Design, Preparation, and Evaluation of a Novel Elastomer with Bio-Based Diethyl Itaconate Aiming at High-Temperature Oil Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(diethyl itaconate/butyl acrylate/ethyl acrylate/glycidyl methacrylate) (PDEBEG)
2.3. Preparation of the PDEBEG/CB Composites
2.4. Measurements and Characterization
2.4.1. Gel Permeation Chromatography (GPC)
2.4.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.3. Proton Nuclear Magnetic Resonance (1H NMR) Spectra
2.4.4. Thermal Performance
2.4.5. Curing Characteristics
2.4.6. Mechanical Properties
2.4.7. Rubber Process Analyzer (RPA)
2.4.8. Scanning Electron Microscopy (SEM)
2.4.9. Oil Resistance Test
3. Results and Discussion
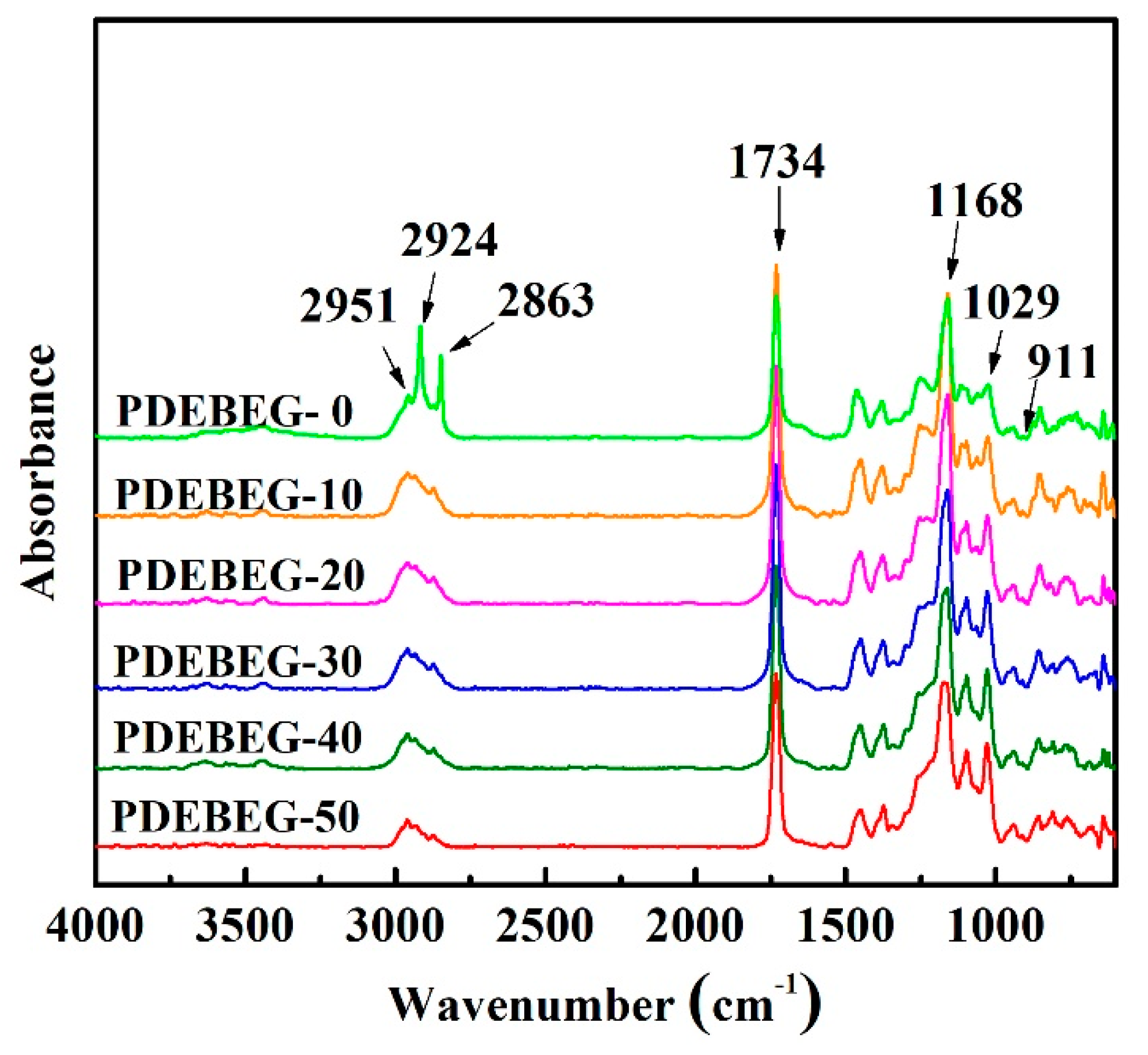
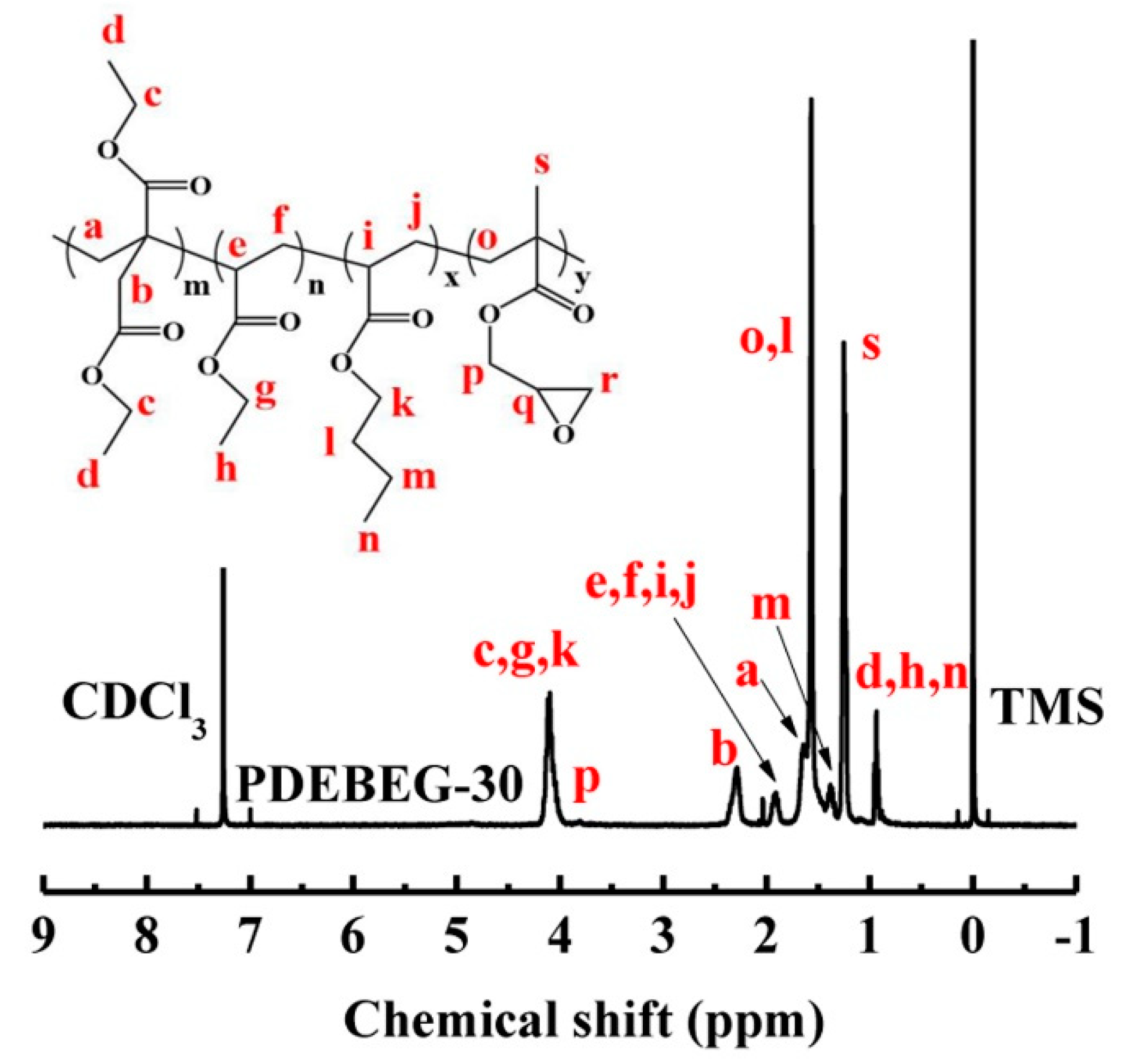
3.1. Synthesis and Characterization of the PDEBEG
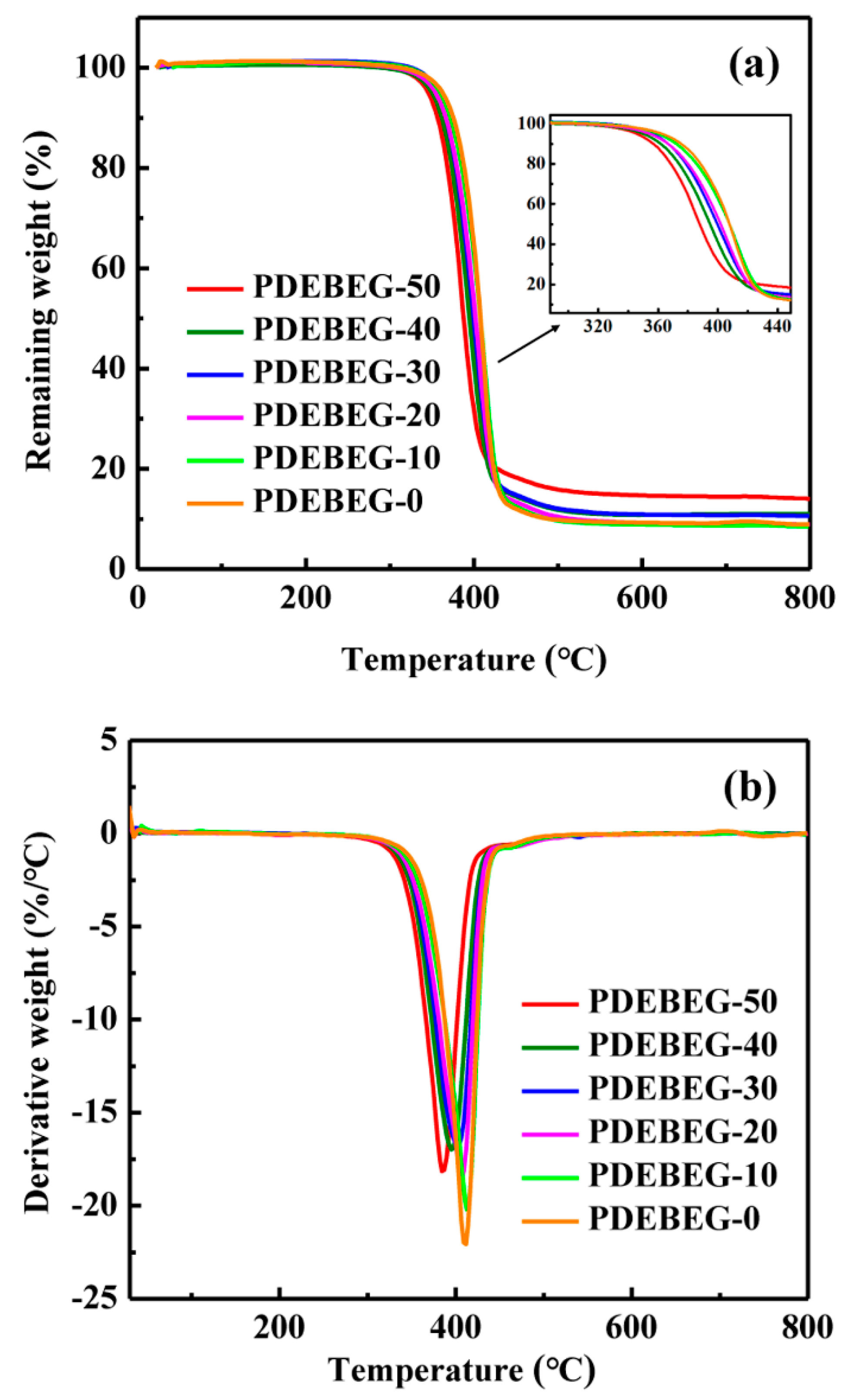
3.2. Thermal Performance of the PDEBEG
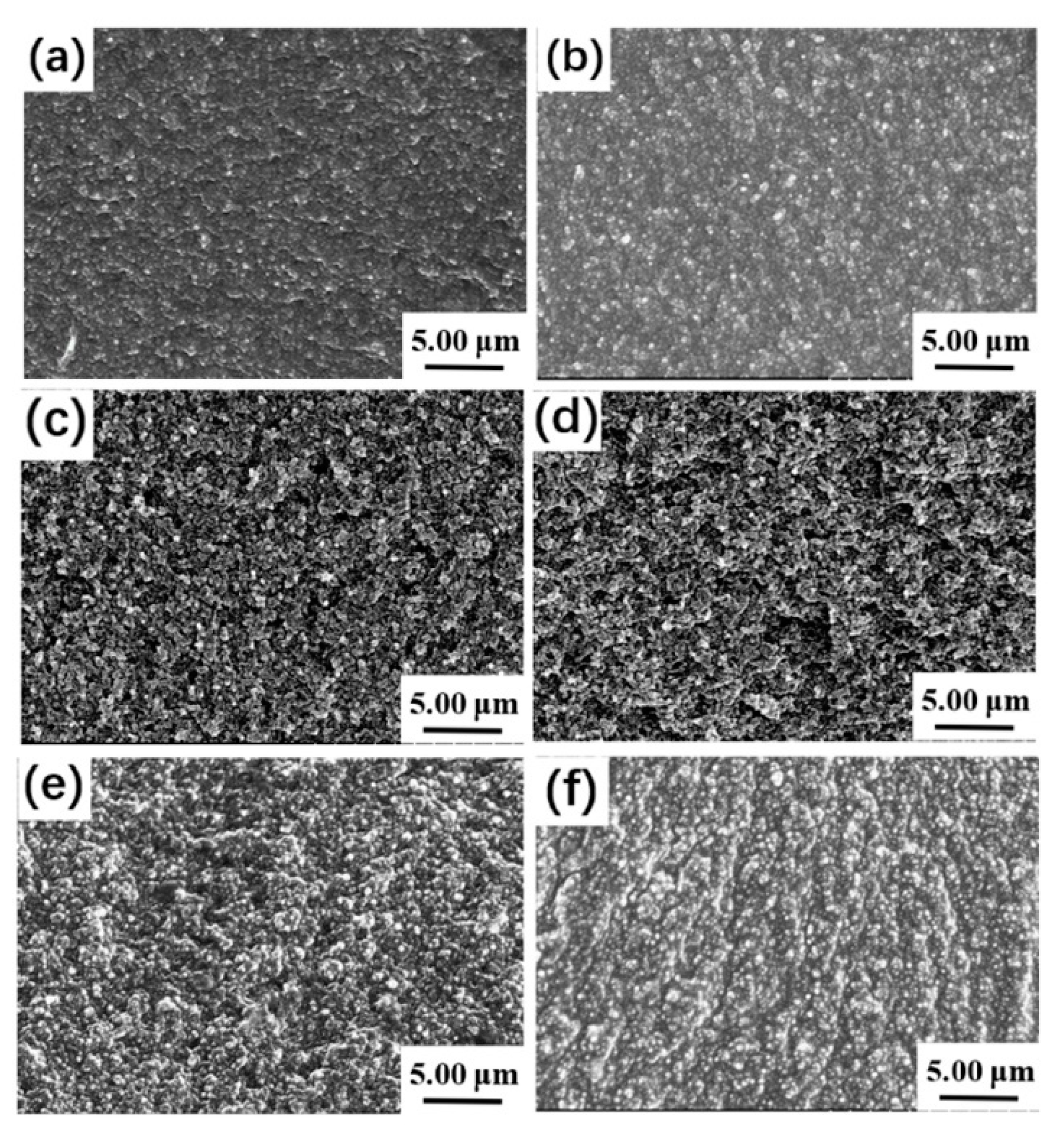
3.3. Vulcanization Characteristics, Morphology, and Mechanical Properties of the PDEBEG/CB Composites
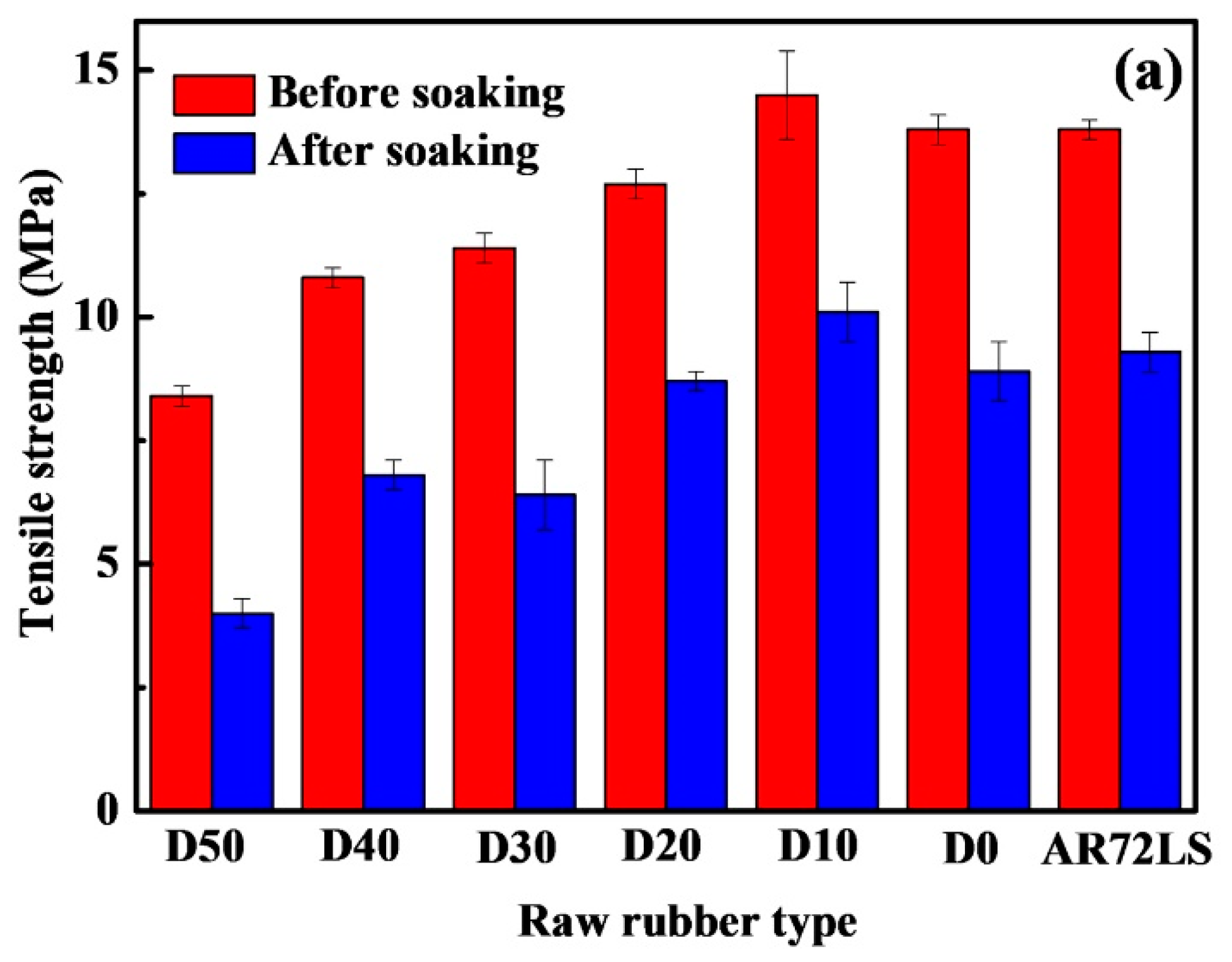
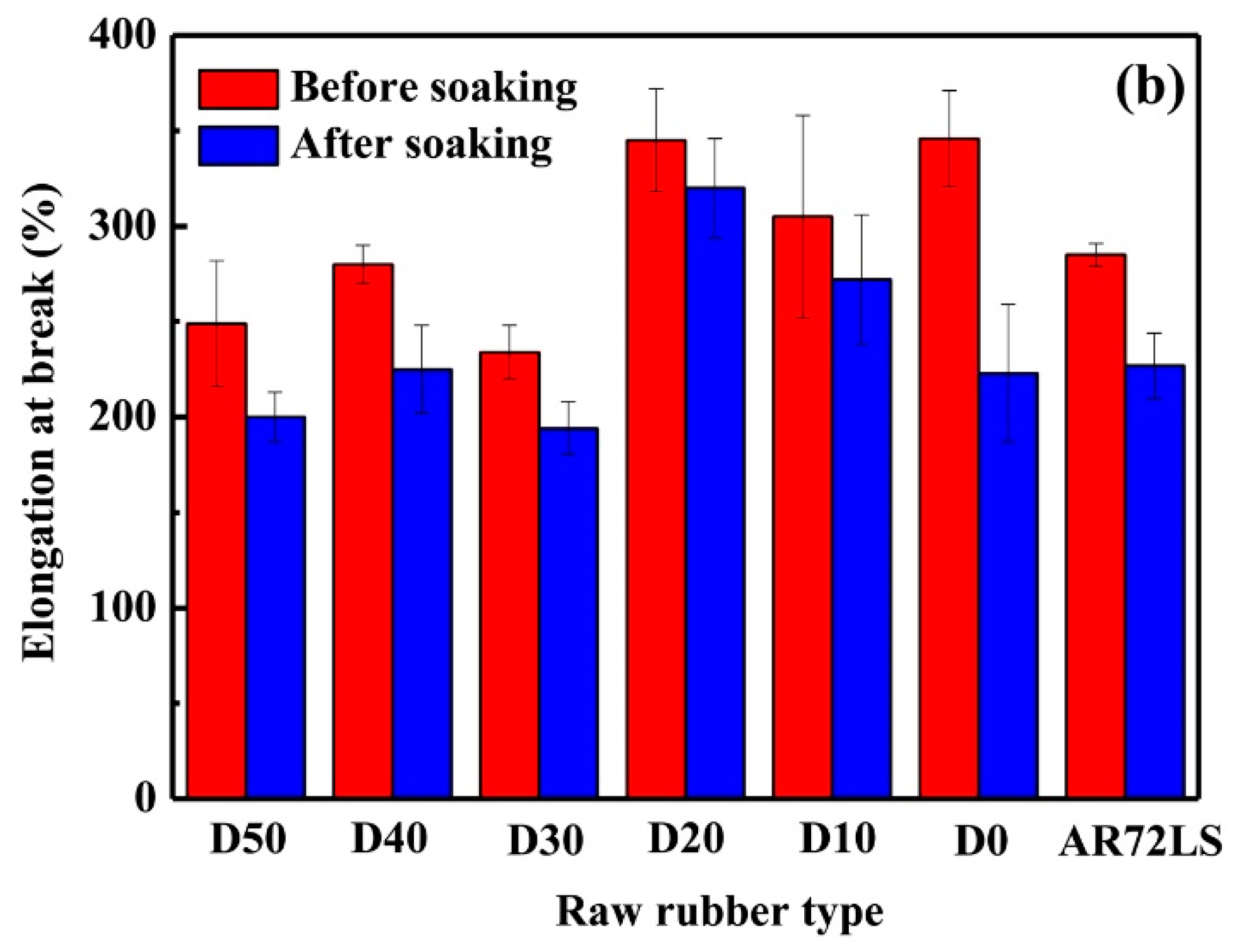
3.4. Oil Resistance of the PDEBEG/CB Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patil, A.O.; Coolbaugh, T.S. Elastomers: A literature review with emphasis on oil resistance. Rubber Chem. Technol. 2005, 78, 516–535. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, J.; Yang, R.; Lervolino, R.; Barbera, S. Effect of lubricating oil on thermal aging of nitrile rubber. Polym. Degrad. Stab. 2018, 151, 136–143. [Google Scholar] [CrossRef]
- Zhao, X.; Xiang, P.; Tian, M.; Fong, H.; Jin, R.; Zhang, L. Nitrile butadiene rubber/hindered phenol nanocomposites with improved strength and high damping performance. Polymer 2007, 48, 6056–6063. [Google Scholar] [CrossRef]
