Comparison of Properties of PVA Nanocomposites Containing Reduced Graphene Oxide and Functionalized Graphene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
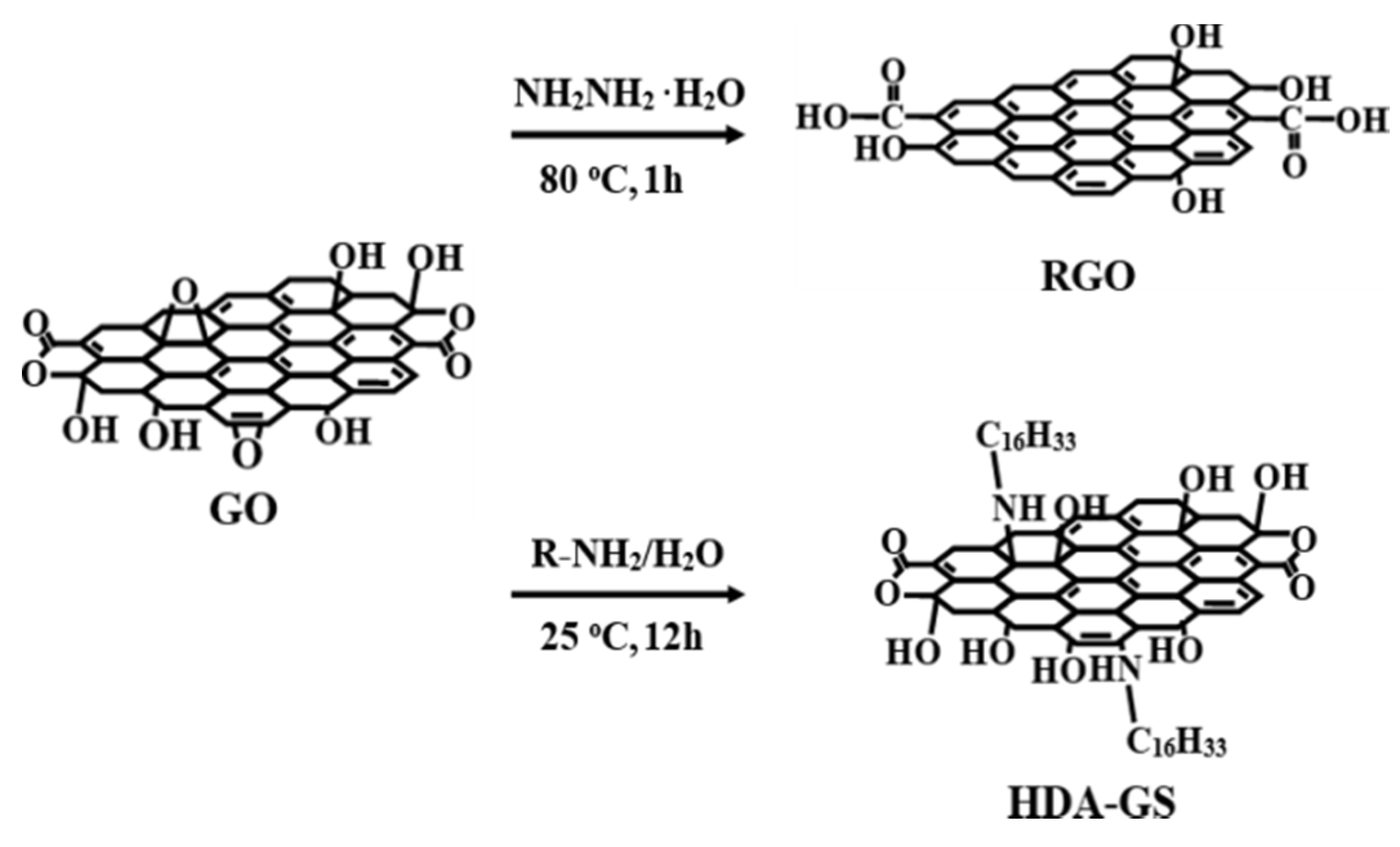
2.2. Preparation of GO
2.3. Synthesis of Reduced GO
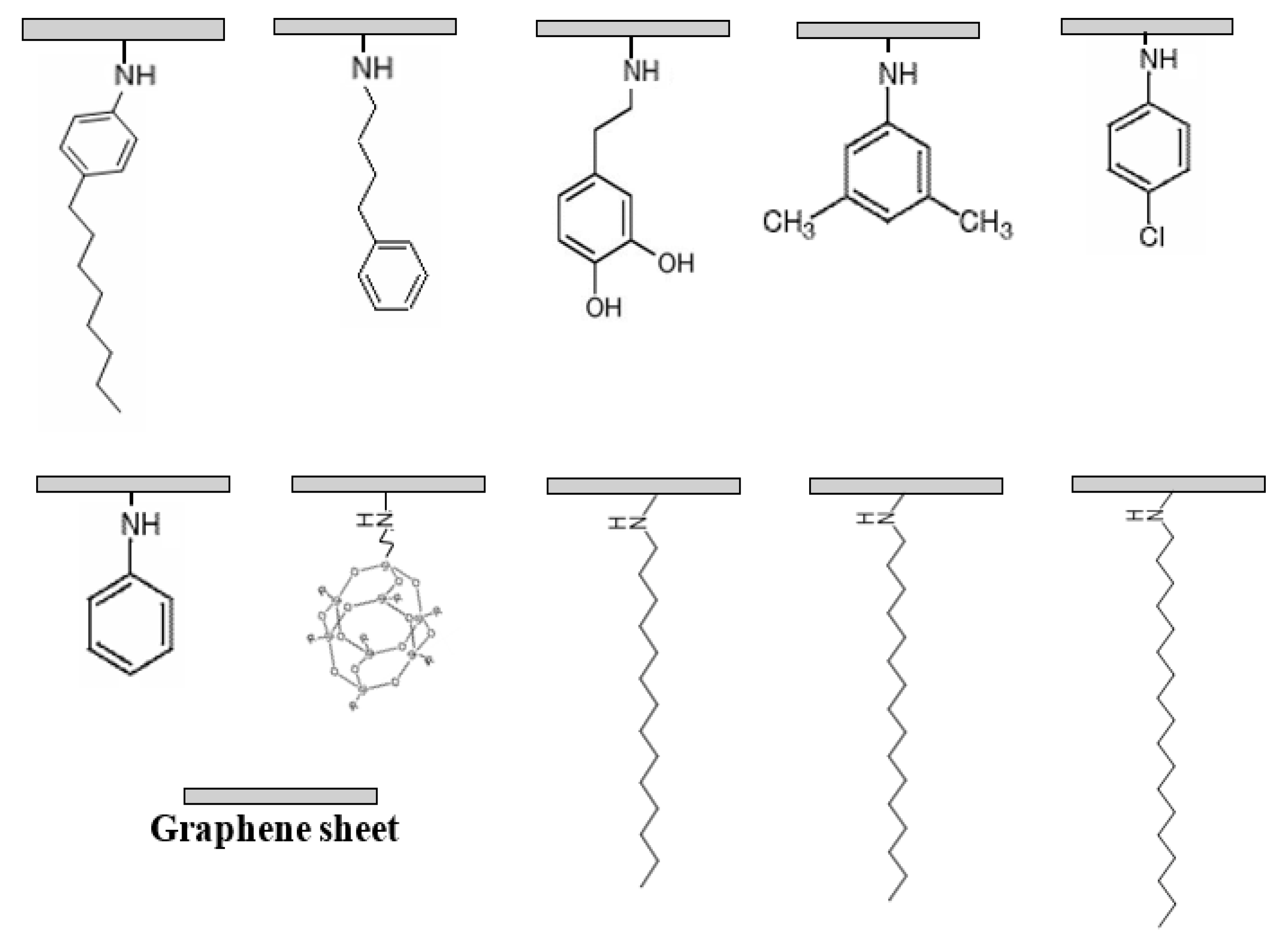
2.4. Synthesis of HDA-GS
2.5. Synthesis of PVA Hybrids with HDA-GS
2.6. Characterizations
3. Results and Discussion
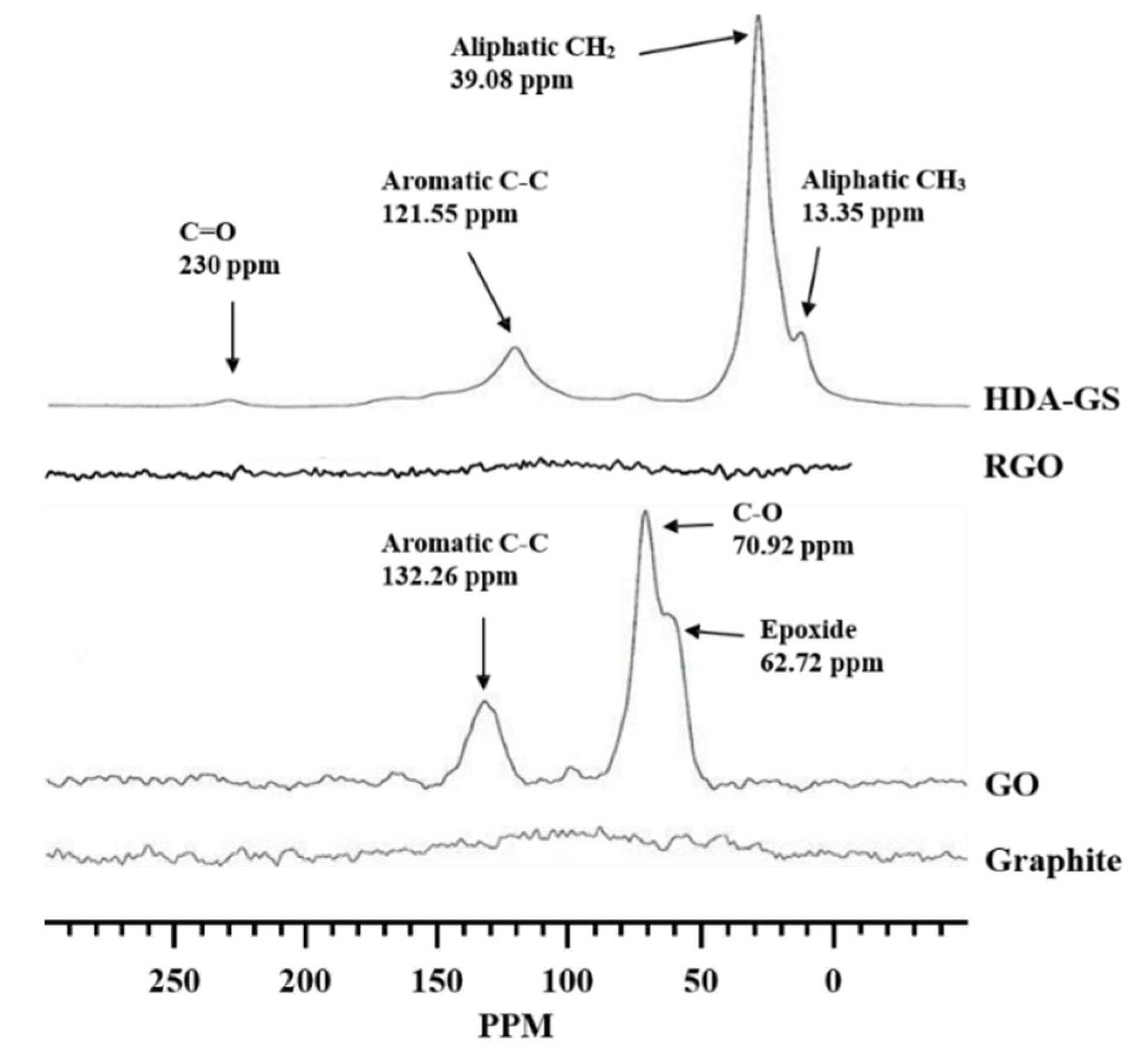
3.1. FT-IR and 13C-NMR Spectroscopy
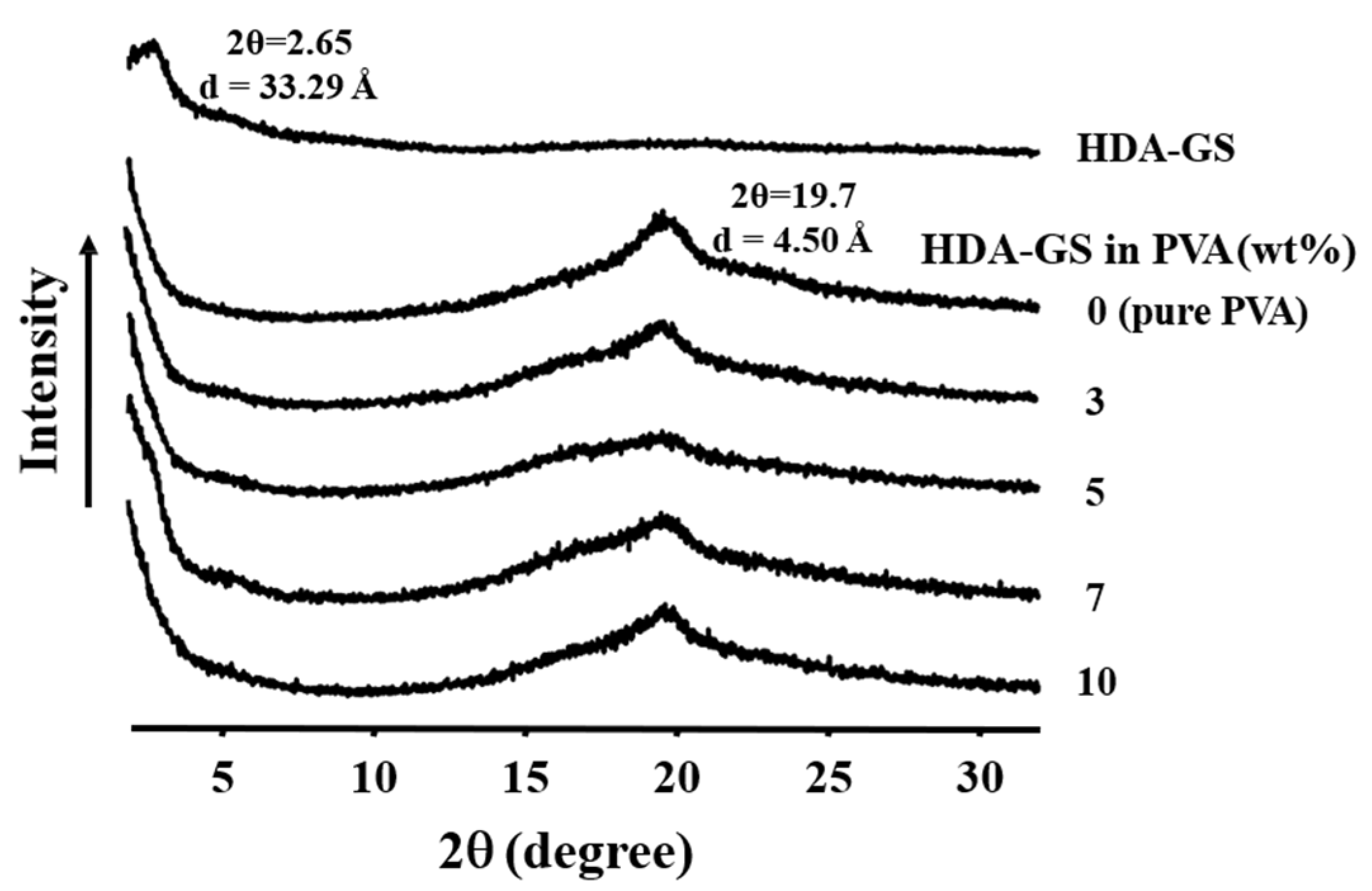
3.2. Wide-Angle X-Ray Diffraction (XRD)
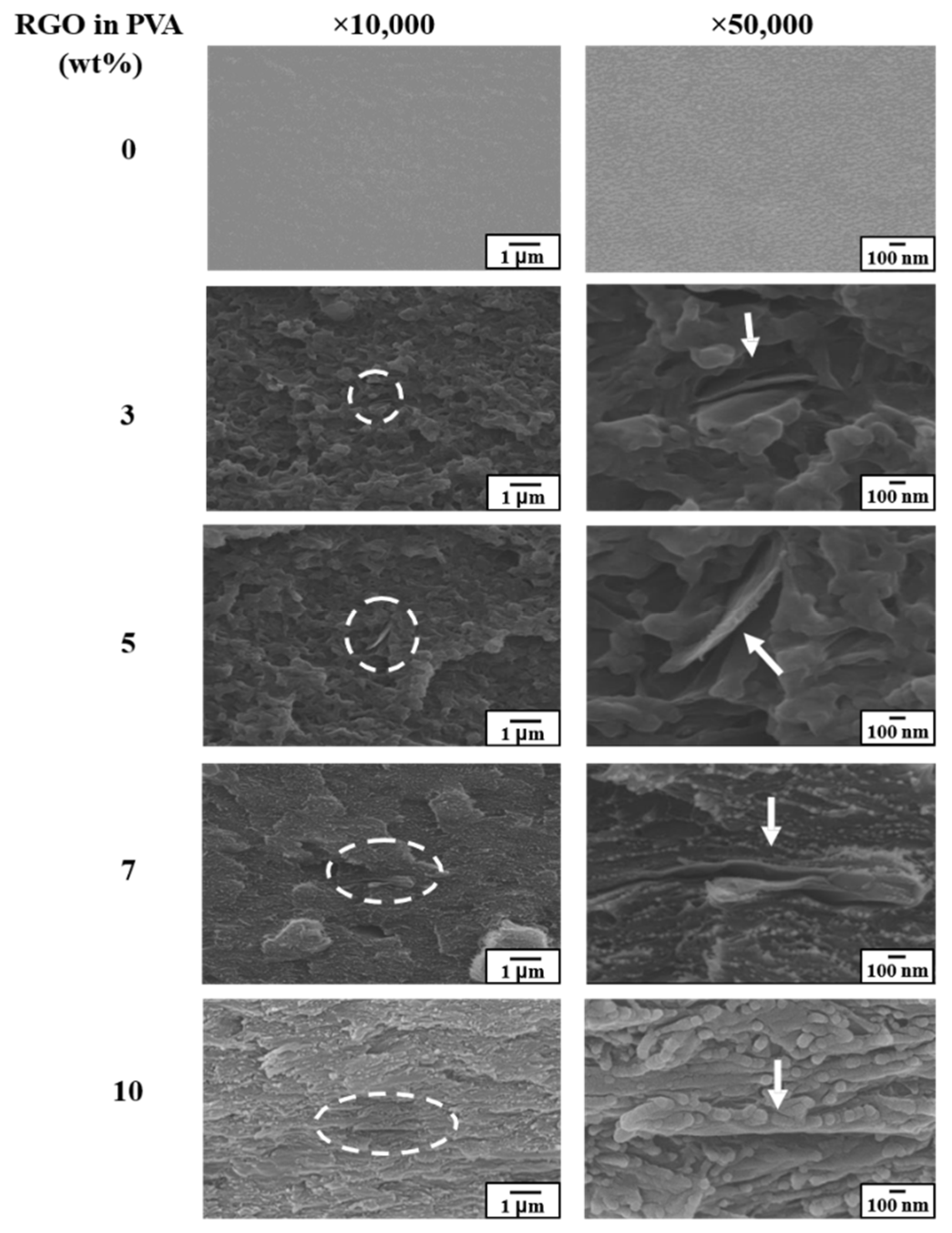
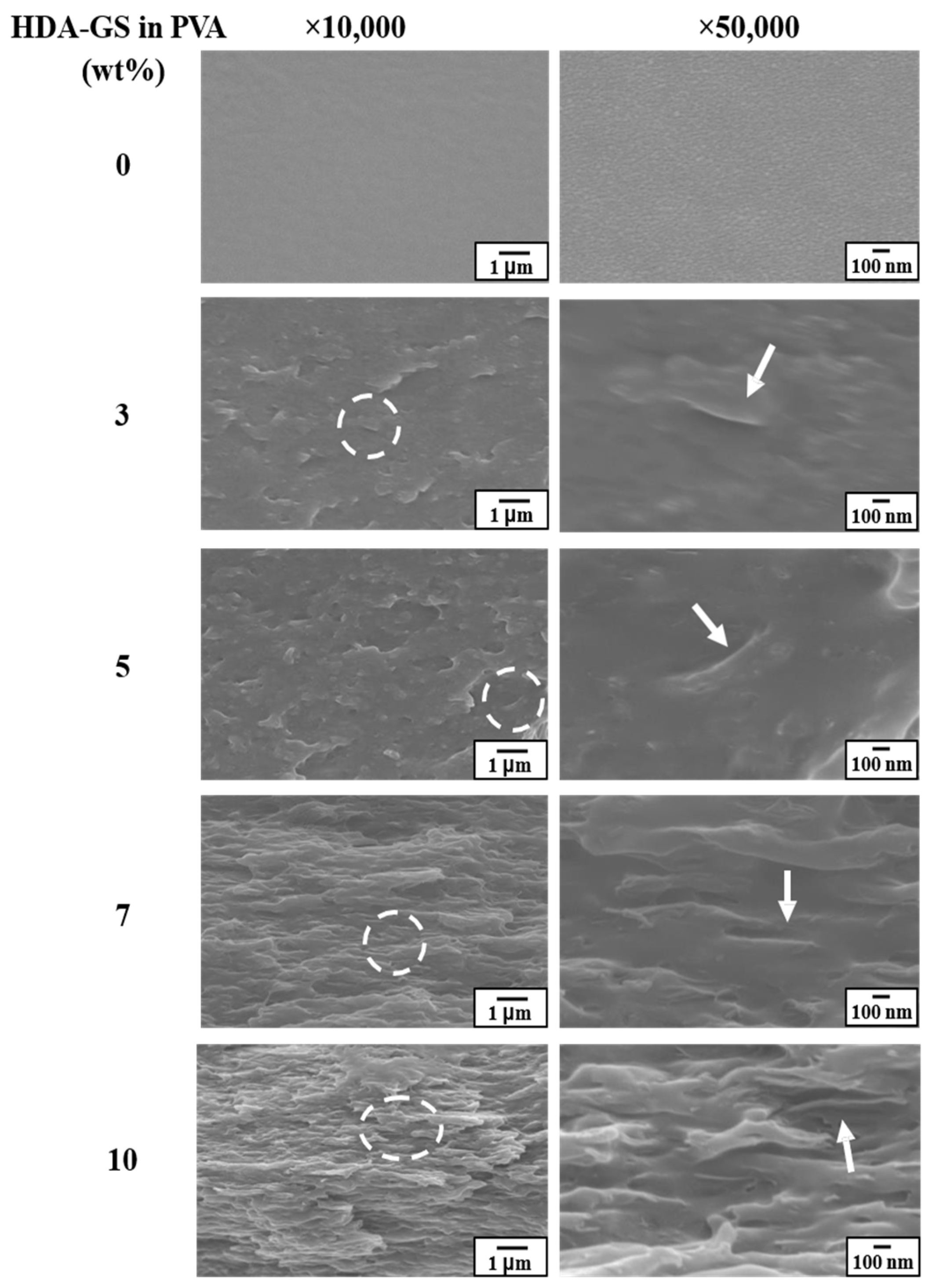
3.3. Morphology
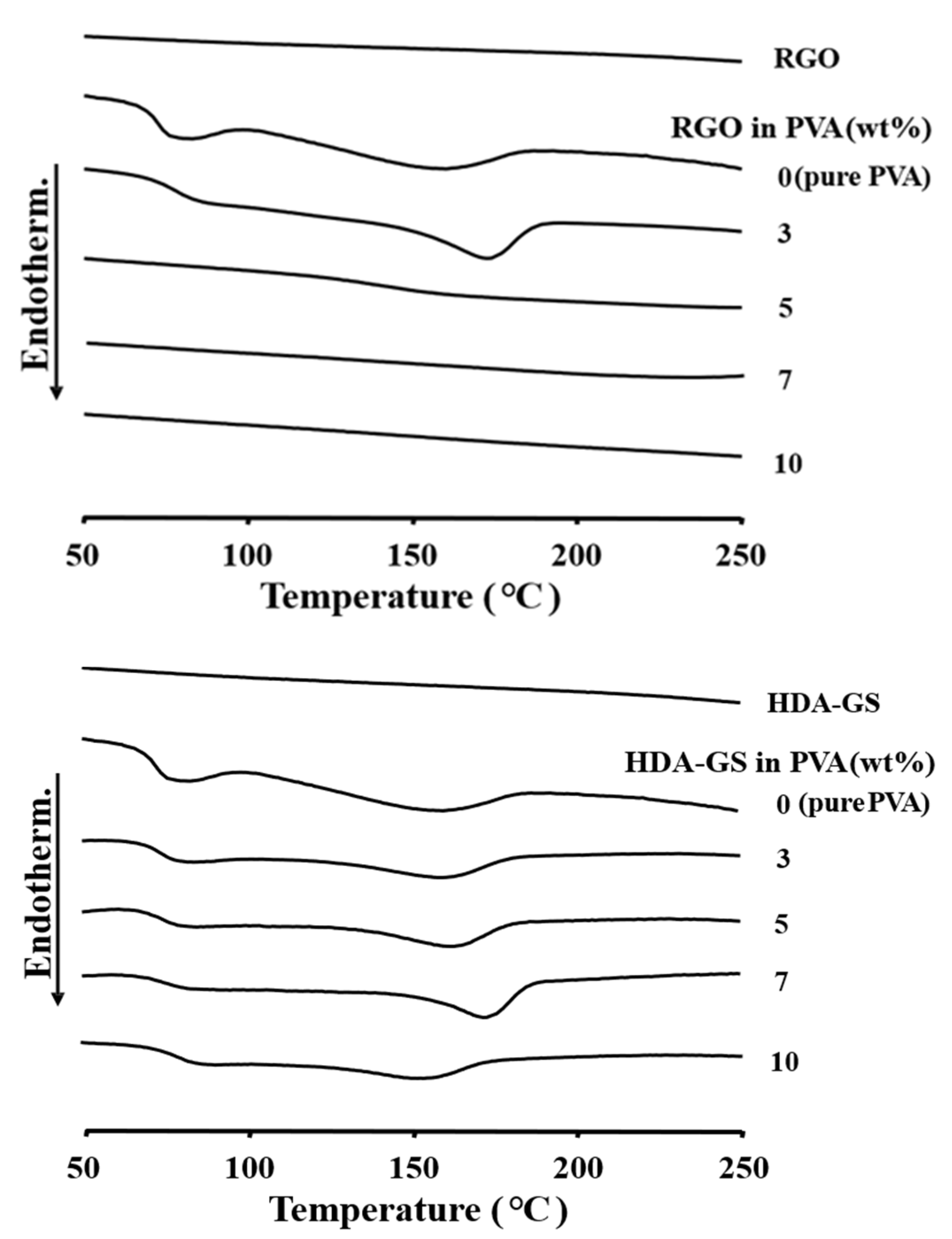
3.4. Thermal Properties
3.5. Gas Permeation
3.6. Electric Conductivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, K.; Gui, Z.; Hu, Y. Facile synthesis of LDH nanoplates as reinforcing agents in PVA nanocomposites. Polym. Adv. Technol. 2017, 28, 386–392. [Google Scholar] [CrossRef]
- Kashyap, S.; Pratihar, S.K.; Behera, S.K. Strong and ductile graphene oxide reinforced PVA nanocomposites. J. Alloys Compd. 2016, 684, 254–260. [Google Scholar] [CrossRef]
- Yahia, I.S.; Mohammed, M.I. Facile synthesis of graphene oxide/PVA nanocomposites for laser optical limiting: Band gap analysis and dielectric constants. J. Mater. Sci. Mater. Electron. 2018, 29, 8555–8563. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, Y.S. Stable fabrication of poly (vinyl alcohol) nanofiber using coaxial electrospinning. Polymer (Korea) 2016, 40, 941–946. [Google Scholar] [CrossRef]
