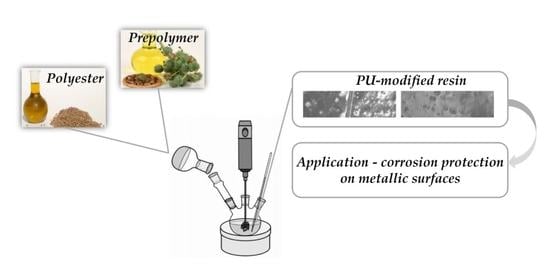
Preparation of Polyurethane Monolithic Resins and Modification with a Condensed Tannin-Yielding Self-Healing Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
2.2.1. Raw Materials
Quantification by Gas Chromatography with Flame-Ionization Detection (GC-FID)
Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (FTIR-ATR)
2.2.2. Polyester
Hydroxyl Groups
2.2.3. Prepolymer
Solid Content and Solvent Content
NCO Groups
2.2.4. PU Resin and PU-Modified Resin
Raman Spectroscopy
Thermogravimetric Analysis (TGA)
Optical Microscopy (OM)
3. Results and Discussion
3.1. Characterization of Raw Material
3.1.1. Quantification by Gas Chromatography with Flame-Ionization Detection (GC-FID)
3.1.2. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (FTIR-ATR)
3.1.3. Thermogravimetric Analysis (TGA)
3.2. Main Chemical Reactions for the Polyester and Prepolymer Preparation and Products Characterization
3.2.1. Polyester Preparation
3.2.2. Prepolymer Preparation
3.2.3. Characterization of Polyester and Prepolymer
Hydroxyl Groups, Solid Content, Solvent Content, and NCO Groups
Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (FTIR-ATR)
3.3. Preparation and Characterization of PU and PU-Modified Resins
3.3.1. Preparation of Polyurethane Resins
3.3.2. Characterization of PU and PU-Modified Resins
Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (FTIR-ATR)
Thermogravimetric Analysis (TGA)
Raman Spectroscopy
Artificial Defected Monoliths in Contact with Deionized Water
3.3.3. Self-Healing Effect
3.4. Applications of the PU and PU-Modified Resins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rabbani, M.M.; Saha, J.K. Polyurethane and Its Derivatives. In Functional Polymers: Polymers and Polymeric Composites: A Reference Series; Mazumder, J., Sheardown, M.H., Al-Ahmed, A., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Szmechtyk, T.; Sienkiewicz, N.; Woźniak, J.; Strzelec, K. Polyurethanes as self-healing materials. Curr. Chem. Lett. 2015, 4, 61–66. [Google Scholar] [CrossRef]
- Yang, Z.; Shuai, B.; Zhang, X.; Yu, H.; Zhang, H.; Jia, Y.; Zhang, C.; Guan, X. Fabrication and performance of a polyurethane hybrid composite with waste red mud. Polym. Compos. 2019, 40, 2424–2431. [Google Scholar] [CrossRef]
- Khalid, T.; Albasha, L.; Qaddoumi, N.; Yehia, S. Feasibility Study of Using Electrically Conductive Concrete for Electromagnetic Shielding Applications as a Substitute for Carbon-Laced Polyurethane Absorbers in Anechoic Chambers. IEEE Trans. Antenn. Propag. 2017, 65, 2428–2435. [Google Scholar] [CrossRef]
- Zhang, C.; Shuai, B.; Zhang, X.; Hu, X.; Zhang, H.; Jia, Y.; Yang, Z.; Guan, X. Polyurethane/Red Mud Composites with Flexibility, Stretchability, and Flame Retardancy for Grouting. Polymers 2018, 10, 906. [Google Scholar] [CrossRef]
- Xiang, X.J.; Qian, J.W.; Yang, W.Y.; Fang, M.H.; Qian, X.Q. Synthesis and properties of nanosilica-reinforced polyurethane for grouting. J. Appl. Polym. Sci. 2006, 100, 4333–4337. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Liu, X.; Guan, X.; Zhang, C.; Niu, Y. Flexible and stretchable polyurethane/waterglass grouting material. Constr. Build. Mater. 2017, 138, 240–246. [Google Scholar] [CrossRef]
- Zaimahwati; Yuniati; Jalal, R.; Rihayat, T.; Zhafiri, S. Synthesis and Characterization Thermal of Polyurethane/MMT from Castor Oil Polyols for Coating. Iop Conf. Ser. Mater. Sci. Eng. 2019, 536, 1–8. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, L.; Feng, L.; Lu, H.; Xu, Y.; Wang, J.; Xiang, B.; Zou, X. Polydopamine functionalized graphene oxide nanocomposites reinforced the corrosion protection and adhesion properties of waterborne polyurethane coatings. Eur. Polym. J. 2019, 109249. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Z.; Ma, L.; Wang, Q.; Chu, A. Zinc oxide array/polyurethane nanocomposite coating: Fabrication, characterization and corrosion resistance. Surf. Coat. Technol. 2019, 358, 497–504. [Google Scholar] [CrossRef]