- Rajasedkar, R.; Pal, K.; Heinrich, G.; Das, A.; Das, C.K. Development of nitrile butadiene rubber-nanoclay composites with epoxidized natural rubber as compatibilizer. Mater. Des. 2009, 30, 3839–3845. [Google Scholar]
- Zhang, X.; Li, H.; Tian, D.; He, X.; Lu, C.H. Enhancement of thermal aging performance and oil resistance of acrylic rubber vulcanisates by adding devulcanised ground fluoroelastomer ultrafine powder as functional filler. Mater. Res. Innov. 2012, 16, 143–149. [Google Scholar] [CrossRef]
- Wen, X.; Yuan, X.; Lan, L.; Hao, L.; Li, S.; Zheng, Z. Effect of transformer oil on room temperature vulcanized silicone rubber. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2337–2343. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, L.; Zhang, H.; Zhu, X.; Liu, G.; Qiu, G.; Liu, S. Predictive power in oil resistance of fluororubber and fluorosilicone rubbers based on three-dimensional solubility parameter theory. Polym. Test. 2019, 75, 380–386. [Google Scholar] [CrossRef]
- Ismail, S.; Chatterjee, T.; Naskar, K. Superior heat-resistant and oil-resistant blends based on dynamically vulcanized hydrogenated acrylonitrile butadiene rubber and polyamide 12. Polym. Adv. Technol. 2017, 28, 665–678. [Google Scholar] [CrossRef]
- Yeo, Y.G.; Park, H.H.; Lee, C.S. A study on the characteristics of a rubber blend of fluorocarbon rubber and hydrogenated nitrile rubber. J. Ind. Eng. Chem. 2013, 19, 1540–1548. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Zhang, L.; Lai, X.; Wu, W.; Zeng, X. In situ preparation of reduced graphene oxide reinforced acrylic rubber by self-assembly. J. Appl. Polym. Sci. 2019, 136, 47187. [Google Scholar] [CrossRef]
