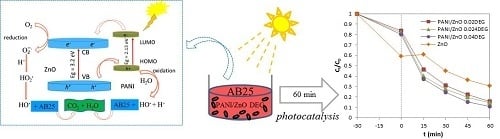
Preparation of a PANI/ZnO Composite for Efficient Photocatalytic Degradation of Acid Blue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Preparation
2.3. Characterizations
2.4. Photodegradation of Acid Blue Dye
3. Results and Discussion
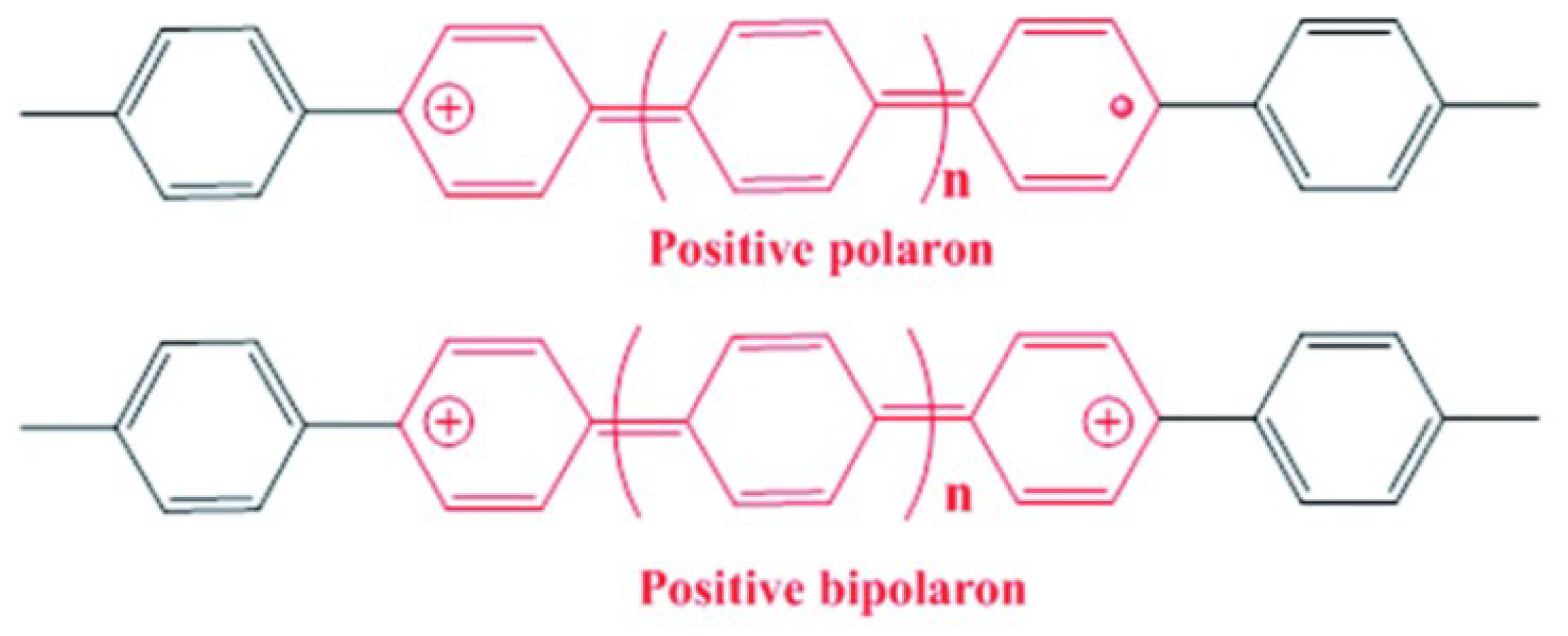
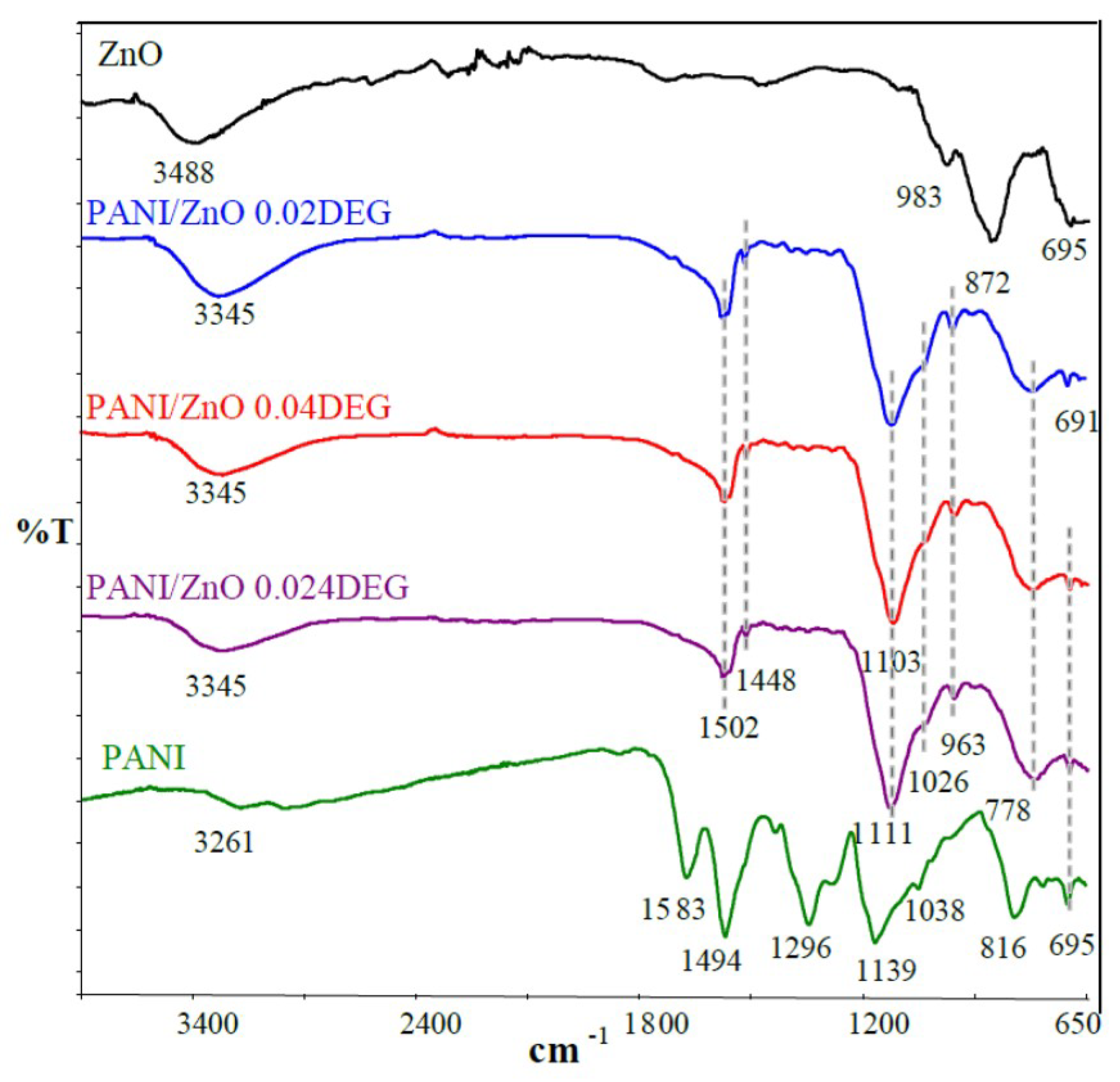
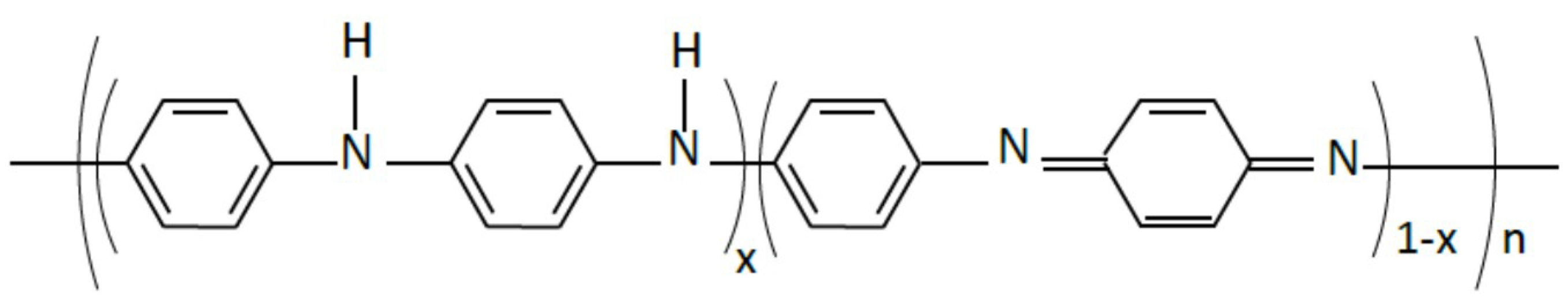
3.1. FTIR Characterization
3.2. Conductivity of PANI/ZnO Composites
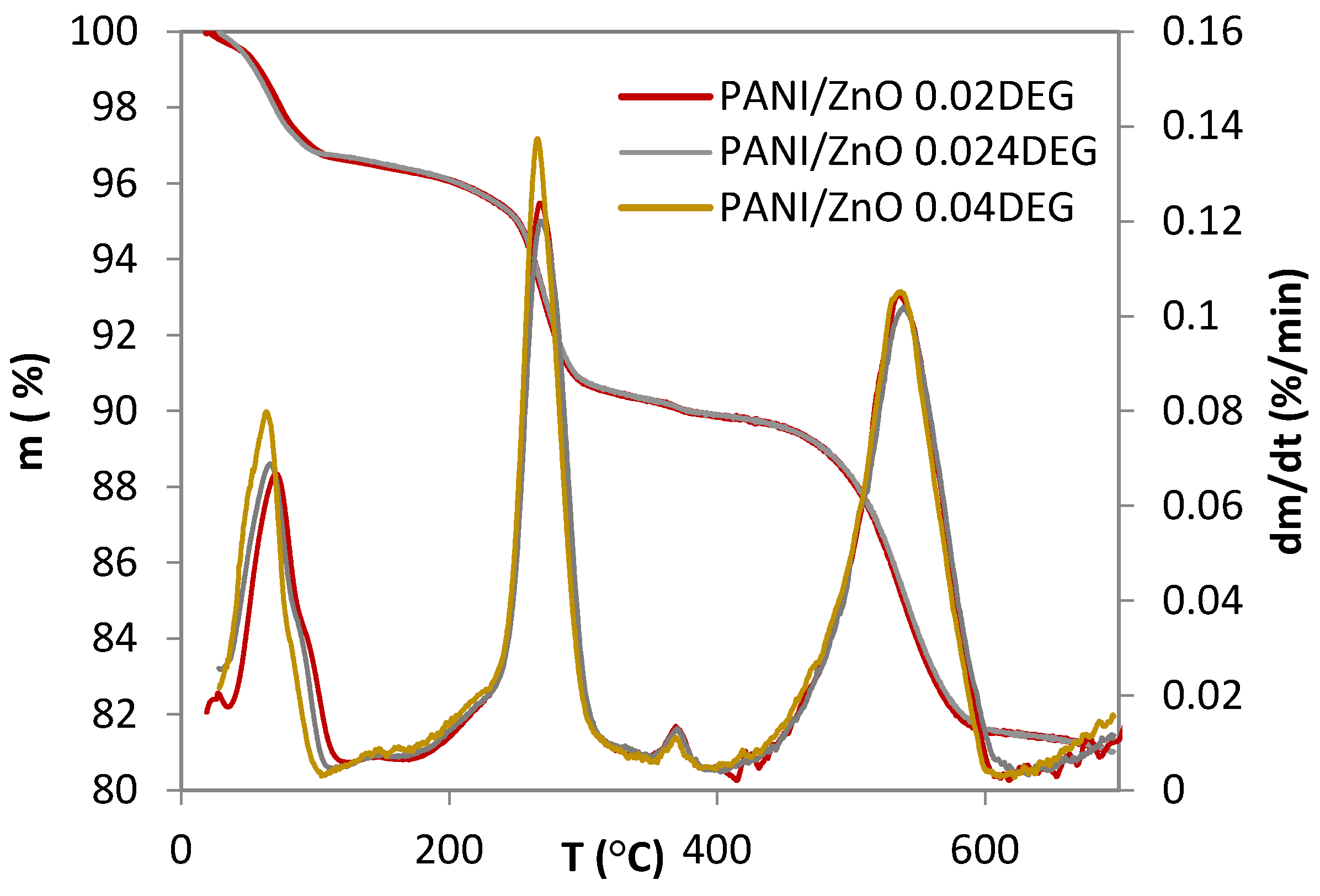
3.3. Thermogravimetric Analysis Characterization
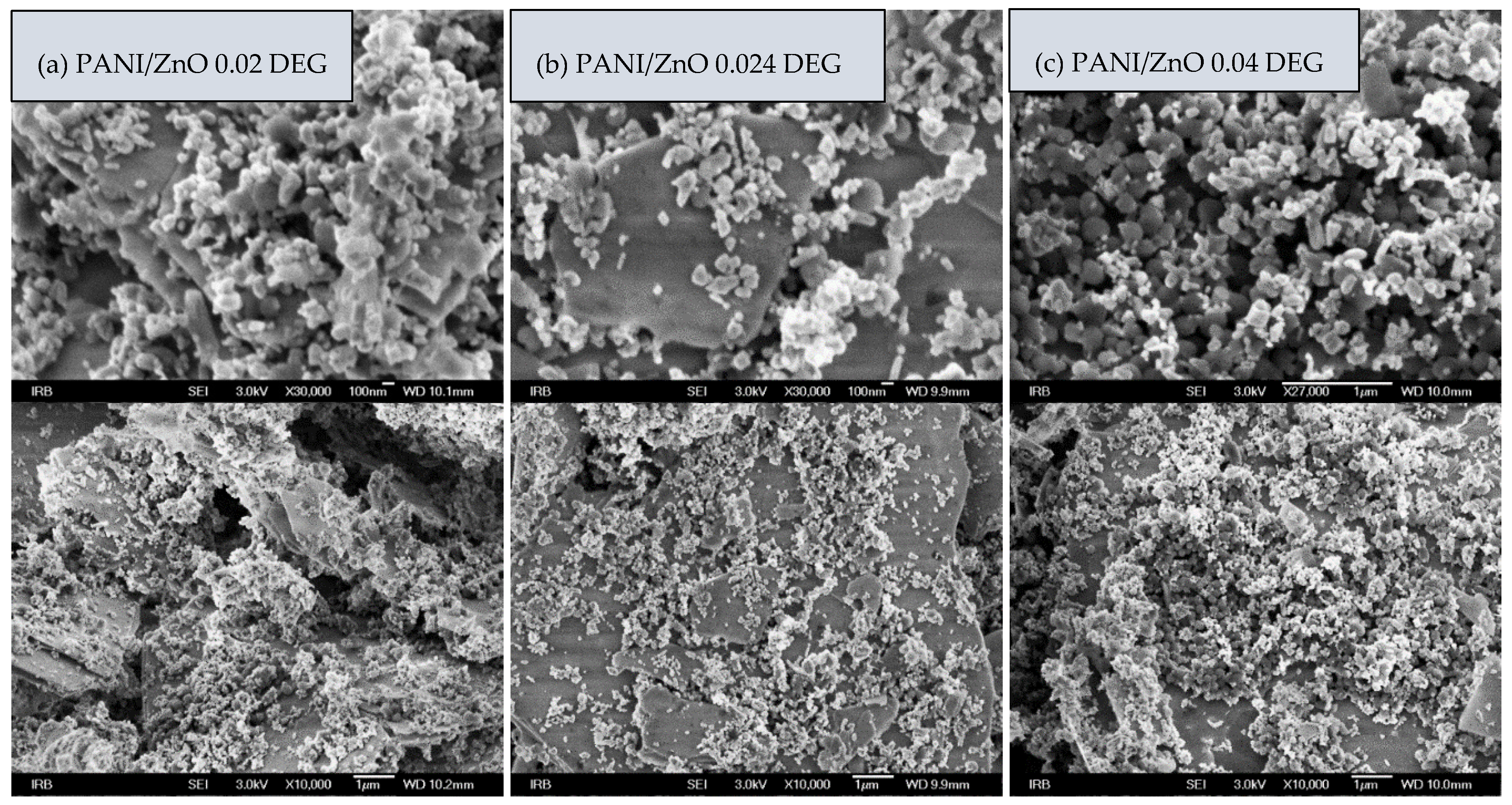
3.4. Morphology of PANI/ZnO Composites
3.5. Surface Area Analysis
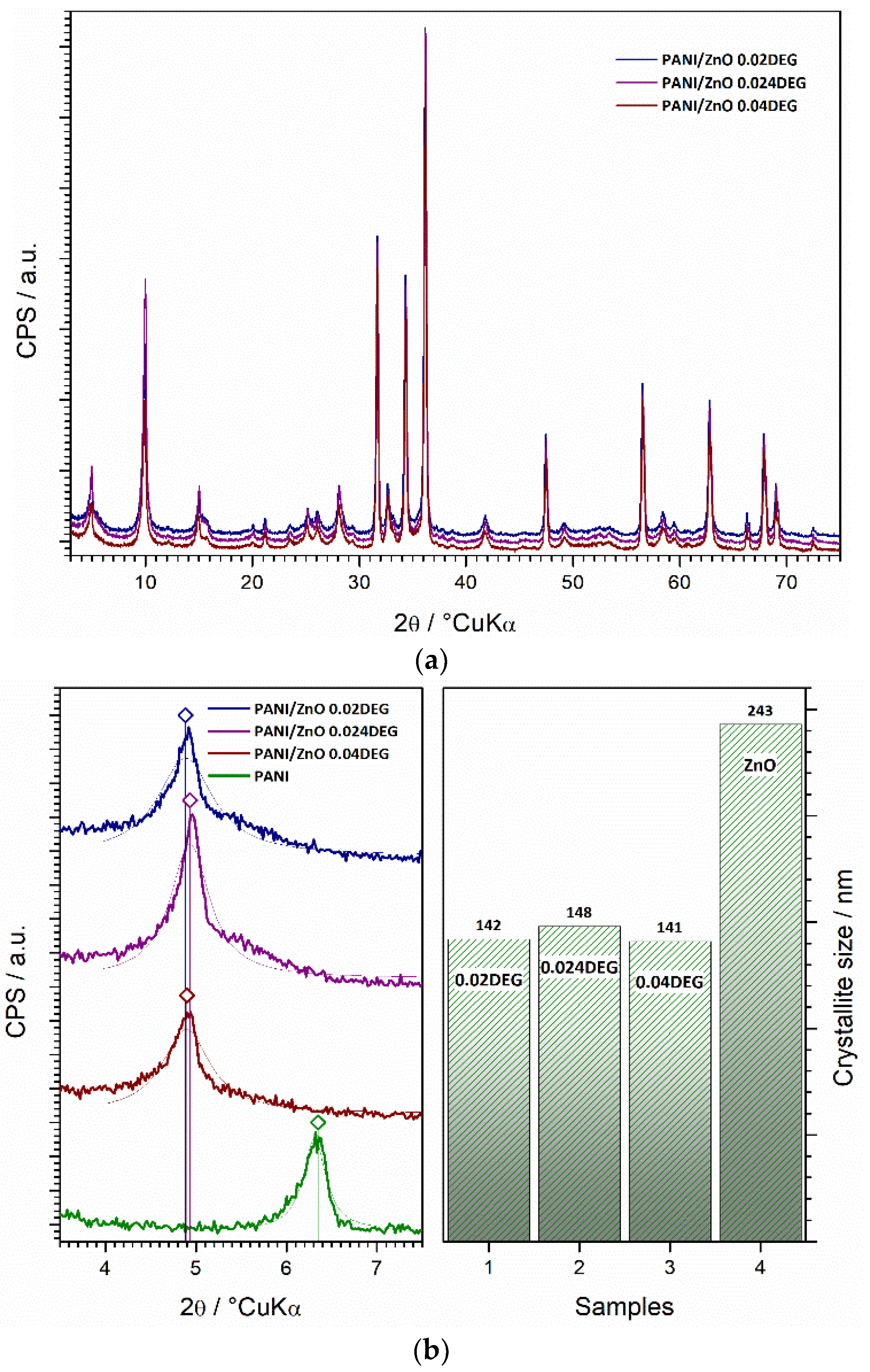
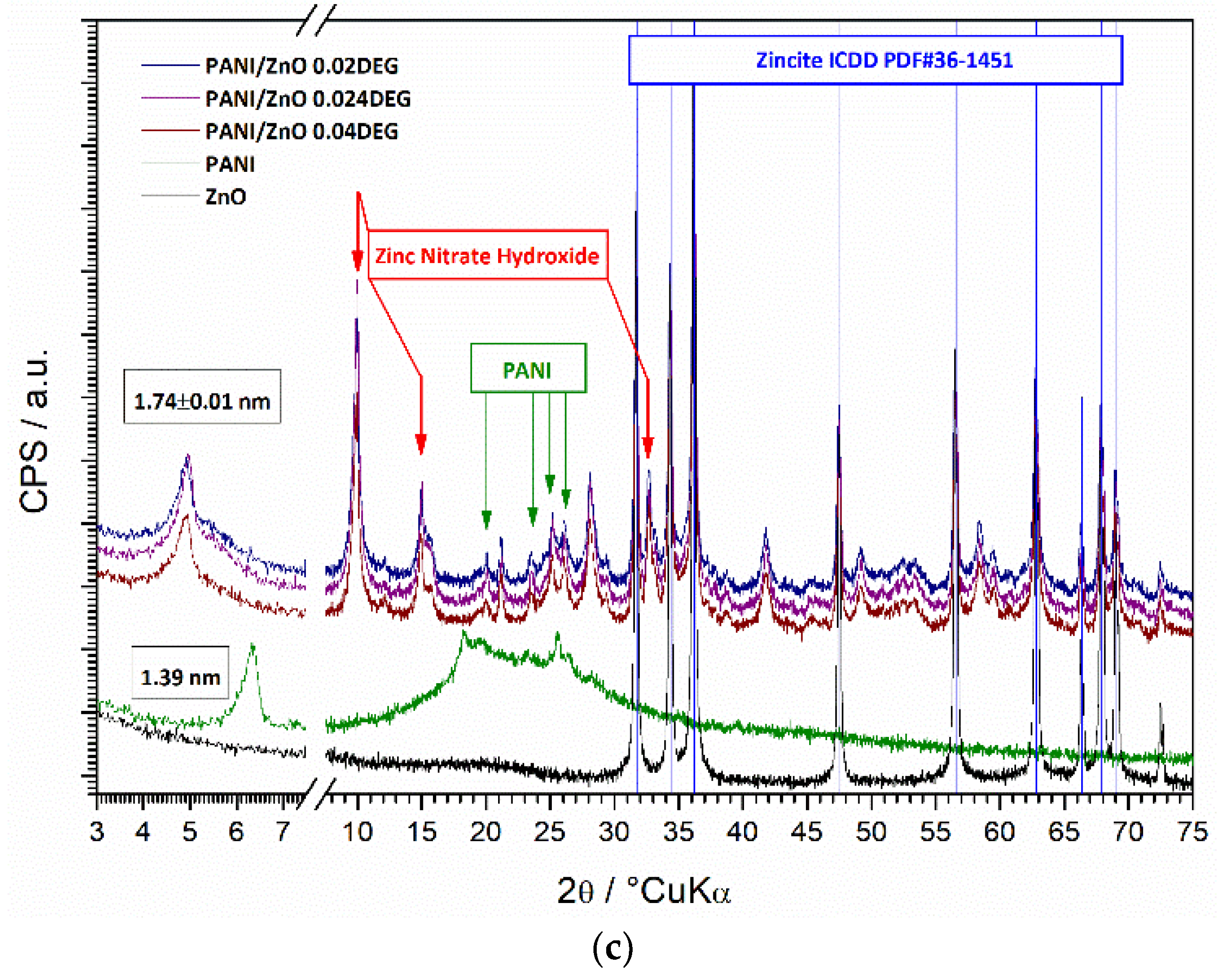
3.6. XRD Characterization
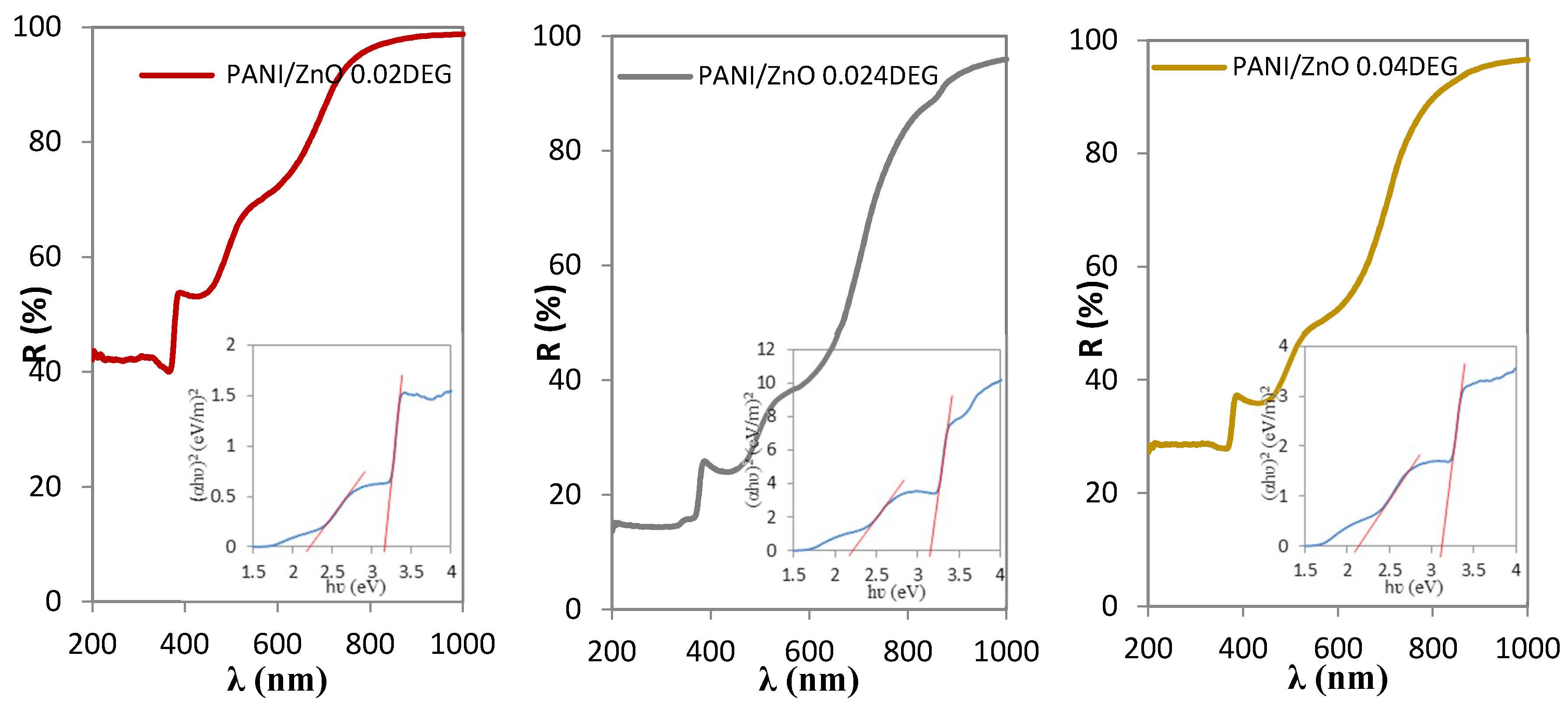
3.7. UV-Vis Spectrophotometry Characterization
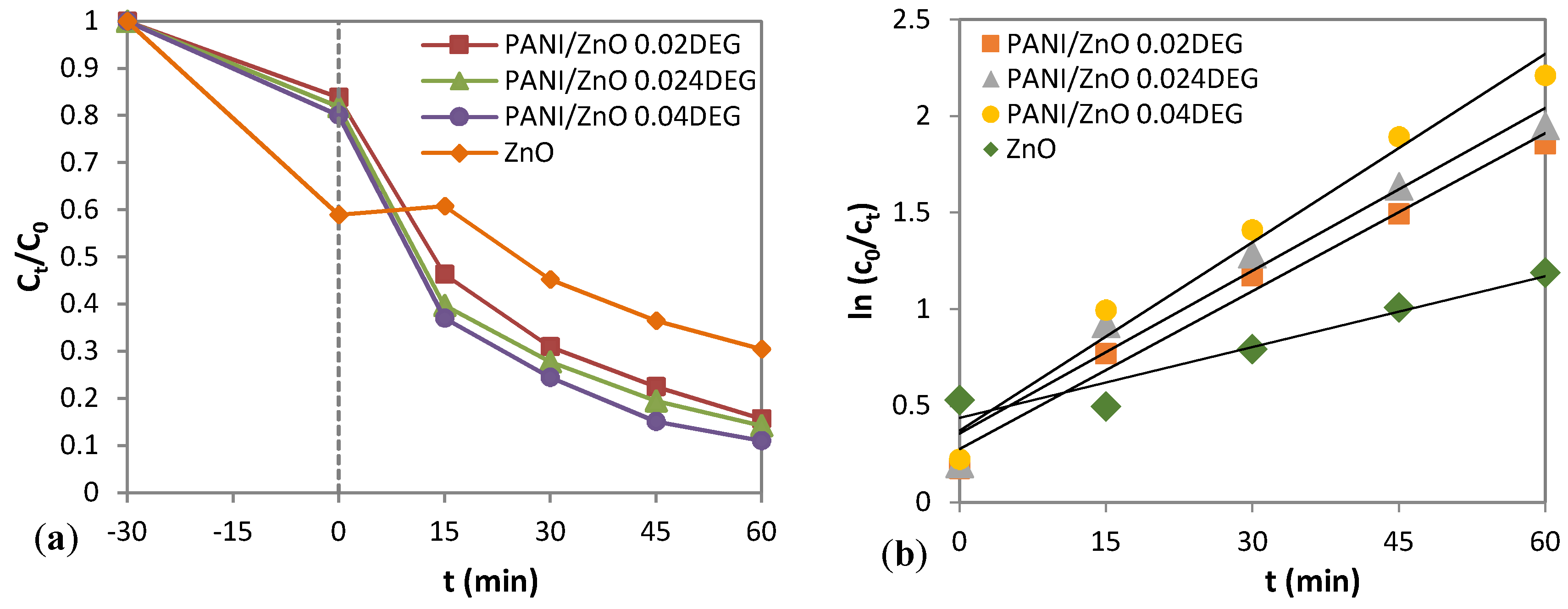
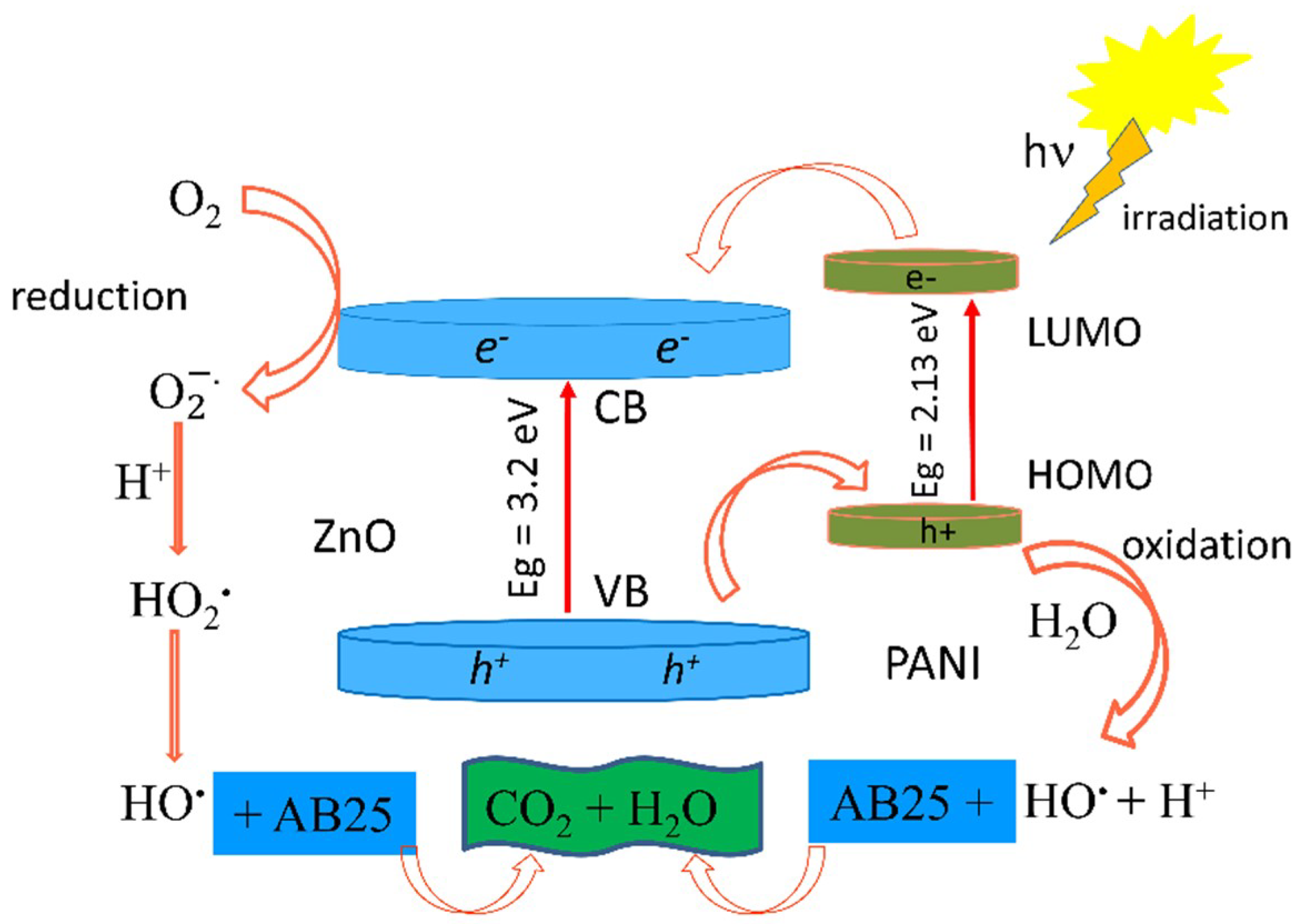
3.8. Photocatalytic Activity of the PANI/ZnO Composite
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aono, M.; Ariga, K. The way to nanoarchitectonics and the way of nanoarchitectonics. Adv. Mater. 2016, 28, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Aono, M. Nanoarchitectonics: A new materials horizon for nanotechnology. Mater. Horiz. 2015, 2, 406–413. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y.; Aono, M. Commentary: Nanoarchitectonics—Think about NANO again. APL Mater. 2015, 3, 061001–061005. [Google Scholar] [CrossRef]
- Jose, J.P.; Malhotra, S.K.; Thomas, S.; Joseph, K.; Goda, K.; Sreekala, M.S. Advances in Polymer Composites: Macro- and Microcomposites State of the Art. In New Challenges, and Opportunities. Polymer Composites, Macro-and Microcomposites, 1st ed.; Wiley-VCH Verlag: Piscataway, NJ, USA, 2012. [Google Scholar]
- Fan, J.C.; Sreekanth, K.M.; Xie, Z.; Chang, S.L.; Rao, K.V. P-Type ZnO materials: Theory, growth, properties and devices. Prog. Mater. Sci. 2013, 58, 874–985. [Google Scholar] [CrossRef]
