Safely Dissolvable and Healable Active Packaging Films Based on Alginate and Pectin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
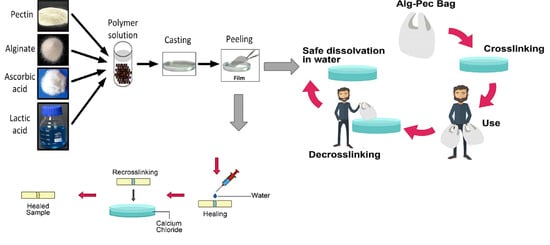
2.2. Preparation of Biocomposite Films
2.3. Crosslinking and Decrosslinking of Biocomposite Films
2.4. Healing of Biocomposite Films
2.5. FE-SEM Observation
2.6. FT-IR Spectrometry
2.7. Hydrophilicity Analysis
2.8. Thermogravimetric Analysis
2.9. Tensile Property
2.10. Antimicrobial Evaluation
2.11. Calcium Concentration
3. Results and Discussion
3.1. Dissolvability and Reusability Analysis
3.2. FT-IR Spectrometry
3.3. Hydrophilicity Analysis
3.4. Tensile Property
3.5. Healing Property
3.6. Thermogravimetric Analysis
3.7. Antimicrobial Evaluation
3.8. Calcium Concentration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- de Souza, A.C.; Ditchfield, C.; Tadini, C. Biodegradable Films Based on Biopolymers for Food Industries. In Innovation in Food Engineering: New Techniques and Products; CRC Press: Boca Raton, FL, USA, 2009; pp. 511–537. ISBN 0309-1740. [Google Scholar]
- López-Rubio, A.; Almenar, E.; Hernandez-Muñoz, P.; Lagarón, J.M.; Catalá, R.; Gavara, R. Overview of active polymer-based packaging technologies for food applications. Food Rev. Int. 2004, 20, 357–387. [Google Scholar] [CrossRef]
- Camilloto, G.P.; Pires, A.C.S.; Soares Nde, F.; Araújo, E.A.; Andrade, N.J.; Ferreira, S.O. Effect of active packaging incorporated with triclosan on bacteria adhesion. J. Food Sci. 2010, 75, E557–E564. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, P. Handbook of Bioplastics and Biocomposites Engineering Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 9781118203699. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- do Sul, J.A.I.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic Biodegradable Polymers as Medical Devices. Med. Device Diagn. Ind. News Prod. Suppliers 1998, 21, 1–8. [Google Scholar]
- Porta, R.; Mariniello, L.; di Pierro, P.; Sorrentino, A.; Giosafatto, C.V.L. Transglutaminase crosslinked pectinand chitosan-based edible films: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 223–238. [Google Scholar] [CrossRef]
- Chen, C.H.; Sheu, M.T.; Chen, T.F.; Wang, Y.C.; Hou, W.C.; Liu, D.Z.; Chung, T.C.; Liang, Y.C. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem. Pharmacol. 2006, 72, 1001–1009. [Google Scholar] [CrossRef]
- Ciriminna, R.; Chavarría-Hernández, N.; Inés Rodríguez Hernández, A.; Pagliaro, M. Pectin: A new perspective from the biorefinery standpoint. Biofuels Bioprod. Biorefin. 2015, 9, 368–377. [Google Scholar] [CrossRef]
- Rao, M.A. Pectins: Structure, Functionality, and Uses. Food Polysacch. Appl. 2006, 160, 353–411. [Google Scholar]
- Chen, J.; Liu, W.; Liu, C.M.; Li, T.; Liang, R.H.; Luo, S.J. Pectin Modifications: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef]
- Saha, N.R.; Sarkar, G.; Roy, I.; Rana, D.; Bhattacharyya, A.; Adhikari, A.; Mukhopadhyay, A.; Chattopadhyay, D. Studies on methylcellulose/pectin/montmorillonite nanocomposite films and their application possibilities. Carbohydr. Polym. 2016, 136, 1218–1227. [Google Scholar] [CrossRef]
- Gorrasi, G. Dispersion of halloysite loaded with natural antimicrobials into pectins: Characterization and controlled release analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Rhim, J.-W. Physical and mechanical properties of water resistant sodium alginate films. LWT Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Hay, I.D.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial alginate production, modification and its applications. Microb. Biotechnol. 2013, 6, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Bedian, L.; Villalba-rodríguez, A.M.; Hernández-vargas, G.; Parra-saldivar, R.; Iqbal, H.M.N. International Journal of Biological Macromolecules Bio-based materials with novel characteristics for tissue engineering applications—A review. Int. J. Biol. Macromol. 2017, 98, 837–846. [Google Scholar] [CrossRef]
