Effects of pH on Nanofibrillation of TEMPO-Oxidized Paper Mulberry Bast Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of PMBFs
2.2.2. TEMPO Oxidation
2.2.3. Analysis of Fiber Property
2.2.4. Ultrasonication and Yield Analysis
2.2.5. Analysis of TEMPO-Oxidized Cellulose Nanofibrils
3. Results and Discussion
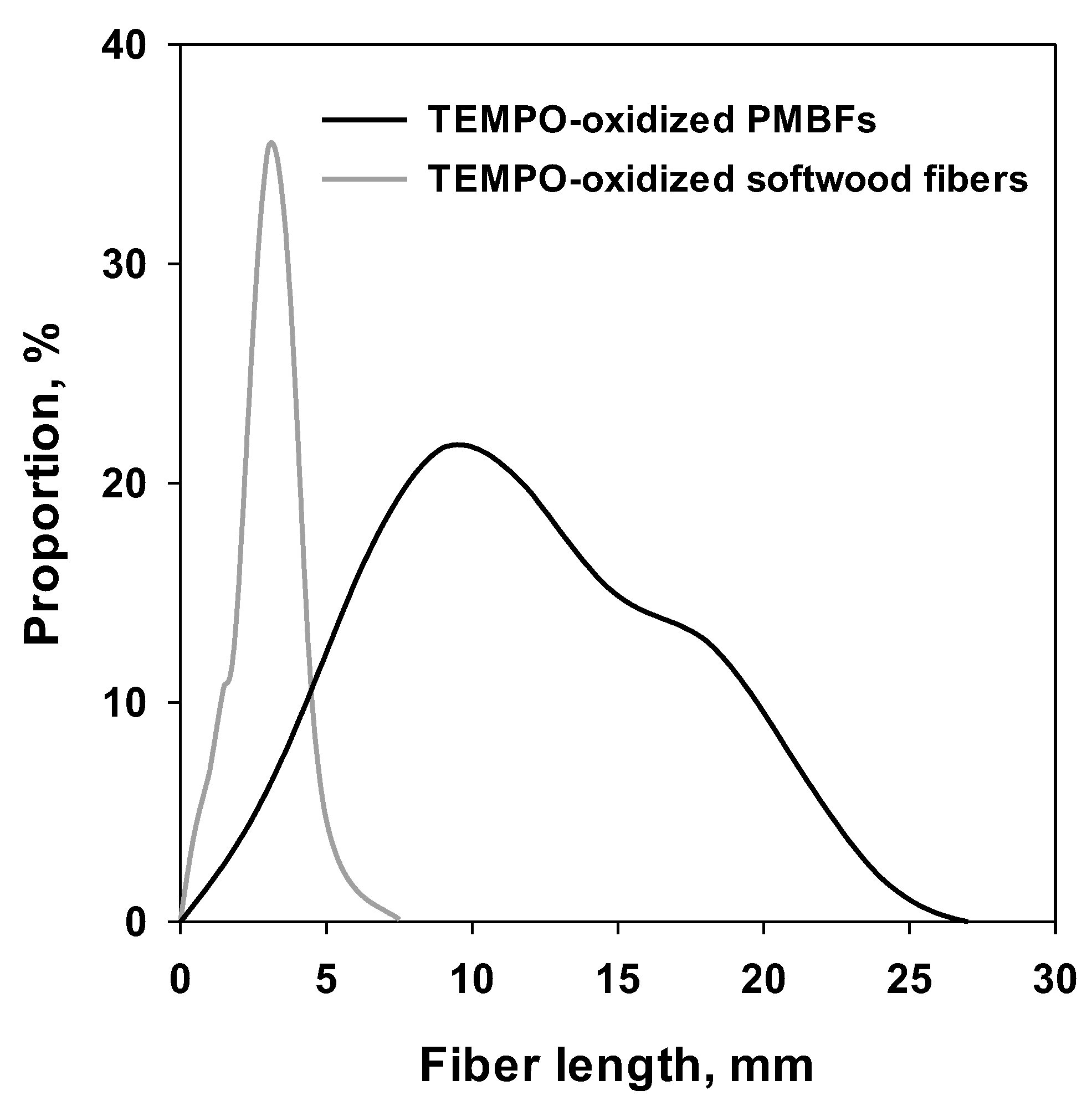
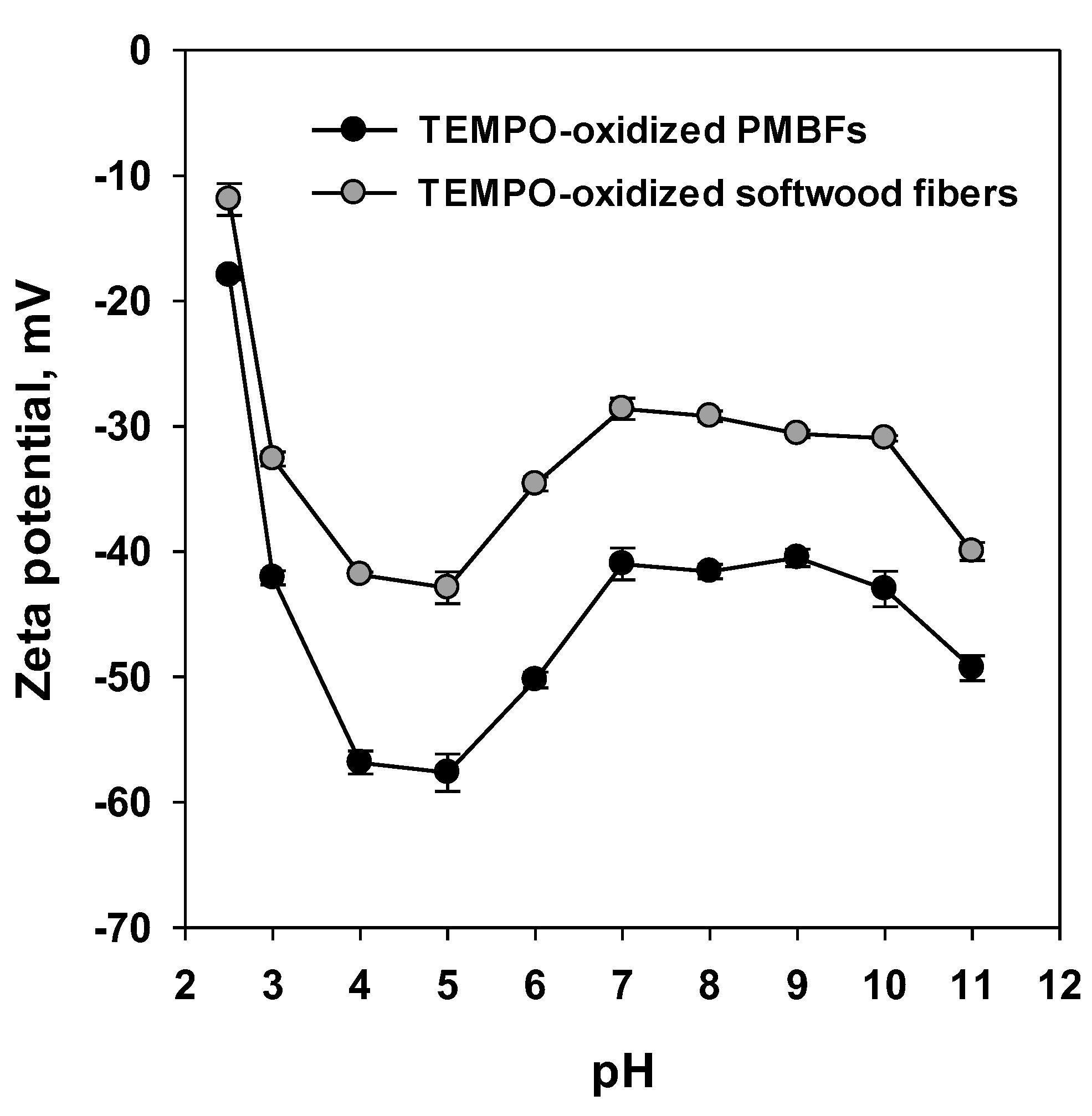
3.1. Fundamental Characteristics of TEMPO-Oxidized Fibers
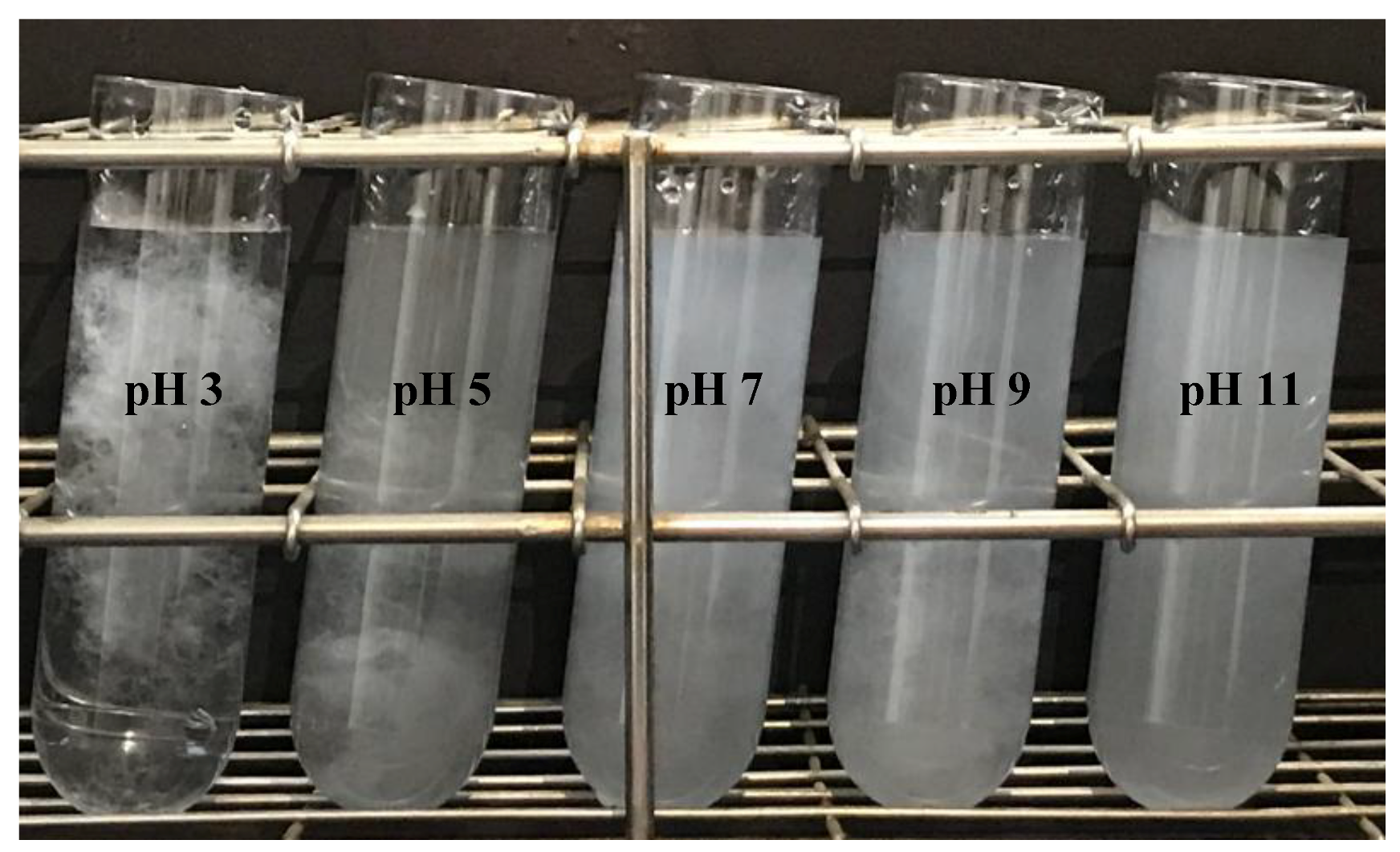
3.2. Effect of pH on Nanofibrillation
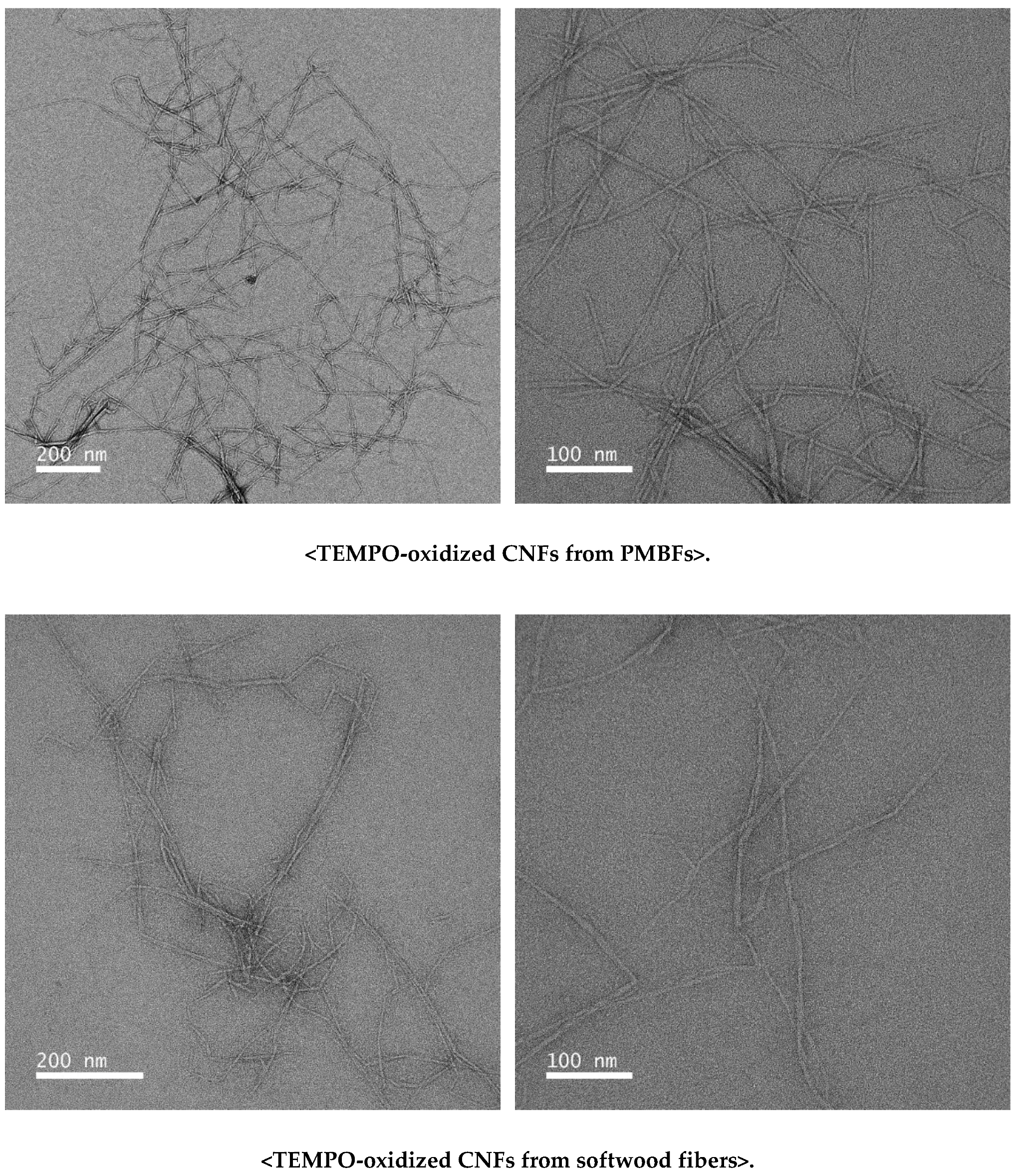
3.3. Characteristics of TEMPO-Oxidized CNFs
3.4. Tensile Properties
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Iwamoto, S.; Nakagaito, A.N.; Yano, H. Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl. Phys. A 2007, 89, 461–466. [Google Scholar] [CrossRef]
- Rodionova, G.; Lenes, M.; Eriksen, Ø.; Gregersen, Ø. Surface chemical modification of microfibrillated cellulose: Improvement of barrier properties for packaging applications. Cellulose 2011, 18, 127–134. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Cai, H.; Sharma, S.; Liu, W.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Plackett, D.V.; Letchford, K.; Jackson, J.K.; Burt, H.M. A review of nanocellulose as a novel vehicle for drug delivery. Nord. Pulp Pap. Res. J. 2014, 29, 105–118. [Google Scholar] [CrossRef]