- Gomez, J.L.; Tigli, O. Zinc oxide nanostructures: From growth to application. J. Mater. Sci. 2013, 48, 612–624. [Google Scholar] [CrossRef]
- Kozuka, Y.; Tsukazaki, A.; Kawasaki, M. Challenges and opportunities of ZnO-related single crystalline heterostructures. Appl. Phys. Rev. 2014, 1, 011303. [Google Scholar] [CrossRef]
- Ludi, B.; Niederberger, M. Zinc oxide nanoparticles: Chemical mechanisms and classical and non-classical crystallization. Dalton Trans. 2013, 35, 12554–12568. [Google Scholar] [CrossRef] [PubMed]
- Klingshirn, C.; Fallert, J.; Zhou, H.; Sartor, J.; Thiele, C.; Fliag, F.M.; Schneider, D.; Kalt, H. 65 years of ZnO research–old and very recent results. Phys. Status Solidi B 2010, 247, 1424–1447. [Google Scholar] [CrossRef]
- Cho, S.; Jung, S.-H.; Lee, K.-H. Morphology-controlled growth of ZnO nanostructures using microwave irradiation: From basic to complex structures. J. Phys. Chem. C 2008, 112, 12769–12776. [Google Scholar] [CrossRef]
- Saleh, R.; Djaja, N.F. Transition-metal-doped ZnO nanoparticles: Synthesis, characterization and photocatalytic activity under UV light. Spectrochim. Acta Part A 2014, 130, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Amini, A.; Zhu, C.; Xu, Z.; Song, H.; Wang, N. Enhanced photocatalytic performance of TiO2-ZnO hybrid nanostructures. Sci. Rep. 2014, 4, 4181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, R.; Lin, J.; Zhu, Y. Enhancement of photocurrent and photocatalytic activity of ZnO hybridized with graphite-like C3N4. Energy Environ. Sci. 2011, 4, 2922–2929. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Zhou, G.; Ang, H.M.; Tade, M.O.; Wang, S. Reduced Graphene Oxide for Catalytic Oxidation of Aqueous Organic Pollutants. ACS Appl. Mater. Interfaces 2012, 4, 5466–5471. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, G.; Cao, T.; Treacy, G.M.; Klavetter, F.; Colaneri, N.; Heeger, A. Flexible light-emitting diodes made from soluble conducting polymers. Nature 1992, 477–479. [Google Scholar] [CrossRef]