- Garrison, T.F.; Kessler, M.R. Chap 3—Plant Oil-Based Polyurethanes, Bio-Based Plant Oil Polymers and Composites; Samy, A., Madbouly, C.Z., Kessler, M.R., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 37–54. [Google Scholar] [CrossRef]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Pfister, D.P.; Xia, Y.; Larock, R.C. Recent advances in vegetable oil-based polyurethanes. Chem. Sus. Chem. 2011, 4, 703–717. [Google Scholar] [CrossRef]
- Panda, S.S.; Panda, B.P.; Nayak, S.K.; Mohanty, S. A Review on Waterborne Thermosetting Polyurethane Coatings Based on Castor Oil: Synthesis, Characterization, and Application. Polym. Plast. Technol. Eng. 2018, 57, 500–522. [Google Scholar] [CrossRef]
- Gällstedt, M.; Pettersson, H.; Johansson, T.; Newson, W.R.; Johansson, E.; Hedenqvist, M.S. Film Extrusion of Crambe abyssinica/Wheat Gluten Blends. J. Vis. Exp. 2017, 119, e54770. [Google Scholar] [CrossRef]
- Rasel, H.; Johansson, T.; Gällstedt, M.; Newson, W.; Johansson, E.; Hedenqvist, M. Development of bioplastics based on agricultural side-stream products: Film extrusion of Crambe abyssinica/wheat gluten blends for packaging purposes. J. Appl. Polym. Sci. 2016, 133, 42442. [Google Scholar] [CrossRef]
- Arbenz, A.; Avérous, L. Tannins: A Resource to Elaborate Aromatic and Biobased Polymers. In Biodegradable and Biobased Polymers for Environmental and Biomedical Applications; Susheel, K., Avérous, L., Eds.; Wiley: Hoboken, NJ, USA, 2016; Chapter 4. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Otaigbe, J.U. Rheokinetics of thermal-induced gelation of waterborne plyurethane dispersions. Macromolecules 2005, 38, 10178–10184. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Xia, Y.; Kessler, M.R. Rheological behavior of environmentally friendly castor oil-based waterborne polyurethane dispersions. Macromolecules 2013, 46, 4606–4616. [Google Scholar] [CrossRef]
- Karak, N.; Rana, S.; Cho, J.W. Synthesis and characterization of castor-oil-modified hyperbranched polyurethanes. J. Appl. Surf. Sci. 2009, 112, 736–743. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Fugivara, C.S.; Benedetti, A.V. Environmentally Friendly coatings applied on aluminum alloy ASTM 1200. In Proceedings of the 64th Annual Meeting of the International Society of Electrochemistry, Santiago de Querétaro, Mexico, 8–13 September 2013; International Society of Electrochemistry: Lausanne, Switzerland, 2013; Volume 1. [Google Scholar]
- Nardeli, J.V.; Fugivara, C.S.; Taryba, M.; Montemor, M.F.; Benedetti, A.V. Zn containing polymer coatings for AA7475: Localized study of anti-corrosion performance. In Proceedings of the the European Corrosion Congress–EUROCORR, Sevilha, Spain, 9–13 September 2019. [Google Scholar]
- Nardeli, J.V.; Snihirova, D.V.; Fugivara, C.S.; Montemor, M.F.; Pinto, E.R.P.; Messaddeq, Y.; Benedetti, A.V. Localised corrosion assessement of crambe-oil-based polyurethane coatings applied on the ASTM 1200 aluminum alloy. Corros. Sci. 2016, 111, 422–435. [Google Scholar] [CrossRef]
- Talbert, R. Paint Technology Handbook; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9781574447033. [Google Scholar]
- Fazenda, J.M.R. Tintas, Ciência e Tecnologia; Abrafati: São Paulo, Brazil, 2009; ISBN 9788521204749. [Google Scholar]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-change and yellowing of polyurethane as a result of UV irradiation. Polym. Deg. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- The Chemistry of Polyurethane Coatings—A General Reference Manual, Bayer® Material Science. Available online: http://digitalcollections.library.cmu.edu/awweb/awarchive?type=file&item=488891 (accessed on 1 May 2019).
