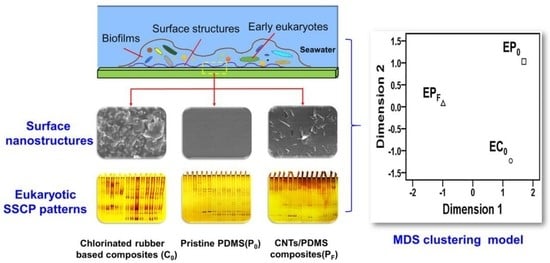
Differential Colonization Dynamics of Marine Biofilm-Forming Eukaryotic Microbes on Different Protective Coating Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Formulation
2.2. Static Ocean Exposure Assays
2.3. Sampling the Natural Biofilms
2.4. Single-Stranded Conformation Polymorphism
2.5. Coating Characterization
2.5.1. Water Contact Angle Measurements
2.5.2. Scanning Electron Microscopy Analysis
2.6. Data Analysis
3. Results and Discussion
3.1. Coating Characterization
3.1.1. Static WCA Measurements
3.1.2. SEM Characterization
3.2. Field Exposure Studies
3.3. Analysis of the Pioneer Eukaryotic Biofilm Communities
3.3.1. The Eukaryotic SSCP Fingerprints
3.3.2. The Eukaryotic Diversity Indices
3.4. Clustering Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cao, S.; Wang, J.D.; Chen, H.S.; Chen, D.R. Progress of marine biofouling and antifouling technologies. Chin. Sci. Bull. 2011, 56, 598–612. [Google Scholar] [CrossRef]
- Lacoste, E.; Gaertner-Mazouni, N. Biofouling impact on production and ecosystem functioning: A review for bivalve aquaculture. Rev. Aquac. 2015, 7, 187–196. [Google Scholar] [CrossRef]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Emira, H.S.; Shakour, A.A.; Abd El Rehim, S.S.; Saleh, I.A.; El-Hashemy, M.A. Evaluation of corrosion protection of carbon steel by anticorrosive paints. Anti-Corros. Methods Mater. 2012, 59, 255–262. [Google Scholar] [CrossRef]
- Sakhri, A.; Perrin, F.X.; Aragon, E.; Lamouric, S.; Benaboura, A. Chlorinated rubber paints for corrosion prevention of mild steel: A comparison between zinc phosphate and polyaniline pigments. Corros. Sci. 2010, 52, 901–909. [Google Scholar] [CrossRef]
- Sakhri, A.; Perrin, F.X.; Benaboura, A.; Aragon, E.; Lamouri, S. Corrosion protection of steel by sulfo-doped polyaniline-pigmented coating. Prog. Org. Coat. 2011, 72, 473–479. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Selim, M.S.; Shenashen, M.A.; El-Safty, S.A.; Higazy, S.A.; Selim, M.M.; Isago, H.; Elmarakbi, A. Recent progress in marine foul-release polymeric nanocomposite coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar] [CrossRef]
- Dobretsov, S.; Abed, R.M.M.; Voolstra, C.R. The effect of surface color on the formation of marine micro and macrofouling communities. Biofouling 2013, 29, 617–627. [Google Scholar] [CrossRef]
- Swain, G.; Herpe, S.; Ralston, E.; Tribou, M. Short-term testing of antifouling surfaces: The importance of colour. Biofouling 2006, 22, 425–429. [Google Scholar] [CrossRef]
- Ong, C.S.; Goh, P.S.; Lau, W.J.; Misdan, N.; Ismail, A.F. Nanomaterials for biofouling and scaling mitigation of thin film composite membrane: A review. Desalination 2016, 393, 2–15. [Google Scholar] [CrossRef]
- Jing, H.; Sahle-Demessie, E.; Sorial, G.A. Inhibition of biofilm growth on polymer-MWCNTs composites and metal surfaces. Sci. Total Environ. 2018, 633, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Gkikas, G.; Lekatou, A.; Sioulas, D.; Paipetis, A.S. Effect of carbon nanotube enhanced adhesives on degradation of bonded joints in corrosive environments. Plast. Rubber Compos. 2014, 43, 322–329. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, Y.F.; Guo, X.P.; Liang, X.; Xu, Y.F.; Ding, D.W.; Bao, W.Y.; Dobretsov, S. The effect of carbon nanotubes and titanium dioxide incorporated in pdms on biofilm community composition and subsequent mussel plantigrade settlement. Biofouling 2016, 32, 763–777. [Google Scholar] [CrossRef]
- Li, Y.C.; Xu, Y.L.; Fleischer, C.C.; Huang, J.; Lin, R.; Yang, L.; Mao, H. Impact of anti-biofouling surface coatings on the properties of nanomaterials and their biomedical applications. J. Mater. Chem. B 2018, 6, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Lovell, C.R. Microbial Surface Colonization and Biofilm Development in Marine Environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef]
- Balqadi, A.A.; Salama, A.J.; Satheesh, S. Microfouling development on artificial substrates deployed in the central Red Sea. Oceanologia 2018, 60, 219–231. [Google Scholar] [CrossRef]
- Hadfield, M.G. Biofilms and Marine Invertebrate Larvae: What Bacteria Produce That Larvae Use to Choose Settlement Sites. In Annual Review of Marine Science; Carlson, C.A., Giovannoni, S.J., Eds.; Annual Reviews: Palo Alto, CA, USA, 2011; Volume 3, pp. 453–470. [Google Scholar]