- Sailor, M.J.; Ginsburg, E.J.; Gorman, C.B.; Kumar, A.; Grubbs, R.H.; Lewis, N.S. Thin Films of n-Si/Poly-(CH3)3Si-Cyclooctatetraene: Conducting-Polymer Solar Cells and Layered Structures. Science 1990, 249, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.; Anwer, T.; Ahmad, S.; Mohammad, F. Preparation, characterization, and dynamic adsorption–desorption studies on polypyrrole encapsulated TiO2 nanopartic. J. Appl. Polym. Sci. 2016, 133, 43411. [Google Scholar] [CrossRef]
- Sapurina, I.Y.; Shishov, M.A. Oxidative polymerization of aniline: Molecular synthesis of polyaniline and the formation of supramolecular structures. In New Polymers for Special Applications; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- MacDiarmid, A.G. “Synthetic metals”: A novel role for organic polymers. Angew. Chem. Int. Ed. 2001, 41, 2581–2590. [Google Scholar] [CrossRef]
- Abdulla, H.S.; Abbo, A.I. Optical and electrical properties of thin films of polyaniline and polypyrrole. Int. J. Electrochem. Sci. 2012, 7, 10666–10678. [Google Scholar]
- Deb, K.; Bera, A.; Bhowmik, K.L.; Saha, B. Conductive polyaniline on paper as a flexible electronic material with controlled physical properties through vapor phase polymerization. Polym. Eng. Sci. 2018. [Google Scholar] [CrossRef]
- Romero, M.; Faccio, R.; Pardo, H.; Tumelero, M.A.; Cid, C.C.P.; Pasa, A.A.; Mombru, A.W. Enhancement of lithium conductivity and evidence of lithium dissociation for LLTO-PMMA nanocomposite electrolyte. Mater. Lett. 2016, 172, 1–5. [Google Scholar] [CrossRef]
