated by electrospinning and solution casting. Compos. Part B Eng. 2013, 44, 613–619. [Google Scholar] [CrossRef]
- Meng, Z.X.; Zheng, W.; Li, L.; Zheng, Y.F. Fabrication and characterization of three-dimensional nanofiber membrane of PCL-MWCNT by electrospinning. Mater. Sci. Eng. C 2010, 30, 1014–1021. [Google Scholar] [CrossRef]
- Guan, X.; Zheng, G.; Dai, K.; Liu, C.; Yan, X.; Shen, C.; Guo, Z. Carbon nanotubes-adsorbed electrospun PA66 nanofiber bundles with improved conductivity and robust flexibility. ACS Appl. Mater. Interfaces 2016, 8, 14150–14159. [Google Scholar] [CrossRef]
- Kostakova, E.; Meszaros, L.; Gregra, J. Composite nanofibers produced by modified needleless electrospinning. Mater. Lett. 2009, 63, 2419–2422. [Google Scholar] [CrossRef]
- Czaniková, K.; Krupa, I.; Ilčíková, M.; Kasák, P.; Chorvát, D.; Valentin, M.; Šlouf, M.; Mosnáček, J.; Mičušík, M.; Omastová, M. Photo-actuating materials based on elastomers and modified carbon nanotubes. J. Nanophotonics 2012, 6, 1–14. [Google Scholar] [CrossRef]
- Czaniková, K.; Torras, N.; Esteve, J.; Krupa, I.; Kasák, P.; Pavlova, E.; Račko, D.; Chodák, I.; Omastová, M. Nanocomposite photoactuators based on an ethylene vinyl acetate copolymer filled with carbon nanotubes. Sens. Actuators B Chem. 2013, 186, 701–710. [Google Scholar] [CrossRef]
- Cikova, E.; Kulicek, J.; Janigova, I.; Omastova, M. Electrospinning of ethylene vinyl acetate/poly(lactic acid) blends on a water surface. Materials 2018, 11, 1737. [Google Scholar] [CrossRef]
- Qian, J.W.; Qi, G.R.; Cheng, R.S. Association of ethylene-vinyl acetate copolymer in dilute solutions—I. Solvent, concentration and annealing temperature effect. Eur. Polym. J. 1997, 33, 1263–1265. [Google Scholar] [CrossRef]
- Smallwood, I.M. Handbook of Organic Solvent Properties; Butterworth-Heinemann: Oxford, UK, 2012; ISBN 0080523781. [Google Scholar]
- Yang, Q.; Li, Z.; Hong, Y.; Zhao, Y.; Qiu, S.; Wang, C.; Wei, Y. Influence of solvents on the formation of ultrathin uniform poly(vinyl pyrrolidone) nanofibers with electrospinning. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3721–3726. [Google Scholar] [CrossRef]
- Armentano, I.; Del Gaudio, C.; Bianco, A.; Dottori, M.; Nanni, F.; Fortunati, E.; Kenny, J.M. Processing and properties of poly(ε-caprolactone)/carbon nanofibre composite mats and films obtained by electrospinning and solvent casting. J. Mater. Sci. 2009, 44, 4789–4795. [Google Scholar] [CrossRef]
- Liu, C.; Shen, J.; Liao, C.Z.; Yeung, K.W.K.; Tjong, S.C. Novel electrospun polyvinylidene fluoride-graphene oxide-silver nanocomposite membranes with protein and bacterial antifouling characteristics. Express Polym. Lett. 2018, 12, 365–382. [Google Scholar] [CrossRef]
- Shimadzu (Shimadzu Corporation) ATR Precautions. Available online: http://www.shimadzu.com/an/ftir/support/ftirtalk/letter2/atr2.html (accessed on 30 October 2017).