- Ha, H.O.; Kim, D.S. Stability change of poly (vinyl alcohol)-iodine complexes due to moisture absorption. Polymer (Korea) 2017, 41, 507–513. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xu, C.; Zhang, M.; Shang, X. Preparation of graphene/poly (vinyl alcohol) nanocomposites with enhanced mechanical properties and water resistance. Polym. Int. 2011, 60, 816–822. [Google Scholar] [CrossRef]
- Borriello, C.; De Maria, A.; Jovic, N.; Montone, A.; Schwarz, M.; Antisari, M.V. Mechanochemical exfoliation of graphite and its polyvinyl alcohol nanocomposites with enhanced barrier properties. Mater. Manuf. Proc. 2009, 24, 1053–1057. [Google Scholar] [CrossRef]
- Nakane, K.; Yamashita, T.; Iwakura, K.; Suzuki, F. Properties and structure of poly (vinyl alcohol)/silica composites. J. Appl. Polym. Sci. 1999, 74, 133–138. [Google Scholar] [CrossRef]
- Chuang, W.Y.; Young, T.H.; Chiu, W.Y.; Lin, C.Y. The effect of polymeric additives on the structure and permeability of poly (vinyl alcohol) asymmetric membranes. Polymer 2000, 41, 5633–5641. [Google Scholar] [CrossRef]
- Bernard, J.; Favier, A.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Synthesis of poly (vinyl alcohol) combs via MADIX/RAFT polymerization. Polymer 2006, 47, 1073–1080. [Google Scholar] [CrossRef]
