Impact of Lignin Content on the Properties of Hemicellulose Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Reactants
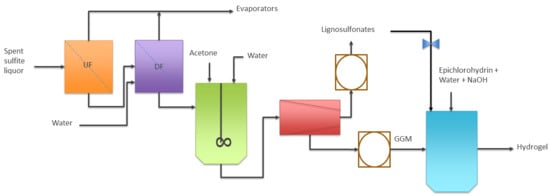
2.2. Ultrafiltration and Anti-solvent Precipitation
2.3. Preparing Hydrogels
2.4. Analysis
2.4.1. Swelling Capacity
2.4.2. Lignin Content and Epichlorohydrin Conversion
2.4.3. Hemicellulose, Acetic Acid, and Glycerol Content
2.4.4. Size-exclusion Chromatography
2.4.5. Fourier Transform Infrared Spectroscopy
2.4.6. Compression Stress and Strain
2.4.7. Thermogravimetric Analysis
2.4.8. Release of BTB from Hydrogels
2.4.9. Liquid Chromatography-mass Spectrometry (LC-MS)
3. Results and discussion
3.1. Ultrafiltration and Anti-solvent Precipitation
3.2. Parameter Study
3.3. Effect of Crosslinking on Hydrogel Content
3.4. Effect of Addition of Lignin on Hydrogel Content
3.5. FTIR on Hydrogels
3.6. Size-Exclusion Chromatography
3.7. Swelling Degree and Drug Release
3.8. Mechanical Strength
3.9. Thermo-gravimetric Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Li, J.; Dong, D.; Zhang, Y.; Yao, F. Ionic starch-based hydrogels for the prevention of nonspecific protein adsorption. Carbohydr. Polym. 2015, 117, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhao, P.; Li, F.; Guo, F.; Li, Z.; Wen, X. Thermosensitive in situ-forming dextran–pluronic hydrogels through Michael addition. Mater. Sci. Eng. C 2010, 30, 1236–1244. [Google Scholar] [CrossRef]
- Wenzl, H. The Chemical Technology of Wood; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Söderqvist Lindblad, M.; Albertsson, A.-C.; Ranucci, E.; Laus, M.; Giani, E. Biodegradable polymers from renewable sources: Rheological characterization of hemicellulose-based hydrogels. Biomacromolecules 2005, 6, 684–690. [Google Scholar] [CrossRef]
- Maleki, L.; Edlund, U.; Albertsson, A.-C. Synthesis of full interpenetrating hemicellulose hydrogel networks. Carbohydr. Polym. 2017, 170, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Maleki, L.; Edlund, U.; Albertsson, A.-C. Unrefined wood hydrolysates are viable reactants for the reproducible synthesis of highly swellable hydrogels. Carbohydr. Polym. 2014, 108, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zasadowski, D.; Yang, J.; Edlund, H.; Norgren, M. Antisolvent precipitation of water-soluble hemicelluloses from TMP process water. Carbohydr. Polym. 2014, 113, 411–419. [Google Scholar] [CrossRef]
- Jacobs, A.; Lundqvist, J.; Stålbrand, H.; Tjerneld, F.; Dahlman, O. Characterization of water-soluble hemicelluloses from spruce and aspen employing SEC/MALDI mass spectroscopy. Carbohydr. Res. 2002, 337, 711–717. [Google Scholar] [CrossRef]
- Krawczyk, H.; Arkell, A.; Jönsson, A.-S. Impact of prefiltration on membrane performance during isolation of hemicelluloses extracted from wheat bran. Sep. Purif. Technol. 2013, 116, 192–198. [Google Scholar] [CrossRef]
- Al Manasrah, M.; Kallioinen, M.; Ilvesniemi, H.; Mänttäri, M. Recovery of galactoglucomannan from wood hydrolysate using regenerated cellulose ultrafiltration membranes. Bioresour. Technol. 2012, 114, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Pranovich, A.; Holmbom, B. Separation of polymeric galactoglucomannans from hot-water extract of spruce wood. Bioresour. Technol. 2013, 130, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Thuvander, J.; Jönsson, A.-S. Extraction of galactoglucomannan from thermomechanical pulp mill process water by microfiltration and ultrafiltration—Influence of microfiltration membrane pore size on ultrafiltration performance. Chem. Eng. Res. Des. 2016, 105, 171–176. [Google Scholar] [CrossRef]