- Jha, A.; Dutta, B.; Bhowmick, A. Effect of fillers and plasticizers on the performance of novel heat and oil-resistant thermoplastic elastomers from nylon-6 and acrylate rubber blends. J. Appl. Polym. Sci. 1999, 74, 1490–1501. [Google Scholar] [CrossRef]
- Jha, A.; Bhowmick, A.K. Mechanical and dynamic mechanical thermal properties of heat- and oil-resistant thermoplastic elastomeric blends of poly (butylene terephthalate) and acrylate rubber. J. Appl. Polym. Sci. 2000, 78, 1001–1008. [Google Scholar] [CrossRef]
- Hu, S.; Chen, S.; Zhao, X.; Guo, M.; Zhang, L. The shape-memory effect of hindered phenol (AO-80)/acrylic rubber (ACM) composites with tunable transition temperature. Materials 2018, 11, 2461. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Qin, C.; Qiu, J. Study on application of the blends of nitrile rubber with acrylate rubber in the coat-metal sealing gasket. Adv. Mater. Res. 2012, 393, 1438–1442. [Google Scholar] [CrossRef]
- Wilfredo, Y.; Anna, S.; Nancy, Y.; Zbigniew, R. Design, synthesis, characterization and optimization of PTT-b-PEO copolymers: A new membrane material for CO2 separation. J. Membr. Sci. 2010, 362, 407–416. [Google Scholar]
- Buchard, A.; Bakewell, C.M.; Weiner, J.; Williams, C.K. Recent developments in catalytic activation of renewable resources for polymer synthesis. Organomet. Renew. 2012, 39, 175–224. [Google Scholar]
- Willke, T. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Choi, S.; Yi, J.; Lee, S. Microbial production of building block chemicals and polymers. Curr. Opin. Biotechnol. 2011, 22, 758–767. [Google Scholar] [CrossRef]
- Choi, S.; Song, C.; Shin, J.; Lee, S. Biorefineries for the production of top building block chemicals and their derivatives. Metab. Eng. 2015, 28, 223–239. [Google Scholar] [CrossRef]