- Chang, J.-H. Permeation properties of water-soluble polymer nanocomposite systems. In Barrier Properties of Polymer Clay Nanocomposites; Mittal, V., Ed.; Nova Science and Publisher: Hauppauge, NY, USA, 2010; Chapter 6; pp. 117–138. [Google Scholar]
- Butnaru, E.; Cheaburu, C.N.; Yilmaz, O.; Pricope, G.M.; Vasile, C. Poly (vinyl alcohol)/chitosan/montmorillonite nanocomposites for food packaging applications: Influence of montmorillonite content. High Perform. Polym. 2016, 28, 1124–1138. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R.; Manias, E.; Giannelis, E.P.; Wuthenow, M.; Hilton, D.; Phillips, S.H. Flammability properties of polymer-layered-silicate nanocomposites. Polypropylene and polystyrene nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; LeBaron, P.C.; Park, I.; Pinnavaia, T.J. Epoxy-clay fabric film composites with unprecedented oxygen-barrier properties. Chem. Mater. 2006, 18, 4393–4398. [Google Scholar] [CrossRef]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2009, 3, 101–105. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. Part B 2006, 110, 8535–8539. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.G.; Bhajantri, R.F.; Ravindrachary, V.; Naik, J.; Kumar, D.J.M. High mechanical and pressure sensitive dielectric properties of graphene oxide dope PVA nanocomposites. RSC Adv. 2016, 6, 77977–77986. [Google Scholar] [CrossRef]