- Lin, Y.M.; Li, D.Z.; Hu, J.H.; Xiao, G.C.; Wang, J.X.; Li, W.J.; Fu, X.Z. Highly efficient photocatalytic degradation of organic pollutants by PANI-modified TiO2 composite. J. Phys. Chem. C 2012, 116, 5764–5772. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, S.C.; Choi, S.; Son, B.; Lee, S.M.; Kim, H.J.; Lee, J. Efficient visible-light induced photocatalysis on nanoporous nitrogen-doped titanium dioxide catalysts. Chem. Eng. J. 2013, 228, 756–764. [Google Scholar] [CrossRef]
- Umar, M.; Aziz, H.A. Photocatalytic degradation of organic pollutants in water. In Organic Pollutants—Monitoring, Risk and Treatment; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Zeng, W.; Liu, Y.G.; Hu, X.J.; Liu, S.B. Decontamination of methylene blue from aqueous solution by magnetic chitosan lignosulfonate grafted with graphene oxide: Effects of environmental conditions and surfactant. RSC Adv. 2016, 6, 19298–19307. [Google Scholar] [CrossRef]
- Mezohegyi, G.; van der Zee, F.P.; Font, J.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manag. 2012, 102, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.J.; Huang, H.Z.; Yuan, B.; Fu, M.L. Decolorization of RhB dye by manganese oxides: Effect of crystal type and solution Ph. Geochem. Trans. 2015, 16, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, S.F.; Li, G.; Konradsson, A. Twist-dependent electronic coupling in an organic intervalence radical cation. Org. Lett. 2001, 3, 1583–1586. [Google Scholar] [CrossRef]
- Chon, H.; Woo, S.I.; Park, S.-E. Recent Advances and New Horizons in Zeolite Science and Technology, 1st ed.; Elservier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Venditti, F.; Cuomo, F.; Ceglie, A.; Avino, P.; Russo, M.V.; Lopez, F. Visible light caffeic acid degradation by carbon-doped titanium dioxide. Langmuir 2015, 31, 3627–3634. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, G.; Cuomo, F.; Ambrosone, L.; Colella, M.; Ceglie, A.; Venditti, F.; Lopez, F. Photocatalytic degradation of a model textile dye using Carbon-doped titanium dioxide and visible light. J. Water Process. Eng. 2017, 20, 71–77. [Google Scholar] [CrossRef]