- Abdul-Khalil, H.P.S.; Tye, Y.Y.; Saurabh, C.K.; Leh, C.P.; Lai, T.K.; Chong, E.W.N.; Fazita, M.R.N.; Hafiidz, J.M.; Banerjee, A.; Syakira, M.I. Seaweed biodegradable polymer films: A review on cellulose as reinforcement materials. Express Polym. Lett. 2016, 11, 1–51. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Kim, J.-H.; Kim, D.-H. Modification of Na-Alginate Films by CaCl2 Treatment. Korean J. Food Sci. Technol. 2003, 35, 217–221. [Google Scholar]
- Parker, S. Principles and Practice. IFLA J. 2006, 32, 179–180. [Google Scholar] [CrossRef]
- García Ibarra, V.; Sendón, R.; Rodríguez-Bernaldo De Quirós, A. Antimicrobial Food Packaging Based on Biodegradable Materials. In Antimicrobial Food Packaging; Academic Press: San Diego, CA, USA, 2016; pp. 363–384. ISBN 9780128007235. [Google Scholar]
- Perdones, Á.; Vargas, M.; Atarés, L.; Chiralt, A. Physical, antioxidant and antimicrobial properties of chitosan-cinnamon leaf oil films as affected by oleic acid. Food Hydrocoll. 2014, 36, 256–264. [Google Scholar] [CrossRef]
- Bof, M.J.; Jiménez, A.; Locaso, D.E.; García, M.A.; Chiralt, A. Grapefruit Seed Extract and Lemon Essential Oil as Active Agents in Corn Starch–Chitosan Blend Films. Food Bioprocess Technol. 2016, 9, 2033–2045. [Google Scholar] [CrossRef]
- Imran, M.; Klouj, A.; Revol-Junelles, A.M.; Desobry, S. Controlled release of nisin from HPMC, sodium caseinate, poly-lactic acid and chitosan for active packaging applications. J. Food Eng. 2014, 143, 178–185. [Google Scholar] [CrossRef]
- Fabra, M.J.; Sánchez-González, L.; Chiralt, A. Lysozyme release from isolate pea protein and starch based films and their antimicrobial properties. LWT Food Sci. Technol. 2014, 55, 22–26. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol-chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Zhong, Q. Physical and antimicrobial properties of chitosan films incorporated with lauric arginate, cinnamon oil, and ethylenediaminetetraacetate. LWT Food Sci. Technol. 2016, 65, 173–179. [Google Scholar] [CrossRef]
- Rhim, J.W.; Wang, L.F. Preparation and characterization of carrageenan-based nanocomposite films reinforced with clay mineral and silver nanoparticles. Appl. Clay Sci. 2014, 97–98, 174–181. [Google Scholar] [CrossRef]
- Liu, L.S.; Jin, T.; Coffin, D.R.; Liu, C.K.; Hicks, K.B. Poly(lactic acid) membranes containing bacteriocins and edta for inhibition of the surface growth of gram-negative bacteria. J. Appl. Polym. Sci. 2010, 117, 486–492. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.X.; Avena-Bustillos, R.d.J.; Soares, N.d.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Avena-Bustillos, R.J.; Friedman, M.; Henika, P.R.; Martín-Belloso, O.; Mchugh, T.H. Mechanical, barrier, and antimicrobial properties of apple puree edible films containing plant essential oils. J. Agric. Food Chem. 2006, 54, 9262–9267. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, S.; Zhu, L.; Olsen, C.W.; McHugh, T.H.; Friedman, M. Edible apple film wraps containing plant antimicrobials inactivate foodborne pathogens on meat and poultry products. J. Food Sci. 2009, 74, M440–M445. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Y.; Meure, S.; Solomon, D. Self-healing polymeric materials: A review of recent developments. Prog. Polym. Sci. 2008, 33, 479–522. [Google Scholar] [CrossRef]