- Golling, F.E.; Pires, R.; Hecking, A.; Weikard, J.; Richter, F.; Danielmeier, K.; Dijkstra, D. Polyurethanes for corrosion & adhesives—Chemistry & applications. Polym. Int. 2019, 68, 848–855. [Google Scholar] [CrossRef]
- Hernes, P.J.; Benner, R.; Cowie, G.L.; Goñi, M.A.; Bergamaschi, B.A.; Hedges, J.I. Tannin diagenesis in mangrove leaves from a tropical estuary: A novel molecular approach. Geochim. Cosmochim. Acta 2001, 65, 3109–3122. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-Based Biofoams-A Review. JRM 2019, 7, 477–492. [Google Scholar] [CrossRef]
- Tondi, G. Tannin-Based Copolymer Resins: Synthesis and Characterization by Solid State 13C NMR and FT-IR Spectroscopy. Polymers 2017, 9, 223. [Google Scholar] [CrossRef]
- Banu, K.; Shimura, T.; Sadeghi, S. Selective detection and recovery of gold at tannin-immobilized non-conducting electrode. Anal. Chim. Acta 2015, 853, 207–213. [Google Scholar] [CrossRef]
- Peres, R.S.; Cassel, E.; Azambuja, D.S. Black wattle tannin as steel corrosion inhibitor. Int. Sch. Res. Net. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Martinez, S.; Štagljar, I. Correlation between the molecular structure and the corrosion inhibition efficiency of chestnut tannin in acidic solutions. J. Molec. Struc. 2003, 640, 167–174. [Google Scholar] [CrossRef]
- Martinez, S. Inhibitory mechanism of mimosa tannin using molecular modeling and substitutional adsorption isotherms. Mater. Chem. Phys. 2002, 77, 97–102. [Google Scholar] [CrossRef]
- Michael, N.C.; Olubunmi, J.A. The corrosion inhibition of mild steel in sulphuric acid solution by flavonoid (catechin) separated from Nypa fruticans Wurmb leaves extract. Sci. J. Chem. 2014, 2, 27–32. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Kassim, M.J.; Adnan, R.; Ibrahim, M.S. Mangrove tannins and their flavanoid monomers as alternative steel corrosion inhibitors in acidic medium. Corros. Sci. 2007, 49, 402–417. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Fugivara, C.S.; Taryba, M.; Pinto, E.R.P.; Montemor, M.F.; Benedetti, A.V. Tannin: A natural corrosion inhibitor for bare and coated aluminum alloys. Prog. Org. Coat. 2019, 135, 368–381. [Google Scholar] [CrossRef]
- Matamala, G.; Smeltzer, W.; Droguett, G. Comparison of steel anticorrosive protection formulated with natural tannins extracted from acacia and from pine bark. Corros. Sci. 2000, 42, 1351–1362. [Google Scholar] [CrossRef]
- Dalmoro, V.; Santos, C.; Santos, J.H.Z. Chap. 20: Smart coatings for corrosion protection. In Industrial Applications for Intelligent Polymers and Coatings; Springer: Berlin, Germany, 2016; pp. 417–435. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Fugivara, C.S.; Taryba, M.; Montemor, M.F.; Ribeiro, S.J.L.; Benedetti, A.V. Novel healing coatings based on natural-derived polyurethane modified with tannins for corrosion protection of AA2024-T3. Corros. Sci. 2019, in press. [Google Scholar] [CrossRef]
- Fundação MS (Mato Grosso do Sul Foundation for Research and Dissemination of Agricultural Technologies). Available online: http://www.fundacaoms.org.br/ (accessed on 1 May 2019).