- Al-Rudainy, B.; Galbe, M.; Wallberg, O. Influence of prefiltration on membrane performance during isolation of lignin-carbohydrate complexes from spent sulfite liquor. Sep. Purif. Technol. 2017, 187, 380–388. [Google Scholar] [CrossRef]
- Al-Rudainy, B.; Galbe, M.; Schagerlöf, H.; Wallberg, O. Antisolvent precipitation of hemicelluloses, lignosulfonates and their complexes from ultrafiltrated spent sulfite liquor (SSL). Holzforschung 2018, 72, 839–850. [Google Scholar] [CrossRef]
- Vermaas, J.V.; Petridis, L.; Qi, X.; Schulz, R.; Lindner, B.; Smith, J.C. Mechanism of lignin inhibition of enzymatic biomass deconstruction. Biotechnol. Biofuels 2015, 8, 217. [Google Scholar] [CrossRef]
- Zhao, W.; Odelius, K.; Edlund, U.; Zhao, C.; Albertsson, A.-C. In situ synthesis of magnetic field-responsive hemicellulose hydrogels for drug delivery. Biomacromolecules 2015, 16, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Palamarchuk, I.; Brovko, O.; Bogolitsyn, K.; Boitsova, T.; Ladesov, A.; Ivakhnov, A. Relationship of the structure and ion-exchange properties of polyelectrolyte complexes based on biopolymers. Russ. J. Appl. Chem. 2015, 88, 103–109. [Google Scholar] [CrossRef]
- Rönnols, J.; Schweinebarth, H.; Jacobs, A.; Stevanic, J.S.; Olsson, A.-M.; Reimann, A.; Aldaeus, F. Structural changes in softwood kraft lignin during thermal treatment. Nord. Pulp Pap. Res. J. 2015, 30, 550–561. [Google Scholar]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, Y.; Yu, Z. Effect of desulfonation of lignosulfonate on the properties of poly (lactic acid)/lignin composites. BioResources 2017, 12, 4810–4829. [Google Scholar] [CrossRef]
- Lemes, A.P.; Soto-Oviedo, M.A.; Waldman, W.R.; Innocentini-Mei, L.H.; Durán, N. Effect of Lignosulfonate on the Thermal and Morphological Behavior of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Polym. Environ. 2010, 18, 250–259. [Google Scholar] [CrossRef]
- Leger, C.A.; Chan, F.D.; Schneider, M.H. Fractionation and characterisation of technical ammonium lignosulphonate. BioResources 2010, 5, 2239–2247. [Google Scholar]
- Beall, F. Thermogravimetric analysis of wood lignin and hemicelluloses. Wood Fiber Sci. 2007, 1, 215–226. [Google Scholar]
- Gebelein, C.; Koblitz, F. Biomedical and Dental Applications of Polymers; Springer Science & Business Media: Berlin, Germany, 2013; Volume 14. [Google Scholar]
- Mussatto, S.I.; Fernandes, M.; Roberto, I.C. Lignin recovery from brewer’s spent grain black liquor. Carbohydr. Polym. 2007, 70, 218–223. [Google Scholar] [CrossRef]
- Swamy, N.K.; Singh, P.; Sarethy, I.P. Precipitation of phenols from paper industry wastewater using ferric chloride. Rasayan J. Chem. 2011, 4, 452–456. [Google Scholar]
- Laine, C. Structures of Hemicelluloses and Pectins in Wood and Pulp; Helsinki University of Technology: Espoo, Finland, 2005. [Google Scholar]
- Kartha, K.; Srivastava, H. Reaction of epichlorhydrin with carbohydrate polymers. Part II. Starch reaction mechanism and physicochemical properties of modified starch. Starch-Stärke 1985, 37, 297–306. [Google Scholar] [CrossRef]