- Ferrari, F.; Greco, A. Thermal analysis of self-healing thermoplastic matrix nanocomposite from cyclic butylene terephthalate. J. Therm. Anal. Calorim. 2018, 134, 567–574. [Google Scholar] [CrossRef]
- Lee, W.; Jin, M.; Yoo, W.; Lee, J. Nanostructuring of a Poymeric Substrate with Wll-defined Nanometer-Scale Topography and Tailored Surface Wettability. Langmuir 2004, 20, 7665–7669. [Google Scholar] [CrossRef]
- Cerna, M.; Barros, A.S.; Nunes, A.; Rocha, S.M.; Delgadillo, I.; Copikova, J.; Coimbra, M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003, 51, 383–389. [Google Scholar] [CrossRef]
- Nešić, A.; Onjia, A.; Davidović, S.; Dimitrijević, S.; Errico, M.E.; Santagata, G.; Malinconico, M. Design of pectin-sodium alginate based films for potential healthcare application: Study of chemico-physical interactions between the components of films and assessment of their antimicrobial activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef]
- Coleman, M.M.; Moskala, E.J. FTi.r. studies of polymer blends containing the poly(hydroxy ether of bisphenol A) and poly(ε-caprolactone). Polymer (Guildf.) 1983, 24, 251–257. [Google Scholar] [CrossRef]
- Cavallaro, G.; Donato, D.I.; Lazzara, G.; Milioto, S. Films of halloysite nanotubes sandwiched between two layers of biopolymer: From the morphology to the dielectric, thermal, transparency, and wettability properties. J. Phys. Chem. C 2011, 115, 20491–20498. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Sustainable nanocomposites based on halloysite nanotubes and pectin/polyethylene glycol blend. Polym. Degrad. Stab. 2013, 98, 2529–2536. [Google Scholar] [CrossRef]
- Farris, S.; Introzzi, L.; Biagioni, P.; Holz, T.; Schiraldi, A.; Piergiovanni, L. Wetting of biopolymer coatings: Contact angle kinetics and image analysis investigation. Langmuir 2011, 27, 7563–7574. [Google Scholar] [CrossRef] [PubMed]
- Oakenfull, D.; Scott, A.; Chai, E. The mechanism of formation of mixed gels by high methoxyl pectins and alginates. Gums Stabilisers Food Ind. 1990, 5, 243–264. [Google Scholar]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of cross-linking with calcium ions on the physical properties of alginate films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef]
- Andersson, C.; Järnström, L.; Fogden, A.; Mira, I.; Voit, W.; Zywicki, S.; Bartkowiak, A. Preparation and incorporation of microcapsules in functional coatings for self-healing of packaging board. Packag. Technol. Sci. 2009, 22, 275–291. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A.; Abu Hasfa, S.H.; Smqadri, S.Q.; Haik, Y. Antimicrobial Activity of Copper Alone and in Combination with Lactic Acid against Escherichia coli O157:H7 in Laboratory Medium and on the Surface of Lettuce and Tomatoes. J. Pathog. 2011, 2011, 650968. [Google Scholar] [CrossRef] [PubMed]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Derouaux, A.; Sauvage, E.; Terrak, M. Peptidoglycan Glycosyltransferase Substrate Mimics as Templates for the Design of New Antibacterial Drugs. Front. Immunol. 2013, 4, 78. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Kennedy, R.A. Swelling and diffusion studies of calcium polysaccharide gels intended for film coating. Int. J. Pharm. 2008, 358, 205–213. [Google Scholar] [CrossRef]
- da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohydr. Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]










| Sample | Thickness (mm) | Crosslinking Time (min) | Decrosslinking Time (min) | Crosslinking Repeatability (times) | Decrosslinking Repeatability (times) | Complete Dissolving after Decrosslinking (min) |
