Dyeing Property and Adsorption Kinetics of Reactive Dyes for Cotton Textiles in Salt-Free Non-Aqueous Dyeing Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Surface Tension
2.3. Cotton Textile Dyeing
2.3.1. Non-Aqueous System
2.3.2. Aqueous Dyeing
2.4. Determination of Adsorption Rate
2.5. Dyeing Evaluations
2.5.1. Color Depth of Dyed Fabric
2.5.2. Dye Uptake of Reactive Dye
2.5.3. Fixation Rate of Reactive Dye
2.5.4. Level Dyeing Property
3. Results and Discussion
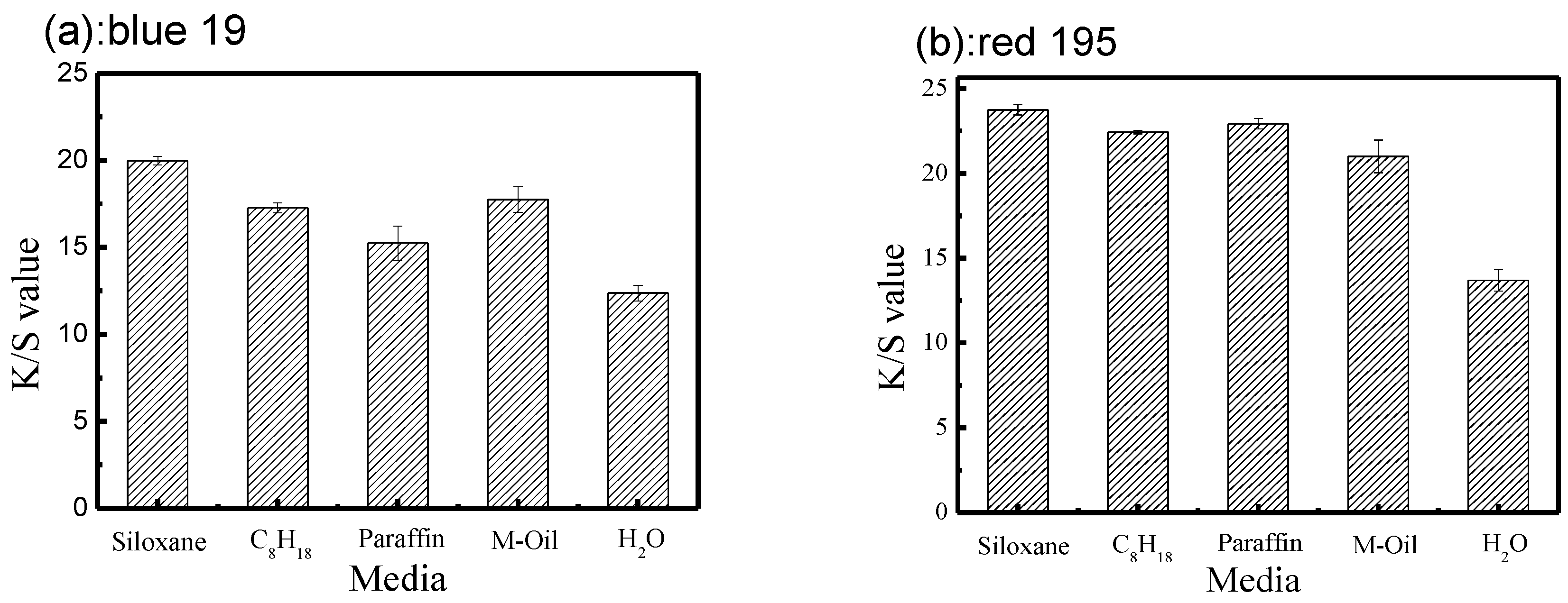
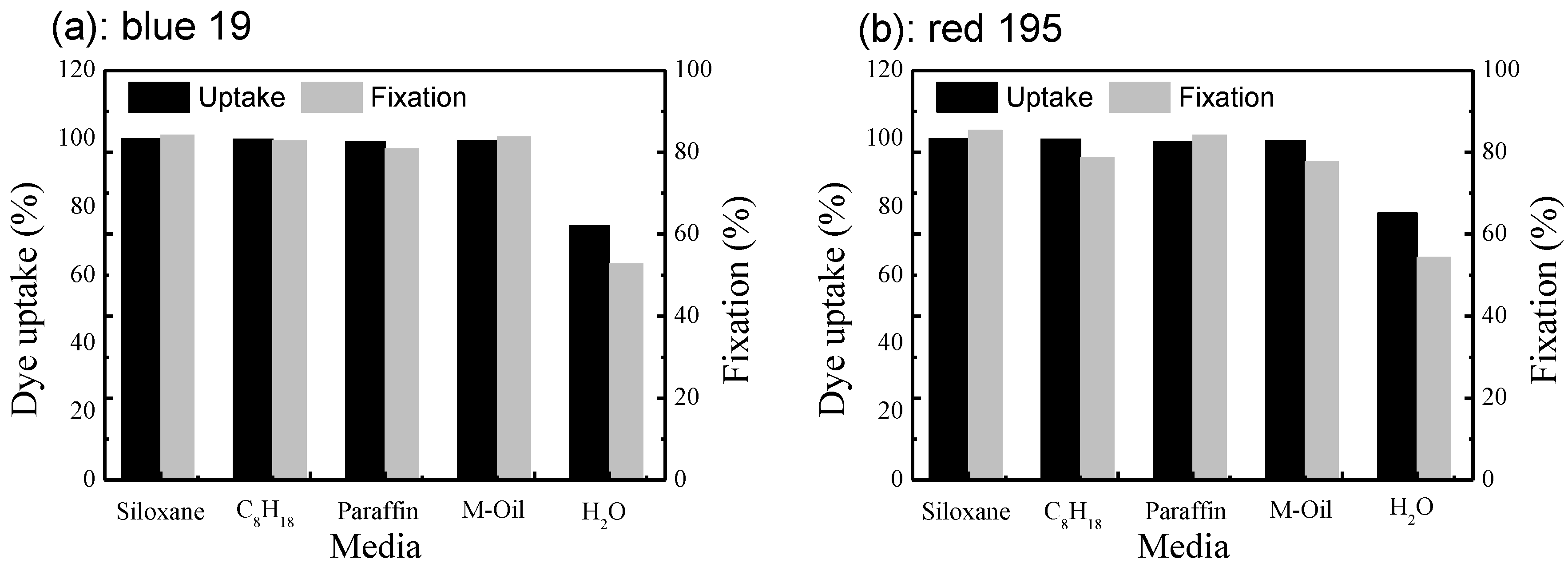