- Han, J.; Qiu, W.; Gao, W. Potential dissolution and photo-dissolution of ZnO thin films. J. Hazard. Mater. 2010, 178, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Rudd, A.L.; Breslin, C.B. Photo-induced dissolution of zinc in alkaline solutions. Electrochim. Acta 2000, 45, 1571–1579. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Wu, F.; Ji, Y. High adsorption capability and selectivity of ZnO nanoparticles for dye removal. Colloids Surf. A 2016, 509, 474–483. [Google Scholar] [CrossRef]
- Tao, Y.; Ju, E.; Ren, J.; Qu, X. Polypyrrole nanoparticles as promising enzyme mimics for sensitive hydrogen peroxide detection. Chem. Commun. 2014, 50, 3030–3032. [Google Scholar] [CrossRef] [PubMed]
- Trchova, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes. Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Nasser, A.A.-S.; Fohad, M.H.; Faheem, A.; Rais, A.K.; Iqbal, A.; Edreese, A.; Mohd, S.K.; Afzal, H.; Tabish, R.; Mohammad, Y.; et al. Biogenic synthesis of Zinc oxide nanostructures from Nigella sativa seed: Prospective role as food packaging material inhibiting broad-spectrum quorum sensing and biofilm. Sci. Rep. 2016, 6, 36761. [Google Scholar] [CrossRef]
- Lohrasbi, M.; Hedayat, N.; Chuang, S.S.C. In-situ infrared study of the synthesis of polyaniline under acid and neutral pH. Top. Catal. 2014, 57, 1570–1575. [Google Scholar] [CrossRef]
- Gilja, V.; Novaković, K.; Travas-Sejdic, J.; Hrnjak-Murgić, Z.; Kraljić Roković, M.; Žic, M. Stability and synergistic effect of polyaniline/TiO2 photocatalysts in degradation of azo dye in wastewater. Nanomaterials 2017, 7, 412. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, K.; Kume, K. Metallic temperature-dependence in the conducting polymer, polyaniline—Spin dynamics study by ESR. Solid State Commun. 1994, 89, 971–975. [Google Scholar] [CrossRef]
- Zic, M. The influence of the PANI structure on the conductive mechanism and on the electrical equivalent circuit analysis. J. Electroanal. Chem. 2009, 635, 29–38. [Google Scholar] [CrossRef]
