Phase Behavior of a Carbon Dioxide/Methyl Trimethoxy Silane/Polystyrene Ternary System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Apparatus and Procedure
3. Results and Discussion
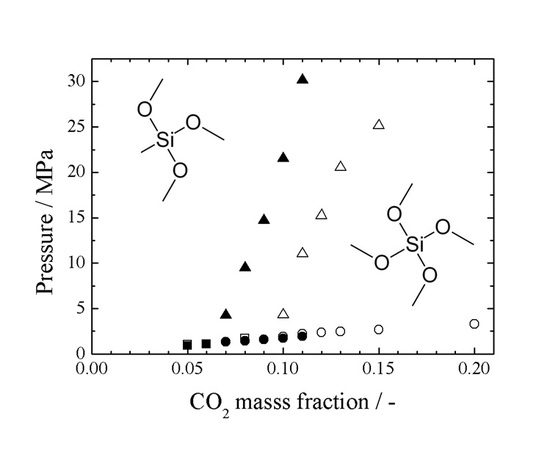
3.1. Effects of Experimental Parameters
3.2. Comparison with the CO2/TMOS/PS System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hüsing, N.; Schubert, U. Aergogels-Airy Materials: Chemistry, Structure, and Properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expand Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Lu, X.; Arduini-Schuster, M.C.; Kuhn, J.; Nilsson, O.; Fricke, J.; Pekala, R.W. Thermal Conductivity of Monolithic Organic Aerogels. Science 1992, 255, 971–972. [Google Scholar] [CrossRef] [PubMed]
- Buratti, C.; Moretti, E. Glazing systems with silica aerogel for energy savings in buildings. Appl. Energy 2012, 98, 396–403. [Google Scholar] [CrossRef]
- Buratti, C.; Moretti, E. Experimental performance evaluation of aerogel glazing systems. Appl. Energy 2012, 97, 430–437. [Google Scholar] [CrossRef]
- Kanamori, K.; Aizawa, M.; Nakanishi, K.; Hanada, T. New Transparent Methylsilsesquioxane Aerogels and Xerogels with Improved Mechanical Properties. Adv. Mater. 2007, 19, 1589–1593. [Google Scholar] [CrossRef]
- Haereid, S.; Anderson, J.; Einarsrud, M.A.; Hua, D.W.; Smith, D.M. Thermal and temporal aging of TMOS-based aerogel precursors in water. J. Non-Crystal. Solids 1995, 185, 221–226. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; Huang, Y.-S.; Shen, M.-Y. Mechanical properties and toughness of carbon aerogel/epoxy polymer composites. J. Mater. Sci. 2015, 50, 3258–3266. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, J.; Feng, J. Synthesis and characterization of ambient-dried microglass fibers/silica aerogel nanocomposites with low thermal conductivity. J. Sol-Gel Sci. Technol. 2017, 83, 64–71. [Google Scholar] [CrossRef]
- Slosarczyk, A. Recent Advances in Research on the Synthetic Fiber Based Silica Aerogel Nanocomposites. Nanomaterials 2017, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Colton, J.S.; Suh, N.P. The Nucleation of Microcelluar Thermoplastic Foam With Additives: Part I: Theoretical Considerations. Polym. Eng. Sci. 1987, 27, 485–492. [Google Scholar] [CrossRef]
- Goel, S.K.; Beckman, E.J. Generation of Microcellular Polymeric Foams Using Supercritical Carbon Dioxide. I: Effect of Pressure and Temperature on Nucleation. Polym. Eng. Sci. 1994, 34, 1137–1147. [Google Scholar] [CrossRef]