- Karabacak, M.; Cinar, M.; Kurt, M.; Sundaraganesan, N. Experimental and theoretical FTIR and FT-Raman spectroscopic analysis of 1-pyrenecarboxylic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers. The Scienta ESCA300 Database; Wiley: Chichester, NY, USA, 1992; ISBN 0-471-93592-1. [Google Scholar]
- Costache, M.C.; Heidecker, M.J.; Manias, E.; Camino, G.; Frache, A.; Beyer, G.; Gupta, R.K.; Wilkie, C.A. The influence of carbon nanotubes, organically modified montmorillonites and layered double hydroxides on the thermal degradation and fire retardancy of polyethylene, ethylene–vinyl acetate copolymer and polystyrene. Polymer 2007, 48, 6532–6545. [Google Scholar] [CrossRef]
- Omastová, M.; Mičušík, M.; Fedorko, P.; Pionteck, J.; Kovářová, J.; Chehimi, M.M. The synergy of ultrasonic treatment and organic modifiers for tuning the surface chemistry and conductivity of multiwalled carbon nanotubes. Surf. Interface Anal. 2014, 46, 940–944. [Google Scholar] [CrossRef]
- Maurin, M.B.; Dittert, L.W.; Hussain, A.A. Thermogravimetric analysis of ethylene-vinyl acetate copolymers with Fourier transform infrared analysis of the pyrolysis products. Thermochim. Acta 1991, 186, 97–102. [Google Scholar] [CrossRef]
- Costache, M.C.; Jiang, D.D.; Wilkie, C.A. Thermal degradation of ethylene-vinyl acetate coplymer nanocomposites. Polymer 2005, 46, 6947–6958. [Google Scholar] [CrossRef]



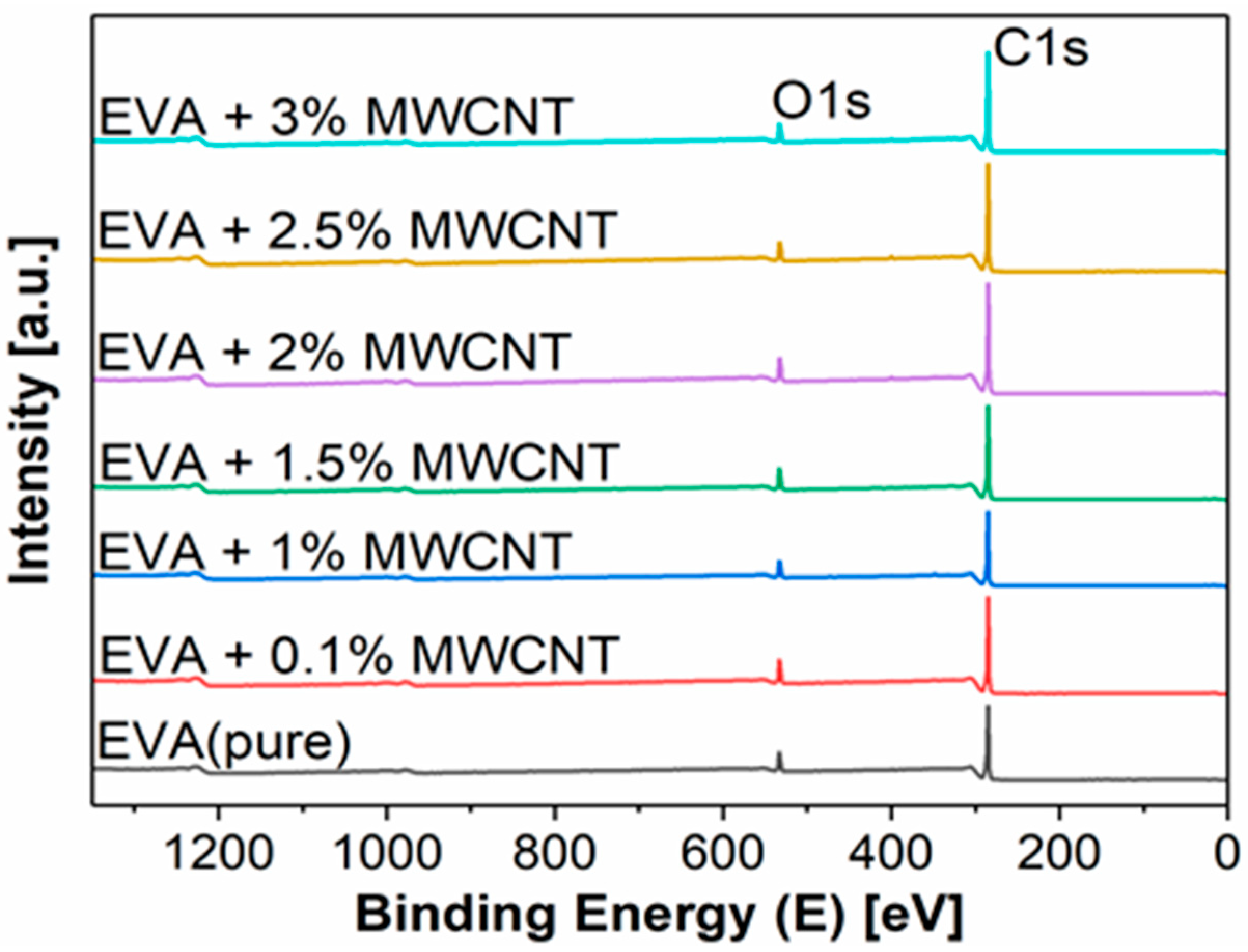


| Sample | Surface Chemical Composition [at %] | |||||
|---|---|---|---|---|---|---|
| C1s | O1s | N1s | Si2p | S2p | F1s/Ca2p | |
| EVA (granules) | 87.2 | 7.7 | 3.1 | 2.0 | - | -/- |
| EVA fibers | 89.2 | 10.5 | 0.2 | 0.1 | - | -/- |
| MWCNT | 99.5 | 0.5 | - | - | - | -/- |
| PyChol | 93.5 | 4.3 | 1.7 | 0.5 | - | -/- |
| PyChol + MWCNTs | 89.6 | 7.8 | 2.5 | - | - | 0.2/- |
| EVA + 0.1 wt % MWCNTs | 89.0 | 10.7 | - | 0.3 | - | -/- |
| EVA + 1.0 wt % MWCNTs | 88.2 | 10.1 | 0.4 | 0.4 | 0.3 | -/0.5 |
| EVA + 1.5 wt % MWCNTs | 89.3 | 10.2 | 0.4 | 0.1 | - | -/- |