- Gao, C.; Vo, C.D.; Jin, Y.Z.; Li, W.; Armes, S.P. Multihydroxy polymer-functionalized carbon nanotubes: Synthesis, derivatization, and metal loading. Macromolecules 2005, 38, 8634–8648. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Dong, R.; Gordiichuk, P.; Zhang, T.; Zhuang, X.; Mai, Y.; Liu, F.; Herrmann, A.; Feng, X. Two-dimensional mesoscale-ordered conducting polymers. Angew. Chem. Int. Ed. 2016, 55, 12516–12521. [Google Scholar] [CrossRef]
- Sun, Y.P.; Huang, W.; Lin, Y.; Fu, K.; Kitaygorodskiy, A.; Riddle, L.A.; Yu, Y.J.; Carroll, D.L. Soluble dendron-functionalized carbon nanotubes: Preparation, characterization, and properties. Chem. Mater. 2001, 13, 2864–2869. [Google Scholar] [CrossRef]
- Ansari, S.; Giannelis, E.P. Functionalized graphene sheet-Poly (vinylidene fluoride) conductive nanocomposites. J. Polym. Sci. Polym. Phys. Ed. 2009, 47, 888–897. [Google Scholar] [CrossRef]
- Raghu, A.V.; Lee, Y.R.; Jeong, H.M.; Shin, C.M. Preparation and physical properties of waterborne polyurethane/functionalized graphene sheet nanocomposites. Macromol. Chem. Phys. 2008, 209, 2487–2493. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Recent advance in functionalized graphene/polymer nanocomposites. J. Mater. Chem. 2010, 20, 7906–7915. [Google Scholar] [CrossRef]