- Wang, J.; Qian, W.; He, Y.; Xiong, Y.; Song, P.; Wang, R.M. Reutilization of discarded biomass for preparing functional polymer materials. Waste Manag. 2017, 65, 11–21. [Google Scholar] [CrossRef]
- Dong, W.; Li, T.; Xiang, S.; Ma, P.; Chen, M. Influence of glutamic acid on the properties of poly (xylitol glutamate sebacate) bioelastomer. Polymers 2013, 5, 1339–1351. [Google Scholar] [CrossRef]
- Dai, L.; Tai, C.; Shen, Y.; Guo, Y.; Tao, F. Biosynthesis of 1,4-butanediol from erythritol using whole-cell catalysis. Biocatal. Biotransform. 2019, 37, 92–96. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, M.; Zhang, Q.; Wang, R.; Liang, Q.; Zhou, G. New bio-based copolyesters derived from 1,4-butanediol, terephthalic acid and 2, 5-thiophenedicarboxylic acid: Synthesis, crystallization behavior, thermal and mechanical properties. Polym. Test. 2019, 75, 213–219. [Google Scholar] [CrossRef]
- Wang, R.; Ma, J.; Zhou, X.; Wang, Z.; Kang, H.; Zhang, L.; Hua, K.C.; Kulig, J. Design and preparation of a novel cross-linkable, high molecular weight, and bio-based elastomer by emulsion polymerization. Macromolecules 2012, 45, 6830–6839. [Google Scholar] [CrossRef]
- Wang, R.; Yao, H.; Lei, W.; Zhou, X.; Zhang, L.; Hua, K.C.; Kulig, J. Morphology, interfacial interaction, and properties of a novel bioelastomer reinforced by silica and carbon black. J. Appl. Polym. Sci. 2013, 129, 1546–1554. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Zhang, L.; Tan, T.; Fong, H. Nonisocyanate biobased poly(ester urethanes) with tunable properties synthesized via an environment-friendly route. ACS Sustain. Chem. Eng. 2016, 4, 2762–2770. [Google Scholar] [CrossRef]