- Li, X.W.; Chen, W.; Bian, C.Q.; He, J.B.; Xu, N.; Xue, G. Surface modification of TiO2 nanoparticles by polyanifine. Appl. Surf. Sci. 2003, 217, 16–22. [Google Scholar] [CrossRef]
- Luo, K.; Shi, N.L.; Sun, C. Thermal transition of electrochemically synthesized polyaniline. Polym. Degrad. Stab. 2006, 91, 2660–2664. [Google Scholar] [CrossRef]
- Singh, N.S.; Kumar, L.; Kumar, A.; Vaisakh, S.; Singh, S.D. Fabrication of zinc oxide/polyaniline (ZnO/PANI) heterojunction and its characterisation at room temperature. Mater. Sci. Semicond. Process. 2017, 60, 29–33. [Google Scholar] [CrossRef]
- Asha, S.L.G.; Nawal, K. Synthesis and X-ray diffraction study of polyaniline doped with chromium oxide. AIP Conf. Proc. 2013, 1536, 617–618. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Sharma, B.K.; Khare, N. Stress-dependent band gap shift and quenching of defects in Al-doped ZnO films. J. Phys. D Appl. Phys. 2010, 43, 465402. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.M. Synergistic effect of microwave irradiation and conjugated polymeric catalyst in the facile degradation of dyes. RSC Adv. 2014, 4, 47153–47162. [Google Scholar] [CrossRef]
- Wang, Y.G.; Li, H.Q.; Xia, Y.Y. Ordered whiskerlike polyaniline grown on the surface of mesoporous carbon and its electrochemical capacitance performance. Adv. Mater. 2006, 18, 2619–2623. [Google Scholar] [CrossRef]
- Yu, S.; Xi, M.; Han, K.; Wang, Z.; Yang, W.; Zhu, H. Preparation and photoelectrocatalytic properties of polyaniline/layered manganese oxide self-assembled film. Thin Solid Films 2010, 519, 357–361. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Wu, Y.; Harima, Y.; Zhang, A.; Tang, H. Hybrid composites of conductive polyaniline and nanocrystalline titanium oxide prepared via self-assembling and graft polymerization. Polymer 2006, 47, 7361–7376. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Wang, J.; Ma, T.; Zhang, Z.; Zhang, X.; Jiang, Y.; Dong, D. Investigation on the sonocatalytic degradation of parathion in the presence of nanometer rutile titanium dioxide (TiO2) catalysis. J. Hazard. Mater. 2006, 137, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Eskizeybek, V.; Sari, F.; Gulce, H.; Gulce, A.; Avcı, A. Preparation of polyaniline/ZnO nanocomposites by using arc-discharge synthesized ZnO nanoparticles and photocatalytic applications. J. Fac. Eng. Arch. Selcuk Univ. 2012, 27, 119–120. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, C.; Sun, H.; Liu, S.; Tad, M.; Wang, S. Photocatalysis of C., N-doped ZnO derived from ZIF-8 for dye degradation and water oxidation. RSC Adv. 2016, 6, 95903–95909. [Google Scholar] [CrossRef]
- Kusvuran, E.; Gulnaz, O.; Irmak, S.; Atanur, O.M.; Yavuz, H.I.; Erbatur, O. Comparison of several advanced oxidation processes for the decolorization of Reactive Red 120 azo dye in aqueous solution. J. Hazard. Mater. 2004, 109, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zongand, R.; Zhu, Y. Photocorrosion inhibition and photoactivity enhancement for zinc oxide via hybridization with monolayer polyaniline. J. Phys. Chem. C 2009, 113, 4605–4611. [Google Scholar] [CrossRef]
- Smela, E. Microfabrication of PPy microactuators and other conjugated polymer devices. J. Micromech. Microeng. 1999, 9. [Google Scholar] [CrossRef]
| Sample | PANI/ZnO 0.02 DEG | PANI/ZnO 0.024 DEG | PANI/ZnO 0.04 DEG |
|---|---|---|---|
| m(PANI):m(ZnO)% | n(ANI):n(APS) = 1:0.25 | ||
| w(ANI/ZnO)/% | 15/85 | 15/85 | 15/85 |
| m(ZnO)/g | 0.800 | 0.800 | 0.800 |
| V(ANI)/mL | 0.588 | 0.588 | 0.588 |
| m (APS)/g | 0.367 | 0.367 | 0.367 |
| V(DEG)/mL | 0.386 | 0.466 | 0.777 |
| Sample | PANI/ZnO 0.020 DEG | PANI/ZnO 0.024 DEG | PANI/ZnO 0.040 DEG | ZnO |
| κ/Scm−1 | 1.862 × 10−6 | 1.935 × 10−6 | 1.691 × 10−6 | 2.955 × 10−7 |