- Sweat, L.H.; Swain, G.W.; Hunsucker, K.Z.; Johnson, K.B. Transported biofilms and their influence on subsequent macrofouling colonization. Biofouling 2017, 33, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Zardus, J.D.; Nedved, B.T.; Huang, Y.; Tran, C.; Hadfield, M.G. Microbial biofilms facilitate adhesion in biofouling invertebrates. Biol. Bull. 2008, 214, 91–98. [Google Scholar] [CrossRef]
- von Ammon, U.; Wood, S.A.; Laroche, O.; Zaiko, A.; Tait, L.; Lavery, S.; Inglis, G.; Pochon, X. The impact of artificial surfaces on marine bacterial and eukaryotic biofouling assemblages: A high-throughput sequencing analysis. Mar. Environ. Res. 2018, 133, 57–66. [Google Scholar] [CrossRef]
- Casse, F.; Swain, G.W. The development of microfouling on four commercial antifouling coatings under static and dynamic immersion. Int. Biodeterior. Biodegrad. 2006, 57, 179–185. [Google Scholar] [CrossRef]
- Briand, J.F.; Djeridi, I.; Jamet, D.; Coupe, S.; Bressy, C.; Molmeret, M.; Le Berre, B.; Rimet, F.; Bouchez, A.; Blache, Y. Pioneer marine biofilms on artificial surfaces including antifouling coatings immersed in two contrasting French Mediterranean coast sites. Biofouling 2012, 28, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J.-F. Marine biofilms on artificial surfaces: Structure and dynamics. Environ. Microbiol. 2013, 15, 2879–2893. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ji, Y.B.; Lang, Y.H.; Wang, L.; Liu, B.; Zhang, Z.Z. A comparative study on the impact of the carbon nanotubes-modified polydimethylsiloxane nanocomposites on the colonization dynamics of the pioneer biofilm communities. Int. Biodeterior. Biodegrad. 2018, 129, 195–201. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.Z. New anti-biofouling carbon nanotubes-filled polydimethylsiloxane composites against colonization by pioneer eukaryotic microbes. Int. Biodeterior. Biodegrad. 2016, 110, 147–154. [Google Scholar] [CrossRef]
- Logares, R.; Audic, S.; Bass, D.; Bittner, L.; Boutte, C.; Christen, R.; Claverie, J.-M.; Decelle, J.; Dolan, J.R.; Dunthorn, M.; et al. Patterns of Rare and Abundant Marine Microbial Eukaryotes. Curr. Biol. 2014, 24, 813–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worden, A.Z.; Follows, M.J.; Giovannoni, S.J.; Wilken, S.; Zimmerman, A.E.; Keeling, P.J. Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes. Science 2015, 347, 1257594. [Google Scholar] [CrossRef]
- Beigbeder, A.; Degee, P.; Conlan, S.L.; Mutton, R.J.; Clare, A.S.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Dubois, P. Preparation and characterisation of silicone-based coatings filled with carbon nanotubes and natural sepiolite and their application as marine fouling-release coatings. Biofouling 2008, 24, 291–302. [Google Scholar] [CrossRef]
- Briand, J.F.; Barani, A.; Garnier, C.; Rehel, K.; Urvois, F.; LePoupon, C.; Bouchez, A.; Debroas, D.; Bressy, C. Spatio-Temporal Variations of Marine Biofilm Communities Colonizing Artificial Substrata Including Antifouling Coatings in Contrasted French Coastal Environments. Microb. Ecol. 2017, 74, 585–598. [Google Scholar] [CrossRef]
- Peng, W.K.; Lin, H.C.; Chen, C.N.; Wang, C.H. DNA identification of two laboratory colonies of the weevils, Sitophilus oryzae (L.) and S-zeamais Motschulsky (Coleoptera: Curculionidae) in Taiwan. J. Stored Prod. Res. 2002, 39, 225–235. [Google Scholar] [CrossRef]
- Han, X.; Wang, L.; Wang, X. Fabrication of Chemical Gradient Using Space Limited Plasma Oxidation and its Application for Droplet Motion. Adv. Funct. Mater. 2012, 22, 4533–4538. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, Y.; Sun, Y.; Yu, M.; Ji, Y.; Wang, L.; Zhang, Z. Differential Colonization Dynamics of Marine Biofilm-Forming Eukaryotic Microbes on Different Protective Coating Materials. Polymers 2019, 11, 161. https://doi.org/10.3390/polym11010161
Lang Y, Sun Y, Yu M, Ji Y, Wang L, Zhang Z. Differential Colonization Dynamics of Marine Biofilm-Forming Eukaryotic Microbes on Different Protective Coating Materials. Polymers. 2019; 11(1):161. https://doi.org/10.3390/polym11010161
Chicago/Turabian StyleLang, Yanhe, Yuan Sun, Miao Yu, Yubin Ji, Lei Wang, and Zhizhou Zhang. 2019. "Differential Colonization Dynamics of Marine Biofilm-Forming Eukaryotic Microbes on Different Protective Coating Materials" Polymers 11, no. 1: 161. https://doi.org/10.3390/polym11010161