| EVA + 2.0 wt % MWCNTs | 88.6 | 9.7 | 1.1 | 0.4 | 0.1 | -/0.2 |
| EVA + 2.5 wt % MWCNTs | 89.0 | 8.4 | 1.7 | 0.9 | - | -/- |
| EVA + 3.0 wt % MWCNTs | 89.7 | 8.8 | 1.2 | 0.4 | - | -/- |
| Sample | Surface Chemical Composition [at %] | |
|---|---|---|
| C1s C–C//C–O//C=O/OC=O (284.7//286.0//287.3/288.9) | O1s C=O/C–O/HO*–CO–O (532.1/533.4/534.7) | |
| EVA (granules) | 87.3 87.4//5.4//5.5//1.7 | 7.7 78.4/21.6/- |
| EVA | 89.2 67.4//19.3//6.7/6.6 | 10.5 48.8/40.2/11.1 |
| EVA/0.1 wt % MWCNT | 89.0 82.1//9.9//2.5/5.5 | 10.7 50.2/49.8/- |
| EVA/1.0 wt % MWCNT | 88.2 85.9//6.5//2.9/4.7 | 10.1 65.0/35.0/- |
| EVA/1.5 wt % MWCNT | 89.3 83.3//8.3//3.0/5.4 | 10.2 50.7/49.3/- |
| EVA/2.0 wt % MWCNT | 88.6 86.2//7.6//1.9/4.3 | 9.7 63.3/36.7/- |
| EVA/2.5 wt % MWCNT | 89.0 86.3//7.0//2.9/3.8 | 8.4 65.7/34.3/- |
| EVA/3.0 wt % MWCNT | 89.7 85.9//7.7//2.1/4.3 | 8.8 63.7/36.3/- |
| Sample | T10% [°C] | T50% [°C] | First Degradation Step (Td1) | Second Degradation Step (Td2) | ||||
|---|---|---|---|---|---|---|---|---|
| Tonset [°C] | Tmax [°C] | Tf [°C] | Tonset [°C] | Tmax [°C] | Tf [°C] | |||
| EVA | 301 | 433 | 270 | 323 | 353 | 410 | 436 | 465 |
| EVA/1.0 wt % MWCNT | 302 | 440 | 251 | 331 | 374 | 414 | 453 | 469 |
| EVA/2.0 wt % MWCNT | 303 | 443 | 222 | 330 | 369 | 416 | 467 | 483 |
| EVA/3.0 wt % MWCNT | 303 | 451 | 252 | 328 | 374 | 425 | 467 | 487 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omastová, M.; Číková, E.; Mičušík, M. Electrospinning of Ethylene Vinyl Acetate/Carbon Nanotube Nanocomposite Fibers. Polymers 2019, 11, 550. https://doi.org/10.3390/polym11030550
Omastová M, Číková E, Mičušík M. Electrospinning of Ethylene Vinyl Acetate/Carbon Nanotube Nanocomposite Fibers. Polymers. 2019; 11(3):550. https://doi.org/10.3390/polym11030550
Chicago/Turabian StyleOmastová, Mária, Eliška Číková, and Matej Mičušík. 2019. "Electrospinning of Ethylene Vinyl Acetate/Carbon Nanotube Nanocomposite Fibers" Polymers 11, no. 3: 550. https://doi.org/10.3390/polym11030550