| Samples | T95 | Tmax1 | Tmax2 | Tmax3 | Tmax4 |
| °C | |||||
| PANI/ZnO 0.020 DEG | 254.5 | 72.3 | 268.8 | 364.9 | 541.2 |
| PANI/ZnO 0.024 DEG | 254.4 | 63.2 | 275.5 | 368.1 | 536.9 |
| PANI/ZnO 0.040 DEG | 245.4 | 70.3 | 266.8 | 365.6 | 529.5 |
| mf * | Δm1 | Δm2 | Δm3 | Δm4 | |
| % | |||||
| PANI/ZnO 0.020 DEG | 78.2 | 3.4 | 6.3 | 0.4 | 8.4 |
| PANI/ZnO 0.024 DEG | 77.5 | 3.1 | 6.1 | 0.7 | 8.3 |
| PANI/ZnO 0.040 DEG | 76.7 | 3.4 | 6.4 | 0.5 | 8.4 |
| Samples | SBET (m2/g) |
|---|---|
| PANI/ZnO 0.02 DEG | 4.4409 ± 0.0559 |
| PANI/ZnO 0.024 DEG | 5.1713 ± 0.0433 |
| PANI/ZnO 0.04 DEG | 5.6680 ± 0.0417 |
| Samples | Eg PANI (eV) | Eg ZnO (eV) |
|---|---|---|
| PANI/ZnO 0.02 DEG | 2.22 | 3.16 |
| PANI/ZnO 0.024 DEG | 2.16 | 3.16 |
| PANI/ZnO 0.04 DEG | 2.13 | 3.11 |
| Samples | R2 | kapp (min−1) |
|---|---|---|
| PANI/ZnO 0.02 DEG | 0.9844 | 0.0272 |
| PANI/ZnO 0.024 DEG | 0.9662 | 0.0281 |
| PANI/ZnO 0.04 DEG | 0.9753 | 0.0325 |
| ZnO | 0.9273 | 0.0062 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilja, V.; Vrban, I.; Mandić, V.; Žic, M.; Hrnjak-Murgić, Z. Preparation of a PANI/ZnO Composite for Efficient Photocatalytic Degradation of Acid Blue. Polymers 2018, 10, 940. https://doi.org/10.3390/polym10090940
Gilja V, Vrban I, Mandić V, Žic M, Hrnjak-Murgić Z. Preparation of a PANI/ZnO Composite for Efficient Photocatalytic Degradation of Acid Blue. Polymers. 2018; 10(9):940. https://doi.org/10.3390/polym10090940
Chicago/Turabian StyleGilja, Vanja, Ivan Vrban, Vilko Mandić, Mark Žic, and Zlata Hrnjak-Murgić. 2018. "Preparation of a PANI/ZnO Composite for Efficient Photocatalytic Degradation of Acid Blue" Polymers 10, no. 9: 940. https://doi.org/10.3390/polym10090940
APA StyleGilja, V., Vrban, I., Mandić, V., Žic, M., & Hrnjak-Murgić, Z. (2018). Preparation of a PANI/ZnO Composite for Efficient Photocatalytic Degradation of Acid Blue. Polymers, 10(9), 940. https://doi.org/10.3390/polym10090940