Electrochromism in Electropolymerized Films of Pyrene-Triphenylamine Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of Compounds
2.2.1. Compound 1
2.2.2. Compound 2
2.3. Electrochemistry
2.4. Spectroelectrochemical Measurements
3. Results
3.1. Synthesis of Monomers
3.2. Electropolymerization
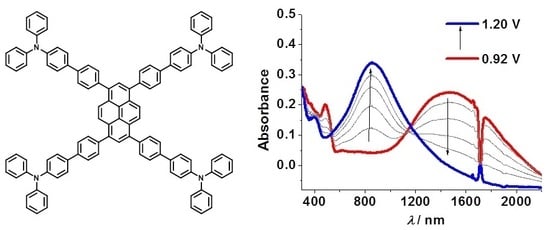
3.3. Spectroelectrochemistry
3.4. Electrochromic Switching
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mortimer, R.J. Electrochromic materials. Chem. Soc. Rev. 1997, 26, 147–156. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in buildings: A state-of-the-art review. Sol. Energy Mater. Sol. Cells 2010, 94, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Sonmez, G.; Sonmez, H.B. Polymeric electrochromics for data storage. J. Mater. Chem. 2006, 16, 2473–2477. [Google Scholar] [CrossRef]
- Krebs, F.C. Electrochromic displays: The new black. Nat. Mater. 2008, 7, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, M.; Ma, J.; Cao, Y.; Deng, Y. Electrochromic metal oxides: Recent progress and prospect. Adv. Electron. Mater. 2018, 4, 1800185. [Google Scholar] [CrossRef]
- Higuchi, M. Stimuli-responsive metallo-supramolecular polymer films: Design, synthesis and device fabrication. J. Mater. Chem. C 2014, 2, 9331–9341. [Google Scholar] [CrossRef]
- Lv, X.; Li, W.; Ouyang, M.; Zhang, Y.; Wright, D.S.; Zhang, C. Polymeric electrochromic materials with donor-acceptor structures. J. Mater. Chem. C 2017, 5, 12–28. [Google Scholar] [CrossRef]
- Cai, S.; Wen, H.; Wang, S.; Niu, H.; Wang, C.; Jiang, X.; Bai, X.; Wang, W. Electrochromic polymers electrochemically polymerized from 2, 5–dithienylpyrrole (DTP) with different triarylamine units: Synthesis, characterization and optoelectrochemical properties. Electrochim. Acta 2017, 228, 332–342. [Google Scholar] [CrossRef]
- Qi, J.; Qiao, W.; Wang, Z.Y. Advances in organic near-infrared materials and emerging applications. Chem. Rec. 2016, 16, 1531–1548. [Google Scholar] [CrossRef]
- Nagasaki, J.; Hiroto, S.; Shinokubo, H. π-Extended dihydrophenazines with three-state NIR electrochromism involving large conformational changes. Chem. Asian J. 2017, 12, 2311–2317. [Google Scholar] [CrossRef]
- Yen, H.-J.; Lin, H.-Y.; Liou, G.-S. Novel starburst triarylamine-containing electroactive aramids with highly stable electrochromism in near-infrared and visible light regions. Chem. Mater. 2011, 23, 1874–1882. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M.; Liao, S.-H. Redox-stable and visible/near-infrared electrochromic aramids with main-chain triphenylamine and pendent 3,6-di-tert-butylcarbazole units. Polym. Chem. 2014, 5, 2473–2483. [Google Scholar] [CrossRef]
- Chuang, Y.-W.; Yen, H.-J.; Wu, J.-H.; Liou, G.-S. Colorless triphenylamine-based aliphatic thermoset epoxy for multicolored and near-infrared electrochromic applications. ACS Appl. Mater. Interfaces 2014, 6, 3594–3599. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiao, W.; Liu, B.; Ren, J.; Wang, Z. Synthesis and near infrared electrochromic properties of metallodithiolene complexes. Sci. China Chem. 2017, 60, 77–83. [Google Scholar] [CrossRef]