- Herrick, F.W.; Casebier, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated cellulose: Morphogy and accessibility. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 797–813. [Google Scholar]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Aulin, C.; Netrval, J.; Wågberg, L.; Lindström, T. Aerogels from nanofibrillated cellulose with tunable oleophobicity. Soft Matter 2010, 6, 3298–3305. [Google Scholar] [CrossRef]
- Zimmermann, T.; Pöhler, E.; Geiger, T. Cellulose fibrils for polymer reinforcement. Adv. Eng. Mater. 2004, 6, 754–761. [Google Scholar] [CrossRef]
- Abe, K.; Iwamoto, S.; Yano, H. Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules 2007, 8, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.; Ryu, J.; Youn, H.J. Effect of the number of passes through grinder on the pore characteristics of nanofibrillated cellulose mat. J. Korea TAPPI 2013, 45, 35–41. [Google Scholar]
- Taniguchi, T.; Okamura, K. New films produced from microfibrillated natural fibres. Polym. Int. 1998, 47, 291–294. [Google Scholar] [CrossRef]
- Chen, P.; Yu, H.; Liu, Y.; Chen, W.; Wang, X.; Ouyang, M. Concentration effects on the isolation and dynamic rheological behavior of cellulose nanofibers via ultrasonic processing. Cellulose 2013, 20, 149–157. [Google Scholar] [CrossRef]
