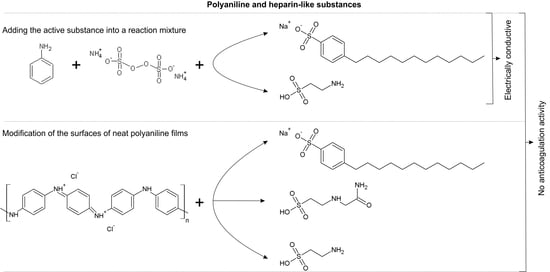
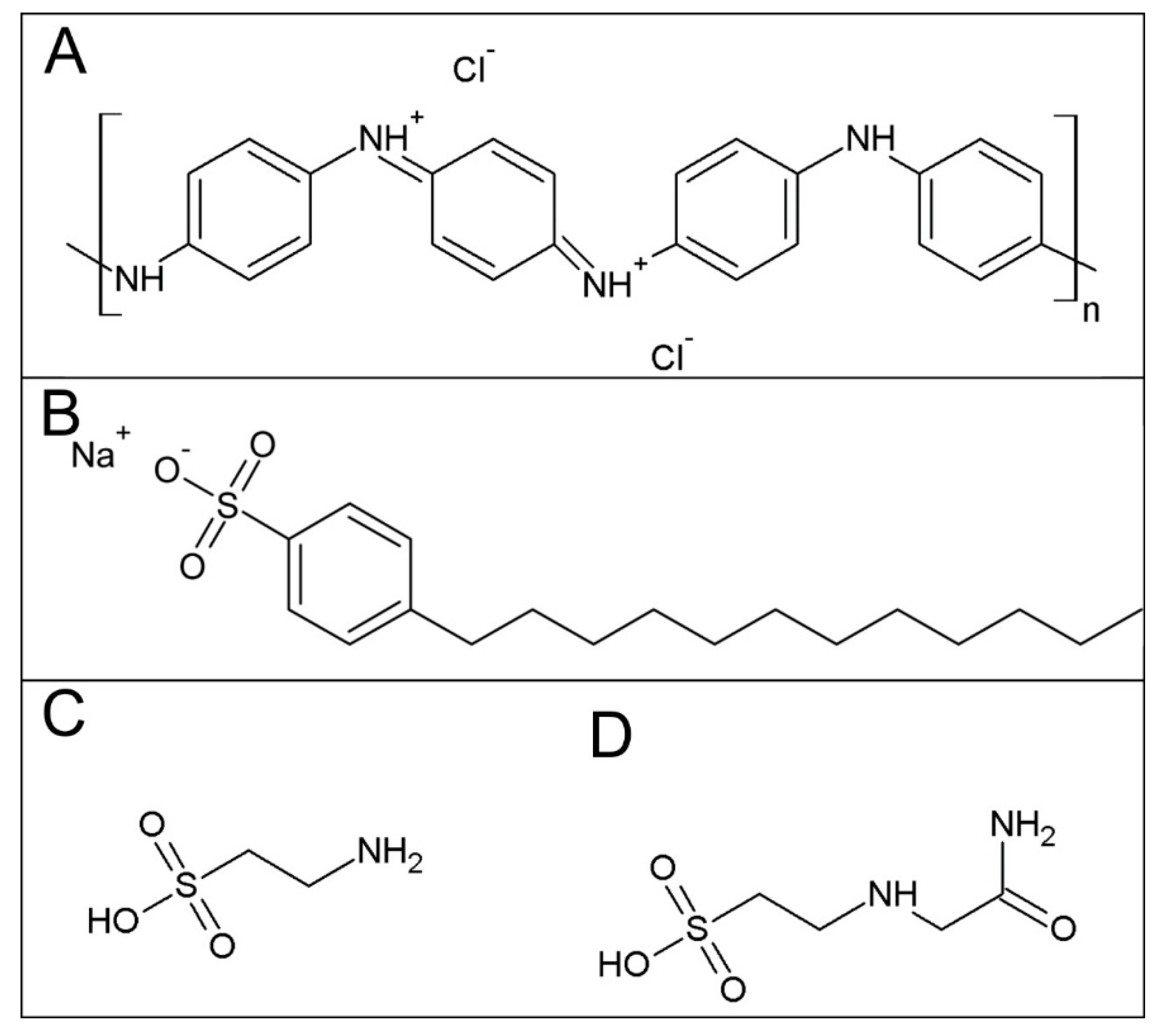
In-Vitro Hemocompatibility of Polyaniline Functionalized by Bioactive Molecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Surface Energy Measurement
2.3. Conductivity
2.4. Anticoagulation Test
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rivnay, J.; Inal, S.; Collins, B.; Sessolo, M.; Stavrinidou, E.; Strakosas, X.; Tassone, C.; Delongchamp, D.; Malliaras, G. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 2016, 7, 11287. [Google Scholar] [CrossRef] [PubMed]
- Ramanaviciene, A.; Kausaite-Minkstimiene, A.; Tautkus, S.; Ramanavicius, A. Biocompatibility of polypyrrole particles: An in-vivo study in mice. J. Pharm. Pharm. 2007, 59, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Vaitkuviene, A.; Kaseta, V.; Voronovic, J.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of cytotoxicity of polypyrrole nanoparticles synthesized by oxidative polymerization. J. Hazard. Mater. 2013, 250, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Vaitkuviene, A.; Ratautaite, V.; Mikoliunaite, L.; Kaseta, V.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Some biocompatibility aspects of conducting polymer polypyrrole evaluated with bone marrow-derived stem cells. Colloid Surf. A 2014, 442, 152–156. [Google Scholar] [CrossRef]
- Humpolicek, P.; Kasparkova, V.; Saha, P.; Stejskal, J. Biocompatibility of polyaniline. Synth. Met. 2012, 162, 722–727. [Google Scholar] [CrossRef]
- Stejskal, J.; Hajná, M.; Kašpárková, V.; Humpolíček, P.; Zhigunov, A.; Trchová, M. Purification of a conducting polymer, polyaniline, for biomedical applications. Synth. Met. 2014, 195, 286–293. [Google Scholar] [CrossRef]
- Humpolíček, P.; Kašpárková, V.; Pacherník, J.; Stejskal, J.; Bober, P.; Capáková, Z.; Radaszkiewicz, K.A.; Junkar, I.; Lehocký, M. The biocompatibility of polyaniline and polypyrrole: A comparative study of their cytotoxicity, embryotoxicity and impurity profile. Mater. Sci. Eng. C 2018, 91, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Neoh, K.G.; Cen, L.; Kang, E.T. Physicochemical and blood compatibility characterization of polypyrrole surface functionalized with heparin. Biotechnol. Bioeng. 