Atmospheric Pressure Plasma Polymerized Oxazoline-Based Thin Films—Antibacterial Properties and Cytocompatibility Performance
Abstract
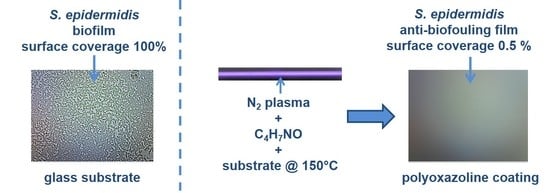
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Deposition
2.3. Surface Characterization
2.4. Characterization of Mechanical Properties
2.5. Antibacterial Tests
2.6. Cytocompatibility Test
3. Results
3.1. Surface Characterization
3.2. FTIR Analysis
3.3. Antibacterial Properties
3.4. Cytocompatibility Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Woodle, M.C.; Engbers, C.M.; Zalipsky, S. New Amphipatic Polymer Lipid Conjugates Forming Long-Circulating Reticuloendothelial System-Evading Liposomes. Bioconj. Chem. 1994, 5, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Zalipsky, S.; Hansen, C.B.; Oaks, J.M.; Allen, T.M. Evaluation of Blood Clearance Rates and Biodistribution of Poly(2-oxazoline)-grafted Liposomes. J. Pharm. Sci. 1996, 85, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Goddard, P.; Hutchinson, L.E.; Brown, J.; Brookman, L.J. Soluble Polymeric Carriers for Drug Delivery. Part 2. Preparation and in Vivo Behaviour of N-acylethylenimine Copolymers. J. Control. Release 1989, 10, 5–16. [Google Scholar] [CrossRef]
- Jordan, R.; Ulman, A. Surface Initiated Living Cationic Polymerization of 2-Oxazolines. J. Am. Chem. Soc. 1998, 120, 243–247. [Google Scholar] [CrossRef]
- Bouten, P.J.M.; Hertsen, D.; Vergaelen, M.; Monnery, B.D.; Boerman, M.A.; Goossens, H.; Catak, S.; van Hest, J.C.M.; Van Speybroeck, V.; Hoogenboom, R. Accelerated Living Cationic Ringopening Polymerization of a Methyl Ester Functionalized 2-Oxazoline Monomer. Polym. Chem. 2015, 6, 514–518. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Tong, Q.; Yan, M. Evaluation of Photochemically Immobilized Poly(2-ethyl-2-oxazoline) Thin Films as Protein-Resistant Surfaces. ACS Appl. Mater. Interfaces 2011, 3, 3463–3471. [Google Scholar] [CrossRef] [Green Version]
- Pidhatika, B.; Rodenstein, M.; Chen, Y.; Rakhmatullina, E.; Mühlebach, A.; Acikgöz, C.; Textor, M.; Konradi, R. Comparative Stability Studies of Poly(2-methyl-2-oxazoline) and Poly(ethyleneglycol) Brush Coatings. Biointerphases 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, K. Nanoengineered Plasma Polymer Films for Biomaterial Applications. Plasma Chem. Plasma Process. 2014, 34, 545–558. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization—A Review. Plasma Processes Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Duan, S.; Liu, X.; Wang, Y.; Meng, Y.; Alsaedi, A.; Hayat, T.; Li, J. Plasma Surface Modification of Materials and Their Entrapment of Water Contaminant: A Review. Plasma Process Polym. 2017, 14, e1600218. [Google Scholar] [CrossRef]
- Yue, M.; Zhou, B.; Jiao, K.; Qian, X.; Xu, Z.; Teng, K.; Zhao, L.; Wang, J.; Jiao, Y. Switchable Hydrophobic/Hydrophilic Surface of Electrospun Poly(l-lactide) Membranes Obtained by CF4 Microwave Plasma Treatment. Appl. Surf. Sci. 2015, 327, 93–99. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, Z.; Chen, L.; Li, W.; Han, Q.; Li, C.; Xu, Z.; Qian, X. PECVD-Induced Growing of Diverse Nanomaterials on Carbon Nanofibers under Various Conditions. Mater. Lett. 2018, 216, 291–294. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Xu, Z.; Lv, H.; Wu, N.; Zhou, B.; Mai, W.; Zhao, L.; Tian, X.; Guo, X. Improvement for Interface Adhesion of Epoxy/Carbon Fibers Endowed With Carbon Nanotubes via Microwave Plasma-Enhanced Chemical Vapor Deposition. Polym. Compos. 2018, 39, E1262–E1268. [Google Scholar] [CrossRef]