- Medeiros, M.A.; Araujo, M.H.; Augusti, R.; de Oliveira, L.C.; Lago, R.M. Acid-catalyzed oligomerization of glycerol investigated by electrospray ionization mass spectrometry. J. Braz. Chem. Soc. 2009, 20, 1667–1673. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Pranovich, A.; Vähäsalo, L.; Hemming, J.; Holmbom, B.; Schols, H.A.; Willför, S. Kinetics of acid hydrolysis of water-soluble spruce O-acetyl galactoglucomannans. J. Agric. Food Chem. 2008, 56, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Nebreda, A.P.; Grénman, H.; Mäki-Arvela, P.; Eränen, K.; Hemming, J.; Willför, S.; Murzin, D.Y.; Salmi, T. Acid hydrolysis of O-acetyl-galactoglucomannan in a continuous tube reactor: A new approach to sugar monomer production. Holzforschung 2016, 70, 187–194. [Google Scholar] [CrossRef]
- Lundqvist, J.; Teleman, A.; Junel, L.; Zacchi, G.; Dahlman, O.; Tjerneld, F.; Stålbrand, H. Isolation and characterization of galactoglucomannan from spruce (Picea abies). Carbohydr. Polym. 2002, 48, 29–39. [Google Scholar] [CrossRef]
- Jyothi, A.N.; Moorthy, S.N.; Rajasekharan, K.N. Effect of cross-linking with epichlorohydrin on the properties of cassava (Manihot esculenta crantz) starch. Starch-Stärke 2006, 58, 292–299. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Muhtadi, A.; Elyani, I.; Musfiroh, I. Epichlorohydrin as Crosslinking Agent for Synthesis of Carboxymethyl Cellulose Sodium (Na-CMC) as Pharmaceutical Excipient from Water Hyacinth (Eichorrnia Crassipes L.). Int. J. Chem. Sci. 2015, 13, 1227–1237. [Google Scholar]
- Körner, A. Dissolution of Polydisperse Polymers in Water; Physical Chemistry 1, Lund University: Lund, Sweden, 2006. [Google Scholar]
- Qi, X.-M.; Chen, G.-G.; Gong, X.-D.; Fu, G.-Q.; Niu, Y.-S.; Bian, J.; Peng, F.; Sun, R.-C. Enhanced mechanical performance of biocompatible hemicelluloses-based hydrogel via chain extension. Sci. Rep. 2016, 6, 33603. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.-W.; Ren, J.-L.; Zhong, L.-X.; Peng, F.; Sun, R.-C. Xylan-rich hemicelluloses-graft-acrylic acid ionic hydrogels with rapid responses to pH, salt, and organic solvents. J. Agric. Food Chem. 2011, 59, 8208–8215. [Google Scholar] [CrossRef]
- Sakai, T.; Matsunaga, T.; Yamamoto, Y.; Ito, C.; Yoshida, R.; Suzuki, S.; Sasaki, N.; Shibayama, M.; Chung, U.-I. Design and fabrication of a high-strength hydrogel with ideally homogeneous network structure from tetrahedron-like macromonomers. Macromolecules 2008, 41, 5379–5384. [Google Scholar] [CrossRef]













| Time (min) | Eluent A (%) | Eluent B (%) |
|---|---|---|
| 1 | 100.0 | 0.0 |
| 8 | 0.0 | 100.0 |
| 10 | 0.0 | 100.0 |
| 12 | 100.0 | 0.0 |
| Fraction | TS of Prepared Solutions (g/L) | Lignin Content (g/L) | Acetic Acid (g/L) | Arabinan (g/L) | Galactan (g/L) | Glucan (g/L) | Xylan (g/L) | Mannan (g/L) |
|---|---|---|---|---|---|---|---|---|
| GGM | 100 | 8.40 | 3.60 | 0.60 | 15.9 | 13.0 | 4.60 | 23.5 |
| Lignin | 99.9 | 94.6 | 0.75 | 0.21 | 1.09 | 1.64 | 0.94 | 4.11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rudainy, B.; Galbe, M.; Arcos Hernandez, M.; Jannasch, P.; Wallberg, O. Impact of Lignin Content on the Properties of Hemicellulose Hydrogels. Polymers 2019, 11, 35. https://doi.org/10.3390/polym11010035
Al-Rudainy B, Galbe M, Arcos Hernandez M, Jannasch P, Wallberg O. Impact of Lignin Content on the Properties of Hemicellulose Hydrogels. Polymers. 2019; 11(1):35. https://doi.org/10.3390/polym11010035
Chicago/Turabian StyleAl-Rudainy, Basel, Mats Galbe, Monica Arcos Hernandez, Patric Jannasch, and Ola Wallberg. 2019. "Impact of Lignin Content on the Properties of Hemicellulose Hydrogels" Polymers 11, no. 1: 35. https://doi.org/10.3390/polym11010035