- Moon, H.G.; Chang, J.-H. Syntheses and characterizations of functionalized graphenes and reduced graphene oxide. Polymer (Korea) 2011, 35, 265–271. [Google Scholar] [CrossRef]
- Heo, C.; Chang, J.-H. Syntheses of functionalized graphene sheets and their polymer nanocomposites. In Handbook of Functional Nanomaterials; Niknam, Z.A., Ed.; Nova Science and Publisher: Hauppauge, NY, USA, 2013; Volume 2, Chapter 4; pp. 85–116. [Google Scholar]
- Heo, C.; Chang, J.-H. Syntheses and characterizations of position specific functionalized graphenes. Polymer (Korea) 2013, 37, 218–224. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Kwon, K.; Chang, J.-H. Comparison of the properties of polyimide nanocomposites containing three different nanofillers: Organoclay, functionalized graphene, and organoclay/functionalized graphene complex. J. Compos. Mater. 2015, 49, 3031–3044. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Zhu, J.; Chang, L.; Ye, L. Nanoindentation and thermal study of polyvinylalcohol/graphene oxide nanocomposite film through organic/inorganic assembly. Appl. Surf. Sci. 2015, 349, 27–34. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, H.M. Enhancement of thermal, mechanical, and barrier properties of ethylene vinyl alcohol copolymer by incorporation of graphene nanosheets: Effect of functionalization of graphene oxide. High Perform. Polym. 2015, 27, 694–704. [Google Scholar] [CrossRef]