- Kumar, V.; Weller, J. Production of Microcellular Polycarbonate Using Carbon Dioxide for Bubble Nucleation. J. Eng. Ind. 1994, 116, 413–420. [Google Scholar] [CrossRef]
- Han, C.D.; Ma, C.-Y. Foam Extrusion Characteristics of Thermoplastic Resin with Fluorocarbon Blowing Agent. I. Low-Density Polyethylene Foam Extrusion. J. Appl. Polym. Sci. 1983, 28, 2961–2982. [Google Scholar] [CrossRef]
- Yoda, S.; Ohara, M.; Furuya, T.; Otake, K. Fabrication of Polymer Form-Silica Nanocomposite Thermal Insulator Via High Pressure Mixture of Polymer/Silicon Alkoxide/Carbon Dioxide. In Proceedings of the 13th European Meeting on Supercritical Fluids, Hague, The Netherlands, 9–13 October 2011. [Google Scholar]
- Matsukawa, H.; Shimada, Y.; Yoda, S.; Okawa, Y.; Naya, M.; Shono, A.; Otake, K. Phase behavior of Carbon dioxide/Tetramethyl orthosilicate/polymer ternary systems. Fluid Phase Equilib. 2018, 457, 1–10. [Google Scholar] [CrossRef]
- Hamada, T.; Kobayashi, D.; Takahashi, T.; Shono, A.; Otake, K.; Tsuji, T.; Yoda, S.; Furuya, T. Phase behavior for carbon dioxide/tetraalkoxysilane systems. Fluid Phase Equilib. 2012, 322–323, 135–141. [Google Scholar] [CrossRef]
- Matsukawa, H.; Shimada, Y.; Yoda, S.; Okawa, Y.; Naya, M.; Shono, A.; Otake, K. Phase behavior of Carbon dioxide/Trimethoxy(methyl)silane and Methylsilicate 51 system. Fluid Phase Equilib. 2018, 455, 6–14. [Google Scholar] [CrossRef]
- Santos, T.M.M.; Rebelatto, E.A.; Chaves, B.B.; Lanza, M.; Oliveira, J.V.; Albuquerque, E.C.M.C.; Vieira de Melo, S.A.B. High pressure phase equilibrium data for carbon dioxide, methyl methacrylate and poly (dimethylsiloxane) systems. J. Supercrit. Fluids 2019, 143, 346–352. [Google Scholar] [CrossRef]
- Latsky, C.; Schwarz, C.E. High pressure bubble- and dew-point data for a system containing CO2 with detergent range alkanes and alcohols. J. Supercrit. Fluids 2018, 141, 265–273. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E.; De Marco, I. PVP microparticles precipitation from acetone-ethanol mixtures using SAS process: Effect of phase behavior. J. Supercrit. Fluids 2019, 143, 321–329. [Google Scholar] [CrossRef]
- Im, T.; Gwon, J.; Kim, S.H.; Shin, H.Y.; Kim, H. High-Pressure Phase Behavior of Poly(l-lactic acid), Trichloromethane, and Carbon Dioxide Ternary Mixture Systems. J. Chem. Eng. Data 2015, 60, 2172–2177. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Yoon, S.-D.; Byun, H.-S. Cloud-Point and Vapor-Liquid Behavior of Binary and Ternary Systems for the Poly(dodecyl acrylate) + Cosolvent and Dodecyl Acrylate in Supercritical Solvents. J. Chem. Eng. Data 2010, 55, 3684–3689. [Google Scholar] [CrossRef]