- Qi, Y.H.; Desjardins, P.; Meng, X.S.; Wang, Z.Y. Electrochromic ruthenium complex materials for optical attenuation. Opt. Mater. 2003, 21, 255–263. [Google Scholar] [CrossRef]
- Li, Z.-J.; Shao, J.-Y.; Zhong, Y.-W. Near-infrared and two-wavelength electrochromism based on nanocrystalline TiO2 films functionalized with ruthenium-amine conjugated complexes. Inorg. Chem. 2017, 56, 8538–8546. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.-L.; Yao, C.-J.; Shao, J.-Y.; Nie, H.-J.; Tang, J.-H.; Zhong, Y.-W. Redox-responsive carbometalated ruthenium and osmium complexes. Sci. China Chem. 2017, 60, 583–590. [Google Scholar] [CrossRef]
- Yao, B.; Chen, F.; Jiang, H.; Zhang, J.; Wan, X. Isomer effect on the near-infrared electrochromism of anthraquinone imides. Electrochim. Acta 2015, 166, 73–81. [Google Scholar] [CrossRef]
- Gu, H.; Ming, S.; Lin, K.; Chen, S.; Liu, X.; Lu, B.; Xu, J. Isoindigo as an electron-deficient for high-performance polymeric electrochromics. Electrochim. Acta 2018, 260, 772–782. [Google Scholar] [CrossRef]
- Lahav, M.; van der Boom, M.E. Polypyridyl metal-organic assemblies for electrochromic applications. Adv. Mater. 2018, 30, 1706641. [Google Scholar] [CrossRef]
- Beverina, L.; Pagani, G.A.; Sassi, M. Multichromophoric electrochromic polymers: Colour tuning of conjugated polymers through the side chain functionalization approach. Chem. Commun. 2014, 50, 5413–5430. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.-H.; Lu, H.-Y. Electrosynthesis of aromatic poly(amide-amine) films from triphenylamine-based electroactive compounds for electrochromic applications. Polymers 2017, 9, 708. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lin, J.-Y. Electrosynthesis of ambipolar electrochromic polymer films from anthraquinone-triarylamine hybrids. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 644–655. [Google Scholar] [CrossRef]
- Qin, L.; Ma, W.; Hanif, M.; Jiang, J.; Xie, Z.; Ma, Y. Donor-node-acceptor polymer with excellent n-doped state for high-performance ambipolar flexible supercapacitors. Macromolecules 2017, 50, 3565–3572. [Google Scholar] [CrossRef]
- Li, M.; Ishihara, S.; Akada, M.; Liao, M.; Sang, L.; Hill, J.P.; Krishnan, V.; Ma, Y.; Ariga, K. Electrochemical-coupling layer-by-layer (ECC-LbL) assembly. J. Am. Chem. Soc. 2011, 133, 7348–7351. [Google Scholar] [CrossRef] [PubMed]
- Li, M. C3-C3′ and C6-C6′ Oxidative couplings of carbazoles. Chem. Eur. J. 2018, 24. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Xu, Z.; Mi, S.; Zheng, Z.; Xu, C. Highly regiosymmetric homopolymer based on dioxythiophene for realizing water-processable blue-to-transmissive electrochrome. ACS Appl. Mater. Interfaces 2015, 7, 11387–11392. [Google Scholar] [CrossRef] [PubMed]
- Rende, E.; Kilic, C.E.; Udum, Y.A.; Toffoli, D.; Toppare, L. Electrochromic properties of multicolored novel polymer synthesized via combination of benzotriazole and N-functionalized 2,5-di(2-thienyl)-1H-pyrrole units. Electrochim. Acta 2014, 138, 454–463. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Recent advances in triphenylamine-based electrochromic derivatives and polymers. Polym. Chem. 2018, 9, 3001–3018. [Google Scholar] [CrossRef]