- Wang, H.; Li, D.; Zhang, R. Preparation of ultralong cellulose nanofibers and optically transparent nanopapers derived from waste corrugated paper pulp. BioResources 2013, 8, 1374–1384. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y. Preparation of millimeter-long cellulose I nanofibers with diameters of 30–80 nm from bamboo fibers. Carbohydr. Polym. 2011, 86, 453–461. [Google Scholar] [CrossRef]
- Pinjari, D.V.; Pandit, A.B. Cavitation milling of natural cellulose to nanofibrils. Ultrason. Sonochem. 2010, 17, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, M.; Henriksson, G.; Berglund, L.A.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Tejado, A.; Alam, M.N.; Antal, M.; Yang, H.; van de Ven, T.G.M. Energy requirements for the disintegration of cellulose fibers into cellulose nanofibers. Cellulose 2012, 19, 831–842. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Fall, A.B.; Lindström, S.B.; Sundman, O.; Ödberg, L.; Wảgberg, L. Colloidal stability of aqueous nanofibrillated cellulose dispersions. Langmuir 2011, 27, 11332–11338. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, C.Y.; Jeong, H.S.; Lee, J.H.; Kim, H.J. Manufacture of conservational machine-made Hanji based on domestic paper mulberry fibers. J. Korea TAPPI 2016, 48, 257–285. [Google Scholar] [CrossRef]