- Kirby, C.F.; McHugh, M.A. Phase Behavior of Polymers in Supercritical Fluid Solvents. Chem. Rev. 1999, 99, 565–600. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, C.; Güner, A. Solubility profiles of poly(ethylene glycol)/solvent systems, I: Qualitive comparison of solubility parameter approaches. Eur. Polym. J. 2007, 43, 3068–3093. [Google Scholar] [CrossRef]
- Krevelen, D.W.V. Properties of Polymers, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Hildebrand, J.H. THE ENTROPY OF VAPORIZATION AS A MEANS OF DISTINGUISHING NORMAL LIQUIDS. J. Am. Chem. Soc. 1915, 37, 970–978. [Google Scholar] [CrossRef]
- Hildebrand, J.H. THE VAPOR PRESSURES OF LIQUID METALS. J. Am. Chem. Soc. 1918, 40, 45–49. [Google Scholar] [CrossRef]
- Fedors, R.F. A Method for Estimating Both the Solubility Parameters and Molar Volumes of Liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Marcus, Y. Solubility Parameter of Carbon Dioxide-An Enigma. ACS Omega 2018, 3, 524–528. [Google Scholar] [CrossRef]






| Component | CAS Number | Supplier | Mole Fraction Purity (Supplier) | |
| Carbon dioxide, CO2 | 124-38-9 | Showa Yozai Co. | 0.9999 | |
| Methyltri(methoxy)silane, MTMS | 1185-55-3 | Tokyo Kasei Co. | 0.98 | |
| Component | CAS Number | Supplier | MW | MW/Mn |
| Polystyrene, PS | 9003-53-6 | Sigma Aldrich Co. | 35,000 | 2.020 |
| Kanto Kagaku Co. | 250,000 | 2.348 | ||
| m1 (CO2) /- | m2 (MTMS) /- | m3 (PS) /- | BP (VLE) /MPa | BP (VLLE) /MPa | CP (LLE) /MPa | ±Δm1 × 103 /- |
|---|---|---|---|---|---|---|
| MTMS:PS = 9.5:0.5 | ||||||
| 0.024 | 0.926 | 0.050 | 0.43 | 1.31 | ||
| 0.050 | 0.902 | 0.048 | 1.02 | 2.79 | 1.26 | |
| 0.062 | 0.890 | 0.048 | 1.20 | 8.36 | 1.24 | |
| 0.070 | 0.883 | 0.047 | 1.30 | 13.34 | 1.24 | |
| 0.079 | 0.874 | 0.047 | 1.46 | 19.51 | 1.23 | |
| 0.091 | 0.863 | 0.046 | 1.59 | 27.22 | 1.22 | |
| MTMS:PS = 9:1 | ||||||
| 0.048 | 0.854 | 0.098 | 0.91 | 1.26 | ||
| 0.058 | 0.845 | 0.097 | 1.09 | 1.25 | ||
| 0.073 | 0.832 | 0.095 | 1.32 | 4.26 | 1.23 | |
| 0.080 | 0.826 | 0.095 | 1.44 | 9.47 | 1.23 | |
| 0.089 | 0.817 | 0.094 | 1.59 | 14.70 | 1.22 | |
| 0.098 | 0.809 | 0.093 | 1.73 | 21.53 | 1.22 | |
| 0.112 | 0.797 | 0.091 | 1.93 | 30.13 | 1.22 | |
| MTMS:PS = 8:2 | ||||||
| 0.046 | 0.764 | 0.190 | 0.95 | 1.27 | ||
| 0.071 | 0.744 | 0.185 | 1.46 | 1.24 | ||
| 0.083 | 0.734 | 0.183 | 1.65 | 1.88 | 1.23 | |
| 0.095 | 0.725 | 0.181 | 1.89 | 6.38 | 1.22 | |
| 0.104 | 0.717 | 0.179 | 2.01 | 11.48 | 1.22 | |
| 0.117 | 0.707 | 0.176 | 2.24 | 21.64 | 1.22 | |
| 0.124 | 0.701 | 0.175 | 2.35 | 25.56 | 1.22 | |
