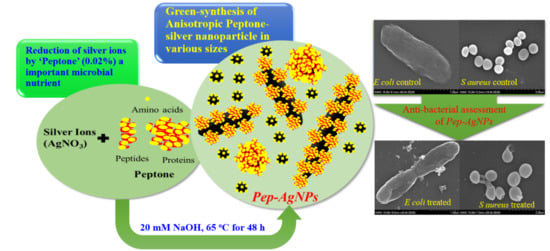
Green-Synthesis of Anisotropic Peptone-Silver Nanoparticles and Its Potential Application as Anti-Bacterial Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Green-Route Synthesis of Pep-AgNPs
2.2. Characterization of Pep-AgNPs
2.3. Microbial Strains
2.4. Antibacterial Test
2.5. SEM Analysis of Bacterial Surface Morphology Observation
3. Results and Discussions
3.1. Pep-AgNPs Synthesis
3.2. Pep-AgNPs Characterizations
3.2.1. FT-IR Analysis
3.2.2. XRD Analysis
3.2.3. TEM Analysis
3.2.4. XPS Analysis
3.3. Anti-Bacterial Assessments of Pep-AgNPs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mao, X.; Cheng, R.; Zhang, H.; Bae, J.; Cheng, L.; Zhang, L.; Deng, L.; Cui, W.; Zhang, Y.; Santos, H.A.; et al. Self-Healing and Injectable Hydrogel for Matching Skin Flap Regeneration. Adv. Sci. 2018, 1801555. [Google Scholar]
- Abu-Saied, M.A.; Taha, T.H.; El-Deeb, N.M.; Hafez, E.E. Polyvinyl alcohol/Sodium alginate integrated silver nanoparticles as probable solution for decontamination of microbes contaminated water. Int. J. Biol. Macromol. 2018, 107, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Rhim, J.W.; Won, K. Preparation of poly(lactide)/lignin/silver nanoparticles composite films with UV light barrier and antibacterial properties. Int. J. Biol. Macromol. 2018, 107, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Raghavendra, G.M.; Kim, D.; Seo, J. One-step synthesis of starch-silver nanoparticle solution and its application to antibacterial paper coating. Int. J. Biol. Macromol. 2018, 107, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, X.; Zheng, Y.; Wang, Z.; Ma, Z.; Yang, Q.; Yao, B.; Zhao, Y.; Zhang, H. A green approach for synthesizing silver nanoparticles, and their antibacterial and cytotoxic activities. New J. Chem. 2018, 42, 2882–2888. [Google Scholar] [CrossRef]
- Michalska, M.; Iwan, A.; Andrzejczuk, M.; Roguska, A.; Sikora, A.; Boharewicz, B.; Tazbir, I.; Hreniak, A.; Popłoński, S.; Korona, K.P.; et al. Analysis of the surface decoration of TiO2 grains using silver nanoparticles obtained by ultrasonochemical synthesis towards organic photovoltaics. New J. Chem. 2018, 42, 7340–7354. [Google Scholar] [CrossRef]
- Yan, L.; Xiang, Y.; Yu, J.; Wang, Y.; Cui, W. Fabrication of Antibacterial and Antiwear Hydroxyapatite Coatings via in Situ Chitosan-Mediated Pulse Electrochemical Deposition. ACS Appl. Mater. Interfaces 2017, 9, 5023–5030. [Google Scholar] [CrossRef]
- Sokołowski, K.; Szynkowska, M.I.; Pawlaczyk, A.; Łukomska-Szymańska, M.; Sokołowski, J. The impact of nanosilver addition on element ions release form light-cured dental composite and compomer into 0.9% NaCl. Acta Biochim. Pol. 2014, 61, 317–323. [Google Scholar]
- Nanda Kumar, D.; Chandrasekaran, N.; Mukherjee, A. Horseradish peroxidase-mediated in situ synthesis of silver nanoparticles: Application for sensing of mercury. New J. Chem. 2018, 42, 13763–13769. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Peng, Y. The interaction of silver nanoparticles with papain and bromelain. New J. Chem. 2018, 42, 4940–4950. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Jung, J.; kim, D.; Seo, J. Step-reduced synthesis of starch-silver nanoparticles. Int. J. Biol. Macromol. 2016, 86, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wu, C.; Wu, T.; Yuan, C.; Chen, S.; Ding, T.; Ye, X.; Hu, Y. Green synthesis of sodium alginate-silver nanoparticles and their antibacterial activity. Int. J. Biol. Macromol. 2018, 111, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Ghodake, G.; Lim, S.R.; Lee, D.S. Casein hydrolytic peptides mediated green synthesis of antibacterial silver nanoparticles. Colloids Surf. B Biointerfaces 2013, 108, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Ma, Y.; Xia, G.; Wang, Y.; Kapadia, W.; Sun, Z.; Wu, W.; Gu, H.; Cui, W.; Huang, X. In vitro and in vivo combined antibacterial effect of levofloxacin/silver co-loaded electrospun fibrous membranes. J. Mater. Chem. B 2017, 5, 7632–7643. [Google Scholar] [CrossRef]
- Tamboli, D.P.; Lee, D.S. Mechanistic antimicrobial approach of extracellularly synthesized silver nanoparticles against gram positive and gram negative bacteria. J. Hazard. Mater. 2013, 260, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Suk Sung, J.; Kim, M.; Ghodake, G. Rapid production of silver nanoparticles at large-scale using gallic acid and their antibacterial assessment. Mater. Lett. 2015, 155, 62–64. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Freitas, F.; Reis, M.A.M.; Prieto, M.A.; Lagaron, J.M. Biosynthesis of silver nanoparticles and polyhydroxybutyrate nanocomposites of interest in antimicrobial applications. Int. J. Biol. Macromol. 2018, 108, 426–435. [Google Scholar] [CrossRef]