- Kang, K.H.; Kim, H.J. Studies on the reinforcement treatment of aged Hanji using cellulose derivative solutions. J. Korea TAPPI 2011, 43, 40–48. [Google Scholar]
- Choi, T.H.; Cho, N.S. New korean traditional papermaking from paper mulberry(I): Pulping characteristics of broussonetia kazinoki siebold. J. Korea TAPPI 1996, 28, 49–59. [Google Scholar]
- Perdue, E.R.; Nieschlag, H.J. Fiber dimentions of nonwoody plant materials. Tappi J. 1961, 44, 776–784. [Google Scholar]
- Chaker, A.; Mutje, P.; Vilaseca, F.; Boufi, S. Reinforcing potential of nanofibrillated cellulose from nonwoody plants. Polym. Compos. 2013, 34, 1999–2007. [Google Scholar] [CrossRef]
- Alila, S.; Besbes, I.; Vilar, M.R.; Mutjé, P.; Boufi, S. Non-woody plants as raw materials for production of microfibrillated cellulose (MFC): A comparative study. Ind. Crops Prod. 2013, 41, 250–259. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Huang, B.; Huang, M.; Ai, X. Isolation and characteristics of cellulose and nanocellulose from lotus leaf stalk agro-wastes. BioResources 2015, 10, 684–696. [Google Scholar] [CrossRef]
- Lin, J.; Yu, L.; Tian, F.; Zhao, N.; Li, X.; Bian, F.; Wang, J. Cellulose nanofibrils aerogels generated from jute fibers. Carbohydr. Polym. 