| MTMS:PS = 7:3 | ||||||
| 0.050 | 0.665 | 0.284 | 0.95 | 1.26 | ||
| 0.071 | 0.651 | 0.278 | 1.44 | 1.24 | ||
| 0.093 | 0.635 | 0.272 | 1.95 | 1.22 | ||
| 0.107 | 0.625 | 0.267 | 2.15 | 3.63 | 1.22 | |
| 0.119 | 0.617 | 0.264 | 2.39 | 12.56 | 1.22 | |
| 0.125 | 0.613 | 0.262 | 2.44 | 16.20 | 1.22 | |
| 0.132 | 0.608 | 0.260 | 2.60 | 23.13 | 1.22 | |
| 0.136 | 0.605 | 0.259 | 2.70 | 27.63 | 1.22 | |
| m1 (CO2) /- | m2 (MTMS) /- | m3 (PS) /- | BP (VLE) /MPa | BP (VLLE) /MPa | CP (LLE) /MPa | ±Δm1 × 103 /- |
|---|---|---|---|---|---|---|
| MTMS:PS = 9:1 | ||||||
| 0.048 | 0.856 | 0.096 | 1.46 | 1.26 | ||
| 0.058 | 0.847 | 0.095 | 1.66 | 1.25 | ||
| 0.068 | 0.838 | 0.094 | 2.00 | 3.60 | 1.24 | |
| 0.077 | 0.829 | 0.093 | 2.21 | 6.28 | 1.23 | |
| 0.087 | 0.821 | 0.092 | 2.32 | 7.58 | 1.22 | |
| 0.098 | 0.810 | 0.091 | 2.68 | 11.91 | 1.22 | |
| 0.110 | 0.800 | 0.090 | 3.05 | 17.07 | 1.22 | |
| 0.121 | 0.790 | 0.089 | 3.45 | 23.51 | 1.22 | |
| m1 (CO2) /- | m2 (MTMS) /- | m3 (PS) /- | BP (VLE) /MPa | BP (VLLE) /MPa | CP (LLE) /MPa | ±Δm1 × 103 /- |
|---|---|---|---|---|---|---|
| MTMS:PS = 9:1 | ||||||
| 0.020 | 0.881 | 0.099 | 0.43 | 1.32 | ||
| 0.039 | 0.864 | 0.097 | 0.74 | 3.62 | 1.28 | |
| 0.049 | 0.855 | 0.096 | 0.92 | 9.91 | 1.26 | |
| 0.062 | 0.843 | 0.095 | 1.05 | 15.50 | 1.25 | |
| 0.072 | 0.834 | 0.094 | 1.22 | 23.06 | 1.24 | |
| 0.082 | 0.826 | 0.093 | 1.34 | 29.01 | 1.23 | |
| MTMS:PS = 8:2 | ||||||
| 0.032 | 0.774 | 0.194 | 0.70 | 1.29 | ||
| 0.057 | 0.754 | 0.189 | 1.08 | 6.60 | 1.25 | |
| 0.066 | 0.747 | 0.187 | 1.28 | 13.83 | 1.24 | |
| 0.075 | 0.740 | 0.185 | 1.41 | 18.01 | 1.23 | |
| 0.080 | 0.736 | 0.184 | 1.60 | 24.10 | 1.23 | |
| 0.091 | 0.727 | 0.182 | 1.70 | 30.67 | 1.22 | |
| T/K | BP (VLLE) /MPa | CP(LLE) /MPa |
|---|---|---|
| 313.2 | 1.70 | 19.78 |
| 333.2 | 2.32 | 16.02 |
| 353.2 | 2.84 | 14.36 |
| 373.2 | 3.36 | 15.01 |
| 393.2 | 4.09 | 17.34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsukawa, H.; Yoda, S.; Okawa, Y.; Otake, K. Phase Behavior of a Carbon Dioxide/Methyl Trimethoxy Silane/Polystyrene Ternary System. Polymers 2019, 11, 246. https://doi.org/10.3390/polym11020246
Matsukawa H, Yoda S, Okawa Y, Otake K. Phase Behavior of a Carbon Dioxide/Methyl Trimethoxy Silane/Polystyrene Ternary System. Polymers. 2019; 11(2):246. https://doi.org/10.3390/polym11020246
Chicago/Turabian StyleMatsukawa, Hiroaki, Satoshi Yoda, Yasuo Okawa, and Katsuto Otake. 2019. "Phase Behavior of a Carbon Dioxide/Methyl Trimethoxy Silane/Polystyrene Ternary System" Polymers 11, no. 2: 246. https://doi.org/10.3390/polym11020246