- Okabe, M.; Lies, D.; Kanamasa, S.; Park, E.Y. Biotechnological production of itaconic acid and its biosynthesis in aspergillus terreus. Appl. Microbiol. Biotechnol. 2009, 84, 597–606. [Google Scholar] [CrossRef]
- Kuenz, A.; Gallenmuller, Y.; Willke, T.; Vorlop, K.D. Microbial production of itaconic acid: Developing a stable platform for high product concentrations. Appl. Microbiol. Biotechnol. 2012, 96, 1209–1216. [Google Scholar] [CrossRef]
- Wei, T.; Lei, L.; Kang, H.; Qiao, B.; Wang, Z.; Zhang, L.; Coates, P.; Hua, K.C.; Kulig, J. Tough bio-based elastomer nanocomposites with high performance for engineering applications. Adv. Eng. Mater. 2012, 14, 112–118. [Google Scholar] [CrossRef]
- Guo, B.; Chen, Y.; Lei, Y.; Zhang, L.; Zhou, W.; Rabie, A.B.; Zhao, J. Biobased poly (propylene sebacate) as shape memory polymer with tunable switching temperature for potential biomedical applications. Biomacromolecules 2011, 12, 1312–1321. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Uyama, H. Full biobased polymeric material from plant oil and poly (lactic acid) with a shape memory property. ACS Sustain. Chem. Eng. 2014, 2, 2057–2062. [Google Scholar] [CrossRef]
- Farmer, T.J.; Castle, R.L.; Clark, J.H.; Macquarrie, D.J. Synthesis of unsaturated polyester resins from various bio-derived platform molecules. Int. J. Mol. Sci. 2015, 16, 14912–14932. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zheng, G.; Nie, X.; Wang, Y. Thermal performance, mechanical property and fire behavior of epoxy thermoset based on reactive phosphorus-containing epoxy monomer. J. Therm. Anal. Calorim. 2017, 127, 1419–1430. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, T.; Xue, X.; He, M.; Xue, J.; Song, M.; Wu, S.; Kang, H.; Zhang, L.; Jia, Q. Synthesis of fully bio-based polyamides with tunable properties by employing itaconic acid. Polymer 2014, 55, 4846–4856. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lebarbe, T.; Grau, E.; Cramail, H. Structure-properties relationship of fatty acid-based thermoplastics as synthetic polymer mimics. Polym. Chem. 2013, 4, 5472–5517. [Google Scholar] [CrossRef] [Green Version]
- Lei, W.; Russell, T.P.; Hu, L.; Zhou, X.; Qiao, H.; Wang, W.; Wang, R.; Zhang, L. Pendant chain effect on the synthesis, characterization, and structure-property relations of poly (di-n-alkyl itaconate-coisoprene) biobased elastomers. ACS Sustain. Chem. Eng. 2017, 5, 5214–5223. [Google Scholar] [CrossRef]
- Qiao, H.; Xu, W.; Chao, M.; Liu, J.; Lei, W.; Zhou, X.; Wang, R.; Zhang, L. Preparation and performance of silica/epoxy group-functionalized biobased elastomer nanocomposite. Ind. Eng. Chem. Res. 2017, 56, 881–889. [Google Scholar] [CrossRef]
- Durant, Y.; Jiang, B.; Tsavalas, J. Emilsion Polymerization of Ester of Itaconic Acid. U.S. Patent US9932421B2, 3 Apirl 2018. [Google Scholar]
- Chakraborty, S.; Ramakrishnan, S. Surface-Functionalized Polystyrene Latexes Using Itaconate-Based Surfmers. Langmuir 2018, 34, 11729–11737. [Google Scholar] [CrossRef]
- Qiao, Z.; Qiu, T.; Liu, W.; Zhang, L.; Tu, J.; Guo, L.; Li, X. A “green” method for preparing ABCBA pentablock elastomers by using RAFT emulsion polymerization. Polym. Chem. 2017, 8, 3013–3021. [Google Scholar] [CrossRef]
- Schmidt, B.; Molinari, V.; Esposito, D.; Tauer, K.; Antonietti, M. Lignin-based polymeric surfactants for emulsion polymerization. Polymer 2017, 112, 418–426. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Held, U.; Willing, A.; Breuer, W.H. New green surfactants for emulsion polymerization. Prog. Org. Coat. 2005, 53, 246–255. [Google Scholar] [CrossRef]