2014, 109, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Azraaie, N.; Abidin, N.A.M.Z.; Aziz, F.A.; Radiman, S.; Ismail, A.; Zin, W.Y.W.M.; Nor, N.M.; Sohaimi, R.M.; Sulaiman, A.Z.; Halim, N.A.; et al. Cellulose Microfibrils/Nanofibrils (CMNF) Produced from Banana (Musa acuminata) Pseudo-Stem Wastes: Isolation and Characterization. Mater. Sci. Forum 2016, 846, 448–453. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Sood, Y.V.; Tyagi, R.; Tyagi, S.; Pande, P.C.; Tondon, R. Surface charge of different paper making raw materials and its influence on paper properties. J. Sci. Ind. Res. 2010, 69, 300–304. [Google Scholar]
- Bismark, A.; Springer, J.; Mohanty, A.K.; Hinrichsen, G.; Khan, M.A. Characterization of several modified jute fibers using zeta-potential mesurements. Colloid Polym. Sci. 2000, 278, 229–235. [Google Scholar] [CrossRef]
- Mendoza, L.; Batchelor, W.; Tabor, R.F.; Garnier, G. Gelation mechanism of cellulose nanofibre gels: A colloids and interfacial perspective. J. Colloid Interface Sci. 2018, 509, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ding, B.; Yu, J.; Al-Deyab, S.S. Cellulose nanowhiskers extracted from TEMPO-oxidized jute fibers. Carbohydr. Polym. 2012, 90, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Puangsin, B.; Yang, Q.; Saito, T.; Isogai, A. Comparative characterization of TEMPO-oxidized cellulose nanofibril films prepared from non-wood resources. Int. J. Biol. Macromol. 