- Elias, M.; Kloc, P.; Jasek, O.; Mazankova, V.; Trunec, D.; Hrdy, R.; Zajickova, L. Atmospheric Pressure Barrier Discharge at High Temperature: Diagnostics and Carbon Nanotubes Deposition. J. Appl. Phys. 2015, 117, 103301. [Google Scholar] [CrossRef]
- Ramiasa, M.; Cavallaro, A.; Mierczynska, A.; Christo, S.; Gleadle, J.; Hayball, J.D.; Vasilev, K. Plasma Polymerised PolyOxazoline Thin Films for Biomedical Applications. Chem. Commun. 2015, 51, 4279–4282. [Google Scholar] [CrossRef] [PubMed]
- Macgregor-Ramiasa, M.N.; Cavallaro, A.A.; Vasilev, K. Properties and Reactivity of Polyoxazoline Plasma Polymer Films. J. Mater. Chem. B 2015, 3, 6327–6337. [Google Scholar] [CrossRef]
- Cavallaro, A.A.; Macgregor-Ramiasa, M.N.; Vasilev, K. Antibiofouling Properties of Plasma-Deposited Oxazoline-Based Thin Films. ACS Appl. Mater. Interfaces 2016, 8, 6354–6362. [Google Scholar] [CrossRef]
- Gherardi, N.; Gouda, G.; Gat, E.; Ricard, A.; Massines, F. Transition from Glow Silent Discharge to Micro-discharges in Nitrogen Gas. Plasma Sources Sci. Technol. 2000, 9, 340–346. [Google Scholar] [CrossRef]
- Gherardi, N.; Martin, S.; Massines, F. A New Approach to SiO2 Deposit using a N2–SiH4–N2O Glow Dielectric Barrier-Controlled Discharge at Atmospheric Pressure. J. Phys. D Appl. Phys. 2000, 33, L104–L108. [Google Scholar] [CrossRef]
- Trunec, D.; Navratil, Z.; Stahel, P.; Zajickova, L.; Bursikova, V.; Cech, J. Deposition of Thin Organosilicon Polymer Films in Atmospheric Pressure Glow Discharge. J. Phys. D Appl. Phys. 2004, 37, 2112–2120. [Google Scholar] [CrossRef]
- Trunec, D.; Zajickova, L.; Bursikova, V.; Studnicka, F.; Stahel, P.; Prysiazhnyi, V.; Perina, V.; Houdkova, J.; Navratil, Z.; Franta, D. Deposition of Hard Thin Films from HMDSO in Atmospheric Pressure Dielectric Barrier Discharge. J. Phys. D Appl. Phys. 2010, 43, 225403. [Google Scholar] [CrossRef]
- Al-Bataineh, S.A.; Cavallaro, A.A.; Michelmore, A.; Macgregor, M.N.; Whittle, J.D.; Vasilev, K. Deposition of 2-oxazoline- based Plasma Polymer Coatings using Atmospheric Pressure Helium Plasma Jet. Plasma Process Polym. 2019, 16, e1900104. [Google Scholar] [CrossRef]
- Van Guyse, J.F.R.; Cools, P.; Egghe, T.; Asadian, M.; Vergaelen, M.; Rigole, P.; Yan, W.; Benetti, E.M.; Jerca, V.; Declercq, H.; et al. Influence of the Aliphatic Side Chain on the Near Atmospheric Pressure Plasma Polymerization of 2-Alkyl-2-oxazolines for Biomedical Applications. ACS Appl. Mater. Interfaces 2019, 11, 31356–31366. [Google Scholar] [CrossRef] [PubMed]
- Obrusnik, A.; Jelinek, P.; Zajickova, L. Modelling of the Gas Flow and Plasma Co-polymerization of Two Monomers in an Atmospheric-Pressure Dielectric Barrier Discharge. Surf. Coat. Technol. 2017, 314, 139–147. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Zlotnikov, I.; Zolotoyabko, E.; Fratzl, P. Nano-scale Modulus Mapping of Biological Composite Materials: Theory and Practice. Prog. Mater. Sci. 2017, 87, 292–320. [Google Scholar] [CrossRef]
- Navratil, Z.; Bursikova, V.; Stahel, P.; Sira, M.; Zverina, P. On the Analysis of Surface Free Energy of DLC Coatings Deposited in Low Pressure RF Discharge. Czech. J. Phys. (Suppl. C) 2004, 54, C877–C882. [Google Scholar] [CrossRef]