- Lei, W.; Qiao, H.; Zhou, X.; Wang, W.; Zhang, L.; Wang, R.; Hua, K.C. Synthesis and evaluation of bio-based elastomer based on diethyl itaconate for oil-resistance applications. Sci. China Chem. 2016, 59, 1376–1383. [Google Scholar] [CrossRef]




| Ingredients | Diethyl Itaconate (wt%) | Ethyl Acrylate (wt%) | Butyl Acrylate (wt%) | Glycidyl Methacrylate (wt%) |
|---|---|---|---|---|
| PDEBEG-50 a | 50 | 10 | 40 | 2 |
| PDEBEG-40 | 40 | 20 | 40 | 2 |
| PDEBEG-30 | 30 | 30 | 40 | 2 |
| PDEBEG-20 | 20 | 40 | 40 | 2 |
| PDEBEG-10 | 10 | 50 | 40 | 2 |
| PDEBEG-0 | 0 | 60 | 40 | 2 |
| Ingredients | Loading (phr) a |
|---|---|
| PDEBEG | 100.0 |
| N330 | 60.0 |
| stearic acid | 1.0 |
| antioxidant 445 | 1.0 |
| zinc dibutyl dithiocaarbamate | 2.5 |
| Sulfur | 1.0 |
| Sample | Mn/104 | Đa | Yield/% | Gel Content/% |
|---|---|---|---|---|
| PDEBEG-50 | 23.7 | 3.86 | 96.1 | 7 |
| PDEBEG-40 | 30.6 | 3.54 | 97.5 | 12 |
| PDEBEG-30 | 31.9 | 3.41 | 98.2 | 14 |
| PDEBEG-20 | 51.7 | 3.03 | 97.8 | 23 |
| PDEBEG-10 | 49.7 | 3.61 | 97.6 | 38 |
| PDEBEG-0 | 70.2 | 2.84 | 98.2 | 46 |
| Samples | PDEBEG-50 | PDEBEG-40 | PDEBEG-30 | PDEBEG-20 | PDEBEG-10 | PDEBEG-0 |
|---|---|---|---|---|---|---|
| Td,5% a | 346 | 350 | 357 | 354 | 360 | 363 |
| Td,max b | 385 | 395 | 403 | 407 | 413 | 416 |
| Sample | Scorch Time (min:s) | Curing Time (min:s) | Torque Increase (dNm) |
|---|---|---|---|
| PDEBEG-50 | 0:40 | 13:24 | 10.3 |
| PDEBEG-40 | 0:11 | 9:46 | 14.0 |
| PDEBEG-30 | 1:23 | 17:23 | 12.7 |
| PDEBEG-20 | 1:04 | 15:23 | 12.5 |
| PDEBEG-10 | 0:16 | 14:23 | 20.0 |
| PDEBEG-0 | 1:46 | 21.50 | 18.5 |
| AR72LS | 3:03 | 19:17 | 19.0 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Permanent Set (%) | Hardness (Shore A) |
|---|---|---|---|---|