2013, 59, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, H.; Saito, T.; Isogai, A. Influence of TEMPO-oxidized cellulose nanofibril length on film properties. Carbohydr. Polym. 2013, 93, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Tarrés, Q.; Boufi, S.; Mutjé, P.; Delgado-Aguilar, M. Enzymatically hydrolyzed and TEMPO-oxidized cellulose nanofibers for the production of nanopapers: Morphological, optical, thermal and mechanical properties. Cellulose 2017, 24, 3943–3954. [Google Scholar] [CrossRef]



| Sample Form | Nanopaper Sheet | |
|---|---|---|
| Source of TEMPO-Oxidized CNFs | PMBFs | Softwood Fibers |
| Tensile strength (MPa) | 110.18 ± 12.88 | 84.19 ± 10.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.Y.; Park, C.-W.; Han, S.-Y.; Kwon, G.-J.; Kim, N.-H.; Lee, S.-H. Effects of pH on Nanofibrillation of TEMPO-Oxidized Paper Mulberry Bast Fibers. Polymers 2019, 11, 414. https://doi.org/10.3390/polym11030414
Park JY, Park C-W, Han S-Y, Kwon G-J, Kim N-H, Lee S-H. Effects of pH on Nanofibrillation of TEMPO-Oxidized Paper Mulberry Bast Fibers. Polymers. 2019; 11(3):414. https://doi.org/10.3390/polym11030414
Chicago/Turabian StylePark, Jung Yoon, Chan-Woo Park, Song-Yi Han, Gu-Joong Kwon, Nam-Hun Kim, and Seung-Hwan Lee. 2019. "Effects of pH on Nanofibrillation of TEMPO-Oxidized Paper Mulberry Bast Fibers" Polymers 11, no. 3: 414. https://doi.org/10.3390/polym11030414