- Leung, M.-K.; Chou, M.-Y.; Su, Y.O.; Chiang, C.L.; Chen, H.-L.; Yang, C.F.; Yang, C.-C.; Lin, C.-C.; Chen, H.T. Diphenylamino group as an effective handle to conjugated donor–acceptor polymers through electropolymerization. Org. Lett. 2003, 5, 839–842. [Google Scholar] [CrossRef]
- Natera, J.; Otero, L.; Sereno, L.; Fungo, F.; Wang, N.-S.; Tsai, Y.-M.; Hwu, T.-Y.; Wong, K.-T. A novel electrochromic polymer synthesized through electropolymerization of a new donor-acceptor bipolar system. Macromolecules 2007, 40, 4456–4463. [Google Scholar] [CrossRef]
- Chang, C.-C.; Leung, M.-K. Photolithographic hole-transport layer derived from electrochemical deposition of oligo(5-vinyl-2-nitrobenzyl triphenylamine-4-carboxylate). Chem. Mater. 2008, 20, 5816–5821. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lin, J.-W. Facile preparation of electrochromic poly(amine–imide) films from diimide compounds with terminal triphenylamino groups via electrochemical oxidative coupling reactions. Polym. Chem. 2014, 5, 6770–6778. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liao, W.-K.; Liou, G.-S. A comparative study of redox-active, ambipolar electrochromic triphenylamine-based polyimides prepared by electrochemical polymerization and conventional polycondensation methods. Polym. Chem. 2018, 9, 236–248. [Google Scholar] [CrossRef]
- Yao, C.-J.; Zhong, Y.-W.; Yao, J. Five-stage near-infrared electrochromism in electropolymerized films composed of alternating cyclometalated bisruthenium and bis-triarylamine segments. Inorg. Chem. 2013, 52, 10000–10008. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.-Q.; Shao, J.-Y.; Ding, J.; Deng, L.-Y.; Zhou, W.-K.; Chen, Y.-X.; Ma, J.-Y.; Wan, L.-J.; Yao, J.; Hu, J.-S.; et al. A two-dimensional hole-transporting material for high-performance perovskite solar cells with 20% average efficiency. Angew. Chem. Int. Ed. 2018, 57, 10959–10965. [Google Scholar] [CrossRef] [PubMed]
- Figueira-Duarte, T.M.; Mullen, K. Pyrene-based materials for organic electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef]
- Feng, N.; Hu, J.-Y.; Redshaw, C.; Yamato, T. Functionalization of pyrene to prepare luminescent materials-typical examples of synthetic methodology. Chem. Eur. J. 2016, 22, 11898–11916. [Google Scholar] [CrossRef]
- Jin, M.; Wu, X.; Xie, J.; Malval, J.P.; Wan, D. One/two-photon-sensitive photoacid generators based on benzene oligomer-containing D–π–A type aryl dialkylsulfonium salts. RSC Adv. 2015, 5, 55340–55347. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.-G.; Li, Z.-J.; Shao, J.-Y.; Zhong, Y.-W. Electrochromism in Electropolymerized Films of Pyrene-Triphenylamine Derivatives. Polymers 2019, 11, 73. https://doi.org/10.3390/polym11010073
Sun T-G, Li Z-J, Shao J-Y, Zhong Y-W. Electrochromism in Electropolymerized Films of Pyrene-Triphenylamine Derivatives. Polymers. 2019; 11(1):73. https://doi.org/10.3390/polym11010073
Chicago/Turabian StyleSun, Tian-Ge, Zhi-Juan Li, Jiang-Yang Shao, and Yu-Wu Zhong. 2019. "Electrochromism in Electropolymerized Films of Pyrene-Triphenylamine Derivatives" Polymers 11, no. 1: 73. https://doi.org/10.3390/polym11010073
APA StyleSun, T.-G., Li, Z.-J., Shao, J.-Y., & Zhong, Y.-W. (2019). Electrochromism in Electropolymerized Films of Pyrene-Triphenylamine Derivatives. Polymers, 11(1), 73. https://doi.org/10.3390/polym11010073