- Grasel, F.S.; Ferrão, M.F. A rapid and non-invasive method for the classification of natural tannin extracts by near-infrared spectroscopy and PLS-DA. Anal. Methods 2016, 8, 644–649. [Google Scholar] [CrossRef]
- Peres, R.S.; Armelin, E.; Alemán, C.; Ferreira, C.A. Modified tannin extracted from black wattle tree as an environmentally friendly antifouling pigment. Ind. Crop. Prod. 2015, 65, 506–514. [Google Scholar] [CrossRef] [Green Version]
- ASTM E222 Standard Test Methods for Hydroxyl Groups Using Acetic Anhydride Acetylation; American Society for Testing Materials: West Conshohocken, PA, USA, 2010.
- Alrashed, M.M.; Jana, S.; Soucek, M.D. Corrosion performance of polyurethane hybrid coatings with encapsulated inhibitor. Prog. Org. Coat. 2019, 130, 235–243. [Google Scholar] [CrossRef]
- ASTM D2572 Standard Test Method for Isocyanate Groups in Urethane Materials or Prepolymers; American Society for Testing Materials: West Conshohocken, PA, USA, 2010.
- Alencar, M.A.S.; Benedetti, A.V.; Fugivara, C.S.; Messaddeq, Y. Construção de célula eletroquímica para observação de amostras in situ em estereomicroscópio. Quim. Nova 2010, 33, 1394–1397. [Google Scholar] [CrossRef] [Green Version]
- Grasel, F.S.; Ferrão, M.F.; Wolf, C.R. Development of methodology for identification the nature of the polyphenolic extracts by FTIR associated with multivariate analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 94–101. [Google Scholar] [CrossRef]
- Pinto, E.R.P. Modificação de óleo Vegetais e de Origem Animal para a Síntese de Poliésteres e Poliuretanos com Baixo teor de Compostos Orgânicos Voláteis. Ph.D. Thesis, Universidade Estadual Paulista Júlio Mesquita Filho (UNESP), Araraquara/SP, Brasil, 2012. [Google Scholar]
- Pinto, E.R.P.; Polito, W.L.; Ribeiro, S.J.L.; Messaddeq, Y. Hydroxylated polyester resin synthesizing from crambe oil by mass polymerization. In Proceedings of the 102nd AOCS Annual Meeting & Expo. 2011, Cincinnati, OH, USA, 1–4 May 2001. [Google Scholar]
- Yeganeh, H.; Shamekhi, M.A. Novel polyurethane insulating coatings based on polyhydroxyl compounds, derived from glycolysed PET and castor oil. J. Appl. Polym. Sci. 2006, 99, 1222–1233. [Google Scholar] [CrossRef]
- Moeini, H.R. Synthesis and properties of novel polyurethane-urea insulating coatings from hydroxyl-terminated prepolymers and blocked isocyanate curing agent. J. Appl. Polym. Sci. 2009, 112, 3714–3720. [Google Scholar] [CrossRef]
- Mannari, V.M.; Massingill, J.L. Two-component high-solid polyurethane coating systems based on soy polyols. J. Coat. Technol. Res. 2006, 3, 151–157. [Google Scholar] [CrossRef]
- Tecnologia dos Poliuretanos. Available online: https://www.poliuretanos.com.br/Cap7/73Tintas.htm (accessed on 1 May 2019).