- Huang, G.; Gao, J.; Wang, X.; Liang, H.; Ge, C. How can graphene reduce the flammability of polymer nanocomposites? Mater. Lett. 2012, 66, 187–189. [Google Scholar] [CrossRef]
- Jiang, L.; Shen, X.P.; Wu, J.L.; Shen, K.C. Preparation and characterization of graphene/poly (vinyl alcohol) nanocomposites. J. Appl. Polym. Sci. 2010, 118, 275–279. [Google Scholar] [CrossRef]
- Dong, S.S.; Wu, F.; Chen, L.; Wang, Y.Z.; Chen, S.C. Preparation and characterization of Poly (vinyl alcohol)/graphene nanocomposite with enhanced thermal stability using PEtVIm-Br as stabilizer and compatibilizer. Polym. Deg. Stab. 2016, 131, 42–52. [Google Scholar] [CrossRef]
- Zoppi, R.A.; Das Neves, S.; Nunes, S.P. Hybrid films of poly (ethylene oxide-b-amide-6) containing sol–gel silicon or titanium oxide as inorganic fillers: Effect of morphology and mechanical properties on gas permeability. Polymer 2000, 41, 5461–5470. [Google Scholar] [CrossRef]
- Jarus, D.; Hiltner, A.; Baer, E. Barrier properties of polypropylene/polyamide blends produced by microlayer coextrusion. Polymer 2002, 43, 2401–2408. [Google Scholar] [CrossRef]
- Joly, C.; Smaihi, M.; Porcar, L.; Noble, R.D. Polyimide-Silica composite materials: How does silica influence their microstructure and gas permeation properties? Chem. Mater. 1999, 11, 2331–2338. [Google Scholar] [CrossRef]