- Calderón-Jiménez, B.; Johnson, M.E.; Montoro Bustos, A.R.; Murphy, K.E.; Winchester, M.R.; Vega Baudrit, J.R. Silver Nanoparticles: Technological Advances, Societal Impacts, and Metrological Challenges. Front. Chem. 2017, 5, 1–26. [Google Scholar] [CrossRef]
- Hutchison, J.E. Greener nanoscience: A proactive approach to advancing applications and reducing implications of nanotechnology. ACS Nano 2008, 2, 395–402. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnology 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, M.; Shinde, S.; Sung, J.S.; Ghodake, G. Cytotoxicity and antibacterial assessment of gallic acid capped gold nanoparticles. Colloids Surf. B Biointerfaces 2017, 149, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Orak, T.; Caglar, O.; Ortucu, S.; Ozkan, H.; Taskin, M. Chicken feather peptone: A new alternative nitrogen source for pigment production by Monascus purpureus. J. Biotechnol. 2018, 271, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Jung, S.; Park, K.-H.; Kim, Y.-R. Microbial Biosynthesis of Silver Nanoparticles in Different Culture Media. J. Agric. Food Chem. 2018, 66, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, W. FTIR Analysis of Protein Structure. Biochemistry 1997, 392, 662–666. [Google Scholar]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Balaji, D.S.; Basavaraja, S.; Deshpande, R.; Mahesh, D.B.; Prabhakar, B.K.; Venkataraman, A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surfaces B Biointerfaces 2009, 68, 88–92. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Yazgan, I.; Akgul, A.; Ontman, R.; Kariuki, V.M.; Eshun, G.B.; Sadik, O.A. Flavonoid-derived anisotropic silver nanoparticles inhibit growth and change the expression of virulence genes in Escherichia coli SM10†. RSC Adv. 2018, 8, 4649–4661. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Singh, H.; Mathiyalagan, R.; Wang, C.; Yang, D.C. Biosynthesis of anisotropic silver nanoparticles by bhargavaea indica and their synergistic effect with antibiotics against pathogenic microorganisms. J. Nanomater. 2015, 2015, 4. [Google Scholar] [CrossRef]
- Sangappa, Y.; Latha, S.; Asha, S.; Sindhu, P.; Parushuram, N.; Shilpa, M.; Byrappa, K.; Narayana, B. Synthesis of anisotropic silver nanoparticles using silk fibroin: Characterization and antimicrobial properties. Mater. Res. Innov. 2017, 23, 79–85. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. J. Biol. Chem. 2015, 290, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Chook, S.W.; Chia, C.H.; Zakaria, S.; Ayob, M.K.; Chee, K.L.; Huang, N.M.; Neoh, H.M.; Lim, H.N.; Jamal, R.; Rahman, R.M.F.R.A. Antibacterial performance of Ag nanoparticles and AgGO nanocomposites prepared via rapid microwave-assisted synthesis method. Nanoscale Res. Lett. 2012, 7, 541. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Silver nanoparticles as an antimicrobial agent: A case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-negative bacteria. J. Gen. Appl. Microbiol. 2017, 63, 36–43. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Jee, S.-C.; Shinde, S.K.; Mistry, B.M.; Saratale, R.G.; Saratale, G.D.; Ghodake, G.S.; Kim, D.-Y.; Sung, J.-S.; Kadam, A.A. Green-Synthesis of Anisotropic Peptone-Silver Nanoparticles and Its Potential Application as Anti-Bacterial Agent. Polymers 2019, 11, 271. https://doi.org/10.3390/polym11020271
Kim M, Jee S-C, Shinde SK, Mistry BM, Saratale RG, Saratale GD, Ghodake GS, Kim D-Y, Sung J-S, Kadam AA. Green-Synthesis of Anisotropic Peptone-Silver Nanoparticles and Its Potential Application as Anti-Bacterial Agent. Polymers. 2019; 11(2):271. https://doi.org/10.3390/polym11020271
Chicago/Turabian StyleKim, Min, Seung-Cheol Jee, Surendra K. Shinde, Bhupendra M. Mistry, Rijuta Ganesh Saratale, Ganesh Dattatraya Saratale, Gajanan S. Ghodake, Dae-Young Kim, Jung-Suk Sung, and Avinash A. Kadam. 2019. "Green-Synthesis of Anisotropic Peptone-Silver Nanoparticles and Its Potential Application as Anti-Bacterial Agent" Polymers 11, no. 2: 271. https://doi.org/10.3390/polym11020271