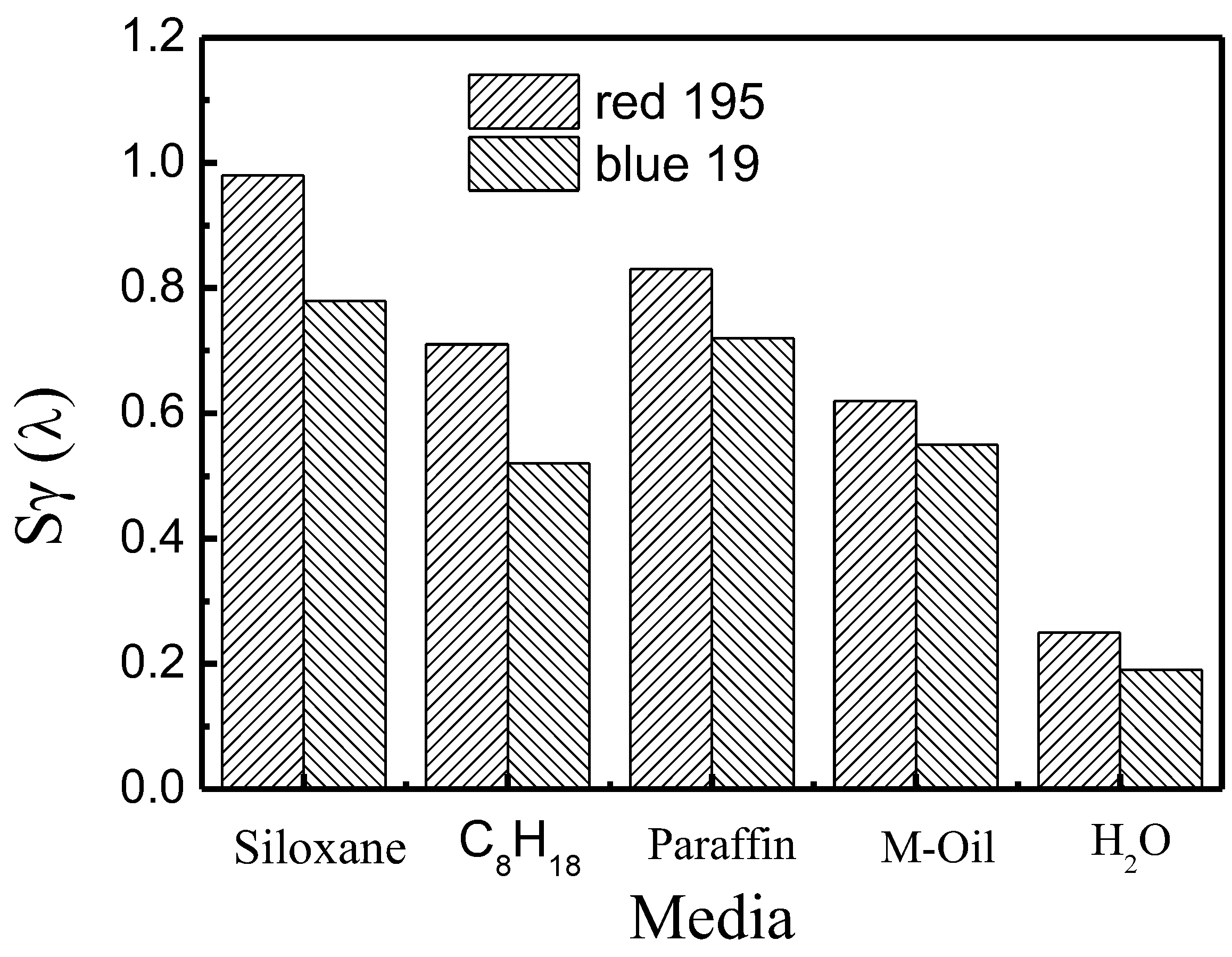
3.1. Comparisons between the Dyeing Property of Reactive Dye in Different Non-Aqueous Dyeing Systems and a Traditional Water Dyeing System
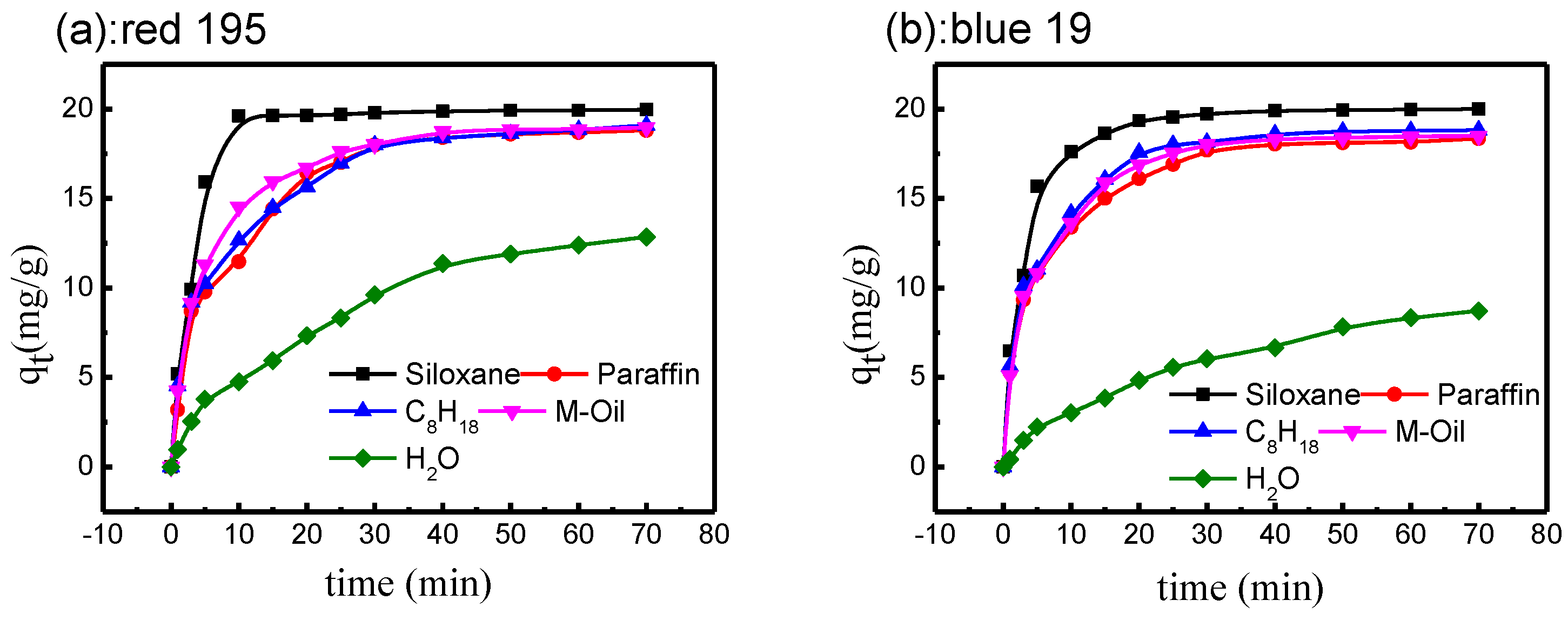