| PDEBEG-50 | 8.4 ± 0.2 | 249 ± 13 | 8 ± 2 | 72 ± 1 |
| PDEBEG-40 | 10.8 ± 0.2 | 280 ± 10 | 8 ± 1 | 69 ± 1 |
| PDEBEG-30 | 11.4 ± 0.3 | 234 ± 14 | 6 ± 2 | 76 ± 2 |
| PDEBEG-20 | 12.7 ± 0.3 | 345 ± 27 | 12 ± 2 | 63 ± 1 |
| PDEBEG-10 | 14.5 ± 0.4 | 305 ± 23 | 10 ± 1 | 63 ± 1 |
| PDEBEG-0 | 13.8 ± 0.3 | 346 ± 25 | 12 ± 1 | 60 ± 1 |
| AR72LS | 13.8 ± 0.2 | 285 ± 6 | 12 ± 2 | 66 ± 1 |
| Sample | Retention Rate of Tensile Strength (%) | Retention Rate of Elongation at Break (%) | Retention Rate of Shore A Hardness (%) | Δm (%) | ΔV (%) |
|---|---|---|---|---|---|
| PDEBEG-50 | 47.6 | 80.3 | 72.2 | +19.6 | +23.7 |
| PDEBEG-40 | 63.0 | 80.4 | 75.4 | +18.5 | +17.6 |
| PDEBEG-30 | 56.1 | 82.9 | 69.7 | +19.2 | +18.8 |
| PDEBEG-20 | 68.5 | 92.8 | 88.9 | +16.9 | +12.5 |
| PDEBEG-10 | 69.7 | 89.2 | 92.1 | +13.8 | +9.9 |
| PDEBEG-0 | 64.5 | 64.5 | 86.7 | +18.5 | +16.7 |
| AR72LS | 67.4 | 79.6 | 86.4 | +18.0 | +14.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Ji, H.; Zhou, X.; Lei, W.; Zhang, L.; Wang, R. Design, Preparation, and Evaluation of a Novel Elastomer with Bio-Based Diethyl Itaconate Aiming at High-Temperature Oil Resistance. Polymers 2019, 11, 1897. https://doi.org/10.3390/polym11111897
Yang H, Ji H, Zhou X, Lei W, Zhang L, Wang R. Design, Preparation, and Evaluation of a Novel Elastomer with Bio-Based Diethyl Itaconate Aiming at High-Temperature Oil Resistance. Polymers. 2019; 11(11):1897. https://doi.org/10.3390/polym11111897
Chicago/Turabian StyleYang, Hui, Haijun Ji, Xinxin Zhou, Weiwei Lei, Liqun Zhang, and Runguo Wang. 2019. "Design, Preparation, and Evaluation of a Novel Elastomer with Bio-Based Diethyl Itaconate Aiming at High-Temperature Oil Resistance" Polymers 11, no. 11: 1897. https://doi.org/10.3390/polym11111897