- Deltombe, R.; Kubiak, K.J.; Bigerelle, M. How to Select the Most Relevant 3D Roughness Parameters of a Surface. Scanning 2014, 36, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Katsikogianni, M.; Missirlis, Y.F. Concise Review of Mechanisms of Bacterial Adhesion to Biomaterials and of Techniques Used in Estimating Bacteria–Material Interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Lerouge, S.; Major, A.; Girault-Lauriault, P.-L.; Raymond, M.-A.; Laplante, P.; Soulez, G.; Mwale, F.; Wertheimer, M.R.; Hébert, M.-J. Nitrogen-Rich Coatings for Promoting Healing around Stent-Grafts after Endovascular Aneurysm Repair. Biomaterials 2007, 28, 1209–1217. [Google Scholar] [CrossRef]







| Element | 60 C | 90 C | 120 C | 150 C |
|---|---|---|---|---|
| C | 43 | 43 | 47 | 48 |
| N | 40 | 41 | 42 | 39 |
| O | 17 | 16 | 11 | 13 |
| Sample | Contact Angle () | Surface Free Energy (mJ/m) | ||||
|---|---|---|---|---|---|---|
| CHI | Glycerol | Water | Total | LW | AB | |
| substrate | 59.8 ± 1.2 | 35.5 ± 2.0 | 33.4 ± 2.3 | 52.6 ± 1.0 | 28.7 ± 0.7 | 23.9 ± 2.0 |
| 60 C | 40.5 ± 1.0 | 28.9 ± 0.8 | 10.0 ± 2.0 | 56.6 ± 0.7 | 39.4 ± 0.6 | 17.3 ± 1.2 |
| 90 C | 40.8 ± 1.4 | 38.3 ± 0.5 | 16.1 ± 0.9 | 50.3 ± 0.9 | 39.2 ± 0.6 | 11.2 ± 1.4 |
| 120 C | 60.8 ± 1.7 | 50.5 ± 1.8 | 40.0 ± 2.8 | 42.4 ± 1.9 | 28.1 ± 1.9 | 14.3 ± 2.8 |
| 150 C | 60.6 ± 4.0 | 46.6 ± 1.7 | 21.9 ± 2.7 | 43.3 ± 1.8 | 27.8 ± 2.8 | 15.5 ± 4.0 |
| Sample | Thickness (m) | Hardness (GPa) | (GPa) |
|---|---|---|---|
| 60 C | 1.5 ± 0.2 | 0.70 ± 0.10 | 15 ± 1 |
| 90 C | 1.7 ± 0.1 | 0.55 ± 0.05 | 11 ± 1 |
| 120 C | 1.1 ± 0.1 | 0.55 ± 0.05 | 11 ± 1 |
| 150 C | 0.6 ± 0.1 | 0.60 ± 0.05 | 15 ± 1 |
| Sample | (GPa) | (GPa) | |
|---|---|---|---|
| 60 C | 16 ± 2 | 0.50 ± 0.05 | 0.031 ± 0.005 |
| 90 C | 11 ± 1 | 0.30 ± 0.04 | 0.027 ±0.006 |
| 120 C | 12 ± 1 | 0.37 ± 0.05 | 0.031 ± 0.005 |
| 150 C | 14 ± 1 | 0.48 ± 0.05 | 0.034 ± 0.005 |
| Sample | (nm) | (nm) | (nm) | (m |
|---|---|---|---|---|
| 60 C | 3.3 | 2.7 | 86.0 | 170 |
| 90 C | 6.2 | 4.2 | 70.6 | 140 |
| 120 C | 7.3 | 6.8 | 48.9 | 88 |
| 150 C | 4.8 | 3.7 | 62.6 | 278 |
| Sample | S. aureus (CFU/cm) | E. coli (CFU/cm) |
|---|---|---|
| substrate | ||
| 60 C | <1 | 4.4 |
| 90 C | <1 | 1.1 |
| 120 C | <1 | 4.4 |
| 150 C | 1.6 | 5.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sťahel, P.; Mazánková, V.; Tomečková, K.; Matoušková, P.; Brablec, A.; Prokeš, L.; Jurmanová, J.; Buršíková, V.; Přibyl, R.; Lehocký, M.; et al. Atmospheric Pressure Plasma Polymerized Oxazoline-Based Thin Films—Antibacterial Properties and Cytocompatibility Performance. Polymers 2019, 11, 2069. https://doi.org/10.3390/polym11122069
Sťahel P, Mazánková V, Tomečková K, Matoušková P, Brablec A, Prokeš L, Jurmanová J, Buršíková V, Přibyl R, Lehocký M, et al. Atmospheric Pressure Plasma Polymerized Oxazoline-Based Thin Films—Antibacterial Properties and Cytocompatibility Performance. Polymers. 2019; 11(12):2069. https://doi.org/10.3390/polym11122069
Chicago/Turabian StyleSťahel, Pavel, Věra Mazánková, Klára Tomečková, Petra Matoušková, Antonín Brablec, Lubomír Prokeš, Jana Jurmanová, Vilma Buršíková, Roman Přibyl, Marián Lehocký, and et al. 2019. "Atmospheric Pressure Plasma Polymerized Oxazoline-Based Thin Films—Antibacterial Properties and Cytocompatibility Performance" Polymers 11, no. 12: 2069. https://doi.org/10.3390/polym11122069