|---|---|---|---|---|---|---|
| Alginate | 0.043 ± 0.008 | 2 | 7 | >5 | >5 | 15 |
| Pectin | 0.038 ± 0.15 | 2 | 1 | - | - | 2 |
| Alg–Pec | 0.043 ± 0.013 | 2 | 6 | >5 | >5 | 10 |
| Alg–Pec–AA–LA | 0.056 ± 0.01 | 2 | 6 | >5 | >5 | 9 |
| Band Frequency (cm−1) | ||||
|---|---|---|---|---|
| Sample | –OH | COO− (asym) | COO− (sym) | COOCH3 |
| Pectin | 3378 | 1605 | 1435 | 1732 |
| Alginate | 3264 | 1591 | 1416 | - |
| Alg–Pec | 3300 | 1601 | 1417 | 1738 |
| Alg–Pec–AA–LA | 3282 | 1594 | 1417 | 1736 |
| Sample | Crosslinking Time (min) | Thickness (mm) | Tensile (MPa) | Elongation (%) |
|---|---|---|---|---|
| Alginate | 0 | 0.024 ± 0.01 | 29.1 ± 3.1 | 8.16 ± 0.9 |
| Alginate | 2 | 0.043 ± 0.008 | 26.05 ± 4.7 | 7.3 ± 1.8 |
| Pectin | 0 | 0.035 ± 0.012 | 18.7 ± 3.2 | 3.9 ± 0.5 |
| Pectin | 2 | 0.038 ± 0.15 | 16.8 ± 2.4 | 3.5 ± 1.2 |
| Alg–Pec | 0 | 0.033 ± 0.014 | 20.1 ± 1.7 | 11.8 ± 1.5 |
| Alg–Pec | 2 | 0.043 ± 0.013 | 23.4 ± 0.9 | 9.7 ± 2.6 |
| Alg–Pec–AA–LA | 0 | 0.039 ± 0.14 | 19.3 ± 2.7 | 10.9 ± 1.3 |
| Alg–Pec–AA–LA | 2 | 0.048 ± 0.01 | 22.7 ± 1.8 | 9.1 ± 0.8 |
| Sample | Crosslinking Time (min) | Thickness (mm) | Tensile (1st healing) (MPa) | Elongation (1st healing) (%) | Healing Efficiency (1st healing) (%) | Tensile (2nd healing) (MPa) | Elongation (2nd healing) (%) | Tensile (3rd healing) (MPa) | Elongation (3rd healing) (%) |
|---|---|---|---|---|---|---|---|---|---|
| Alginate | 2 | 0.043 ± 0.008 | 24.1 ± 2.4 | 6.5 ± 1.1 | 92.5 | 20.5 ± 1.8 | 5.1 ± 0.3 | 16.4 ± 1.3 | 3.8 ± 0.4 |
| Alg–Pec | 2 | 0.043 ± 0.013 | 20.5 ± 1.7 | 9.1 ± 1.5 | 87.6 | 15.8 ± 1.5 | 7.4 ± 1.2 | 12.1 ± 0.9 | 4.9 ± 0.5 |
| Alg–Pec–AA–LA | 2 | 0.048 ± 0.01 | 19.1 ± 1.8 | 8.5 ± 0.8 | 84.1 | 14.5 ± 0.7 | 6.1 ± 0.5 | 10.7 ± 1.1 | 4.2 ± 0.7 |
| Sample | Temperature at 5% Weight Loss (°C) | Weight loss at 100 °C (%) | Residual Matter at 700 °C (%) | Temperature at Maximum Weight Loss Rate (°C) |
|---|---|---|---|---|
| Alginate | 41.05 | 18.96 | 22.95 | 249, 587 |
| Pectin | 42.51 | 14.72 | 5.33 | 228, 498 |
| Alg–Pec | 49.10 | 12.98 | 29.10 | 229, 690 |
| Crosslinked Alginate | 51.18 | 15.13 | 12.07 | 203, 553 |
| Crosslinked Pectin | 40.97 | 20.17 | 10.67 | 232, 537 |
| Crosslinked Alg–Pec | 78.11 | 7.48 | 13.21 | 240, 527 |
| Bacteria | Diameter of Zone of Inhibition (mm) |
|---|---|
| E. coli | 9.0 ± 1.5 |
| P. aeruginosa | 6.0 ± 2.0 |
| S. aureus | 8.0 ± 1.0 |
| Sample | Calcium Content (mg/g sample) |
|---|---|
| Alg | 32.18 |
| Pec | 22.7 |
| Alg–Pec | 27.8 |
| Alg–Pec–AA–LA | 30.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makaremi, M.; Yousefi, H.; Cavallaro, G.; Lazzara, G.; Goh, C.B.S.; Lee, S.M.; Solouk, A.; Pasbakhsh, P. Safely Dissolvable and Healable Active Packaging Films Based on Alginate and Pectin. Polymers 2019, 11, 1594. https://doi.org/10.3390/polym11101594
Makaremi M, Yousefi H, Cavallaro G, Lazzara G, Goh CBS, Lee SM, Solouk A, Pasbakhsh P. Safely Dissolvable and Healable Active Packaging Films Based on Alginate and Pectin. Polymers. 2019; 11(10):1594. https://doi.org/10.3390/polym11101594
Chicago/Turabian StyleMakaremi, Maziyar, Hosnieh Yousefi, Giuseppe Cavallaro, Giuseppe Lazzara, Calvin Bok Sun Goh, Sui Mae Lee, Atefeh Solouk, and Pooria Pasbakhsh. 2019. "Safely Dissolvable and Healable Active Packaging Films Based on Alginate and Pectin" Polymers 11, no. 10: 1594. https://doi.org/10.3390/polym11101594
APA StyleMakaremi, M., Yousefi, H., Cavallaro, G., Lazzara, G., Goh, C. B. S., Lee, S. M., Solouk, A., & Pasbakhsh, P. (2019). Safely Dissolvable and Healable Active Packaging Films Based on Alginate and Pectin. Polymers, 11(10), 1594. https://doi.org/10.3390/polym11101594