2003, 84, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, N.; Carlsson, D.O.; Hong, J.; Larsson, R.; Fellstrom, B.; Nyholm, L.; Stromme, M.; Mihranyan, A. Haemocompatibility and ion exchange capability of nanocellulose polypyrrole membranes intended for blood purification. J. R. Soc. Interface 2012, 9, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Zhu, A.P.; Wu, Q.; Chen, X.B.; Kim, J.H.; Shen, J. New biocompatible polypyrrole-based films with good blood compatibility and high electrical conductivity. Colloid Surf. B 2008, 67, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Humpolíček, P.; Kuceková, Z.; Kašpárková, V.; Pelková, J.; Modic, M.; Junkar, I.; Trchová, M.; Bober, P.; Stejskal, J.; Lehocký, M. Blood coagulation and platelet adhesion on polyaniline films. Colloid Surf. B 2015, 133, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Paneva, D.; Stoilova, O.; Manolova, N.; Danchev, D.; Lazarov, Z.; Rashkov, I. Copolymers of 2-acryloylamido-2-methylpropanesulfonic acid and acrylic acid with anticoagulant activity. E-Polymers 2003, 3, 11. [Google Scholar] [CrossRef]
- Yancheva, E.; Paneva, D.; Danchev, D.; Mespouille, L.; Dubois, P.; Manolova, N.; Rashkov, I. Polyelectrolyte complexes based on (quaternized) poly (2-dimethylamino)ethyl methacrylate: Behavior in contact with blood. Macromol. Biosci. 2007, 7, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Šorm, M.; Nešpůrek, S.; Mrkvičková, L.; Kálal, J.; Vorlová, Z. Anticoagulation activity of some sulfate-containing polymers of the methacrylate type. J. Polym. Sci. C 1979, 66, 349–356. [Google Scholar] [CrossRef]
- Stejskal, J.; Gilbert, R.G. Polyaniline. Preparation of a conducting polymer (IUPAC technical report). Pure Appl. Chem. 2002, 74, 857–867. [Google Scholar] [CrossRef]
- Secomb, T.W. Hemodynamics. Compr. Physiol. 2016, 6, 975–1003. [Google Scholar] [PubMed]
- Bober, P.; Humpolíček, P.; Pacherník, J.; Stejskal, J.; Lindfors, T. Conducting polyaniline based cell culture substrate for embryonic stem cells and embryoid bodies. RSC Adv. 2015, 5, 50328–50335. [Google Scholar] [CrossRef]