- Haghdadeh, P.; Ghaffari, M.; Ramezanzadeh, B.; Bahlakeh, G.; Saeb, M.R. Polyurethane coatings reinforced with 3-(triethoxysilyl)propyl isocyanate functionalized graphene oxide nanosheets: Mechanical and anti-corrosion properties. Prog. Org. Coat. 2019, 136, 105243. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Y.; Li, W.; Wang, W.; Zhao, X.; Song, L. Multi-level self-healing ability of shape memory polyurethane coating with microcapsules by induction heating. Chem. Eng. J. 2019, 368, 1033–1044. [Google Scholar] [CrossRef]
- Han, Y.; Hu, J.; Xin, Z. Facile preparation of high solid content waterborne polyurethane and its application in leather surface finishing. Prog. Org. Coat. 2019, 130, 8–16. [Google Scholar] [CrossRef]
- Koh, E.; Kim, N.K.; Shin, J.; Kim, Y.W. Polyurethane Microcapsules for Self-Healing Paint Coatings. RSC Adv. 2014, 4, 15830–15834. [Google Scholar] [CrossRef]
- Brochu, A.B.; Chyan, W.J.; Reichert, W.M. Microencapsulation of 2-octylcyanoacrylate tissue adhesive for self-healing acrylic bone cement. J. Biomed. Mater. Res. B 2012, 100, 1764–1772. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.E.; Archibald, D.D.; Myrick, M.L.; Angel, S.M. Determination of physucal properties of reaction-injection-molded polyurethanes by NIR-FT-Raman spectroscopy. Appl. Spectrosc. 1990, 44, 1297–1300. [Google Scholar] [CrossRef]
- Romanova, V.; Begishev, V.; Karmanov, V.; Kondyurin, A.; Maitz, M.F. Fourier transform Raman and Fourier transform infrared spectra of cross-linked polyurethaneurea films synthesized from solutions. J. Raman Spectrosc. 2002, 33, 769–777. [Google Scholar] [CrossRef]
- Janik, H.; Palys, B.; Petrovic, Z.S. Multiphase-separated polyurethanes studied by micro-raman spectroscopy. Macromol. Rapid Commun. 2003, 24, 265–268. [Google Scholar] [CrossRef]
- Parnell, S.; Min, K.; Cakmak, M. Kinetic studies of polyurethane polymerization with Raman spectroscopy. Polymer 2003, 44, 5137–5144. [Google Scholar] [CrossRef]
- Weakley, A.T.; Warwick, P.C.T.; Bitterwolf, T.E.; Aston, D.E. Multivariate analysis of micro-raman spectra of thermoplastic polyurethane blends using principal component analysis and principal component regression. Appl. Spectrosc. 2012, 66, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Bruckmoser, K.; Resch, K. Investigation of ageing mechanisms in thermoplastic polyurethanes by means of IR and Raman spectroscopy. Macromol. Symp. 2014, 339, 70–83. [Google Scholar] [CrossRef]
- Navarchian, A.H.; Najafipoor, N.; Ahangaran, F. Surface-modified poly(methyl methacrylate) microcapsules containing linseed oil for application in self-healing epoxy-based coatings. Prog. Org. Coat. 2019, 132, 288–297. [Google Scholar] [CrossRef]
- Khan, A.; Ubaid, F.; Fayyad, E.M.; Ahmad, Z.; Shakoor, R.A.; Montemor, M.F.; Kahraman, R.; Mansour, S.; Hassan, M.K.; Hasan, A.; et al. Synthesis and properties of polyelectrolyte multilayered microcapsules reinforced smart coatings. J. Mater. Sci. 2019, 54, 12079–12094. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, A.B.; Tatiya, P.D.; Hedaoo, R.K.; Kulkarni, R.D.; Gite, V.V. Polyurethane Prepared from Neem Oil Polyesteramides for Self-Healing Anticorrosive Coatings. Ind. Eng. Chem. Res. 2013, 52, 10189–10197. [Google Scholar] [CrossRef]