- Ebeling, T.; Norek, S.; Hasan, A.; Hiltner, A.; Baer, E. Effect of a tie layer on the delamination toughness of polypropylene and polyamide-66 microlayers. J. Appl. Polym. Sci. 1999, 71, 1461–1467. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, J.K.; Lee, H.S. Transparent and high gas barrier films based on poly (vinyl alcohol)/graphene oxide composites. Thin Sol. Film 2011, 519, 7766–7771. [Google Scholar] [CrossRef]
- Huang, H.D.; Ren, P.G.; Chen, J.; Zhang, W.Q.; Ji, X.; Li, Z.M. High barrier graphene oxide nanosheet/poly (vinyl alcohol) nanocomposite films. J. Membr. Sci. 2012, 409, 156–163. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]



| Filler in PVA (% w/w) | RGO | HDA-GS | ||||||
|---|---|---|---|---|---|---|---|---|
| Tg (°C) | Tm (°C) | TDi a (°C) | wtR600 b (%) | Tg (°C) | Tm (°C) | TDi (°C) | wtR600 (%) | |
| 0 (pure PVA) | 67 | 161 | 226 | 6 | 67 | 161 | 226 | 6 |
| 3 | 71 | 174 | 231 | 6 | 68 | 161 | 236 | 7 |
| 5 | 134 | n.o. | 233 | 17 | 70 | 165 | 237 | 11 |
| 7 | n.o.c | n.o. | 243 | 24 | 71 | 173 | 252 | 12 |
| 10 | n.o. | n.o. | 251 | 29 | 73 | 156 | 238 | 12 |
| Filler in PVA (% w/w) | RGO | HDA-GS | ||||
|---|---|---|---|---|---|---|
| Thickness (µm) | O2TR a (cc/m2/day) | Pc/Ppb | Thickness (µm) | O2TR (cc/m2/day) | Pc/Pp | |
| 0 (pure PVA) | 20 | 7.29 | 1 | 20 | 7.29 | 1 |
| 3 | 23 | <10−2 | - | 19 | 6.41 | 0.88 |
| 5 | 24 | 3.42 | 0.47 | 24 | 0.98 | 0.13 |