- Li, Y.M.; Zhao, R.; Li, X.; Wang, C.Y.; Bao, H.W.; Wang, S.D.; Fang, J.; Huang, J.Q.; Wang, C. Blood-compatible polyaniline coated electrospun polyurethane fiber scaffolds for enhanced adhesion and proliferation of human umbilical vein endothelial cells. Fiber. Polym. 2019, 20, 250–260. [Google Scholar] [CrossRef]
- Zhang, F.; Kang, E.T.; Neoh, K.G.; Wang, P.; Tan, K.L. Reactive coupling of poly(ethylene glycol) on electroactive polyaniline films for reduction in protein adsorption and platelet adhesion. Biomaterials 2002, 23, 787–795. [Google Scholar] [CrossRef]
- Li, Z.F.; Ruckenstein, E. Grafting of poly(ethylene oxide) to the surface of polyaniline films through a chlorosulfonation method and the biocompatibility of the modified films. J. Colloid Interface Sci. 2004, 269, 62–71. [Google Scholar] [CrossRef]
- Humpolíček, P.; Radaszkiewicz, K.; Kašpárková, V.; Stejskal, J.; Trchová, M.; Kuceková, Z.; Vičarová, H.; Pachernik, J.; Lehocký, M.; Minarik, A. Stem cell differentiation on conducting polyaniline. RSC Adv. 2015, 5, 68796–68805. [Google Scholar] [CrossRef]
- Burtis, C.A.; Ashwood, E.R.; Bruns, D.E. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 4th ed.; Elsevier: St. Louis, MO, USA, 2006; p. 2412. [Google Scholar]
- Stejskal, J.; Sapurina, I. Polyaniline: Thin films and colloidal dispersions - (IUPAC technical report). Pure Appl. Chem. 2005, 77, 815–826. [Google Scholar] [CrossRef]
- Kay, C.; Schwan, H. Specific resistance of body tissues. Circul. Res. 1956, 4, 664–670. [Google Scholar]
- Kaushansky, K.; Lichtman, M.; Beutler, E.; Kipps, T.; Prchal, J.; Seligsohn, U. Principles of antithrombotic therapy. In Williams Hematology, 7th ed.; McGraw-Hill Professional: New York, NY, USA, 2006. [Google Scholar]
- Lam, L.H.; Silbert, J.E.; Rosenberg, R.D. Separation of active and inactive forms of heparin. Biochem. Biophys. Res. Commun. 1976, 69, 570–577. [Google Scholar] [CrossRef]
- Mosier, P.; Krishnasamy, C.; Kellogg, G.; Desai, U. On the specificity of heparin/heparan sulfate binding to proteins. Anion-binding sites on antithrombin and thrombin are fundamentally different. PLoS ONE 2012, 7, e48632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| γtot ± SD (mN·m−1) | |||