- Hailong, F.; Wang, J.; Jin, Z. Tough, Swelling-Resistant, Self-Healing, and Adhesive Dual-Cross-Linked Hydrogels Based on Polymer–Tannic Acid Multiple Hydrogen Bonds. Macromolecules 2018, 51, 1696–1705. [Google Scholar] [CrossRef]
- Burattini, S.; Colquhoun, H.M.; Fox, J.D.; Friedmann, D.; Greenland, B.W.; Harris, P.J.F.; Hayes, W.; Mackay, M.E.; Rowan, S.J. A self-repairing, supramolecular polymer system: Healability as a consequence of donor–acceptor π–π stacking interactions. Chem. Commun. 2009, 44, 6717–6719. [Google Scholar] [CrossRef]
- Mauldin, T.C.; Kessler, M.R. Self-healing polymers and composites. Int. Mater. Rev. 2010, 55, 317–346. [Google Scholar] [CrossRef]



















| Correspondent Fatty Acid | Chemical Name | Crambe | Castor |
|---|---|---|---|
| (%) | |||
| Palmitic acid | hexadecanoic acid | 3.5 | 1.2 |
| Stearic acid | octadecanoic acid | 1.7 | 0.9 |
| Oleic acid | cis-9-octadecenoic acid | 15.1 | 3.6 |
| Ricinoleic acid | 12-hydroxy-9-cis octadecenoic acid | - | 94.9 |
| Linoleic acid | cis-,cis-9,12-octadecadienoic acid | 7.4 | 6.2 |
| Linolenic acid | cis,9,12,15-octadecatrienoic acid | 5.1 | 0.7 |
| Arachidic acid | eicosanoic acid | 1.9 | - |
| Gadoleic acid | cis,9-eicosenoic acid | 6.9 | 0.5 |
| - | 11,14-Eicosadienoic acid | 0.4 | - |
| Behenic acid | docosanoic acid | 3.6 | - |
| Erucic acid | 13-cis-docosenoic acid | 52.9 | - |
| DHA | cis-4,7,10,13,16,19-docosahexanoic acid | 1.1 | - |
| Lignoceric acid | tetracosanoic acid | 5.2 | - |
| - | cis-15-tetracenoic acid | 2.3 | - |
| Properties | |||
|---|---|---|---|
| Polyester | Prepolymer | ||
| Hydroxyl groups (mg KOH/g sample) | 151.6 | Solid content (%) | 56.1 |
| Solvent content (%) | 43.8 | ||
| NCO groups (%) | 6.26 | ||
| Temperature (°C) | Weight Loss Due to: |
|---|---|
| 260–350 | Decomposition of active H sources (chain extenders) |
| 360–490 | Breakup of isocyanate (rigid segment) of the polyester (flexible segment) |
| 200–650 (two steps) | Decomposition of polyurethane (PU) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardeli, J.V.; Fugivara, C.S.; Pinto, E.R.P.; Polito, W.L.; Messaddeq, Y.; José Lima Ribeiro, S.; Benedetti, A.V. Preparation of Polyurethane Monolithic Resins and Modification with a Condensed Tannin-Yielding Self-Healing Property. Polymers 2019, 11, 1890. https://doi.org/10.3390/polym11111890
Nardeli JV, Fugivara CS, Pinto ERP, Polito WL, Messaddeq Y, José Lima Ribeiro S, Benedetti AV. Preparation of Polyurethane Monolithic Resins and Modification with a Condensed Tannin-Yielding Self-Healing Property. Polymers. 2019; 11(11):1890. https://doi.org/10.3390/polym11111890
Chicago/Turabian StyleNardeli, Jéssica Verger, Cecílio Sadao Fugivara, Elaine Ruzgus Pereira Pinto, Wagner Luiz Polito, Younes Messaddeq, Sidney José Lima Ribeiro, and Assis Vicente Benedetti. 2019. "Preparation of Polyurethane Monolithic Resins and Modification with a Condensed Tannin-Yielding Self-Healing Property" Polymers 11, no. 11: 1890. https://doi.org/10.3390/polym11111890