| 7 | 23 | 11.25 | 1.54 | 21 | 5.85 | 0.80 |
| 10 | 22 | 39.23 | 5.38 | 20 | 31.27 | 4.29 |
| Filler in PVA (% w/w) | RGO | HDA-GS | ||
|---|---|---|---|---|
| Thickness (µm) | Conductivity (S/cm) | Thickness (µm) | Conductivity (S/cm) | |
| 0 (pure PVA) | 42 | 10−10 | 42 | 10−10 |
| 3 | 42 | 10−10 | 45 | 10−10 |
| 5 | 46 | 1.2 × 10−4 | 48 | 2.2 × 10−5 |
| 7 | 48 | 8.2 × 10−3 | 48 | 2.6 × 10−4 |
| 10 | 47 | 1.1 × 10−2 | 47 | 7.3 × 10−3 |
| 20 | 43 | 1.2 × 10−1 | ||
| 40 | 48 | 1.1 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, G.T.; Chang, J.-H. Comparison of Properties of PVA Nanocomposites Containing Reduced Graphene Oxide and Functionalized Graphene. Polymers 2019, 11, 450. https://doi.org/10.3390/polym11030450
Park GT, Chang J-H. Comparison of Properties of PVA Nanocomposites Containing Reduced Graphene Oxide and Functionalized Graphene. Polymers. 2019; 11(3):450. https://doi.org/10.3390/polym11030450
Chicago/Turabian StylePark, Gi Tae, and Jin-Hae Chang. 2019. "Comparison of Properties of PVA Nanocomposites Containing Reduced Graphene Oxide and Functionalized Graphene" Polymers 11, no. 3: 450. https://doi.org/10.3390/polym11030450
APA StylePark, G. T., & Chang, J.-H. (2019). Comparison of Properties of PVA Nanocomposites Containing Reduced Graphene Oxide and Functionalized Graphene. Polymers, 11(3), 450. https://doi.org/10.3390/polym11030450