|---|---|---|---|
| Day 0 | Day 7 | Day 14 | |
| PANI-BSDBS | 51 ± 3 | 51 ± 2 | 50 ± 7 |
| PANI-SSDBS | 51 ± 3 | 47 ± 4 | 44 ± 4 |
| PANI-BTaurine | 51 ± 7 | 53 ± 2 | 38 ± 12 |
| PANI-STaurine | 55 ± 8 | 45 ± 7 | 47 ± 2 |
| PANI-BACES | 41 ± 1 | 37 ± 1 | 45 ± 0 |
| PANI-MSDBS | 46 ± 5 | 47 ± 4 | 46 ± 4 |
| PANI-MTaurine | 53 ± 4 | 44 ± 10 | 46 ± 4 |
| PANI-S (a) | 52.54 | n.d. | n.d. |
| PANI-B (a) | 50.88 | n.d. | n.d. |
| Sample | Day 0 | Day 7 | Day 14 |
|---|---|---|---|
| PANI-MSDBS | 7.0 ± 0.03 | 5.7 ± 0.002 | 5.2 ± 0.004 |
| PANI-MTaurine | 17.9 ± 0.01 | 16.5 ± 0.004 | 15.8 ± 0.003 |
| PT [s] | aPPT [s] | TCT [s] | |
|---|---|---|---|
| Reference | 12.1 ± 0.1 | 25.2 ± 0.5 | 16.7 ± 0.3 |
| PANI-BSDBS | 12.2 ± 0.6 | 29.5 ± 4.7 | 20.0 ± 2.2 |
| PANI-SSDBS | 11.8 ± 0.2 | 25.6 ± 0.3 | 18.3 ± 0.3 |
| PANI-BTaurin | 11.8 ± 0.0 | 25.4 ± 0.5 | 17.7 ± 0.5 |
| PANI-STaurin | 11.8 ± 0.2 | 26.9 ± 0.6 | 19.2 ± 0.4 |
| PANI-BACES | 12.3 ± 0.0 | 25.4 ± 0.3 | 15.4 ± 0.4 |
| PANI-MSDBS | 12.0 ± 0.1 | 25.8 ± 0.6 | 18.2 ± 0.5 |
| PANI-MTaurin | 12.1 ± 0.3 | 30.0 ± 2.5 | 19.7 ± 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skopalová, K.; Capáková, Z.; Bober, P.; Pelková, J.; Stejskal, J.; Kašpárková, V.; Lehocký, M.; Junkar, I.; Mozetič, M.; Humpolíček, P. In-Vitro Hemocompatibility of Polyaniline Functionalized by Bioactive Molecules. Polymers 2019, 11, 1861. https://doi.org/10.3390/polym11111861
Skopalová K, Capáková Z, Bober P, Pelková J, Stejskal J, Kašpárková V, Lehocký M, Junkar I, Mozetič M, Humpolíček P. In-Vitro Hemocompatibility of Polyaniline Functionalized by Bioactive Molecules. Polymers. 2019; 11(11):1861. https://doi.org/10.3390/polym11111861
Chicago/Turabian StyleSkopalová, Kateřina, Zdenka Capáková, Patrycja Bober, Jana Pelková, Jaroslav Stejskal, Věra Kašpárková, Marián Lehocký, Ita Junkar, Miran Mozetič, and Petr Humpolíček. 2019. "In-Vitro Hemocompatibility of Polyaniline Functionalized by Bioactive Molecules" Polymers 11, no. 11: 1861. https://doi.org/10.3390/polym11111861