3.2. Dye Adsorption in Different Non-Aqueous Dyeing Systems
3.3. Dye Adsorption Kinetics
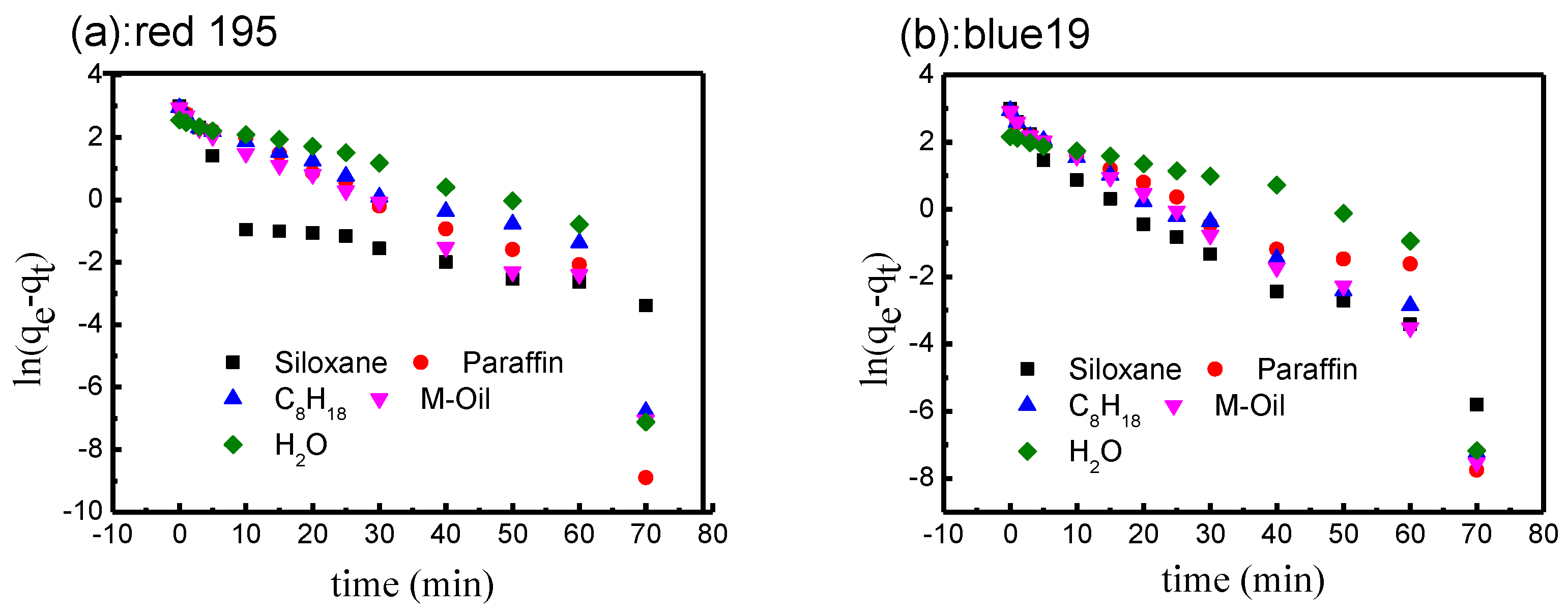
3.3.1. Fitting of Pseudo-First-Order Kinetic Model
3.3.2. Fitting of Pseudo-Second-Order Kinetic Model
3.3.3. Effect of Non-Aqueous Media on the Dyeing Level Property
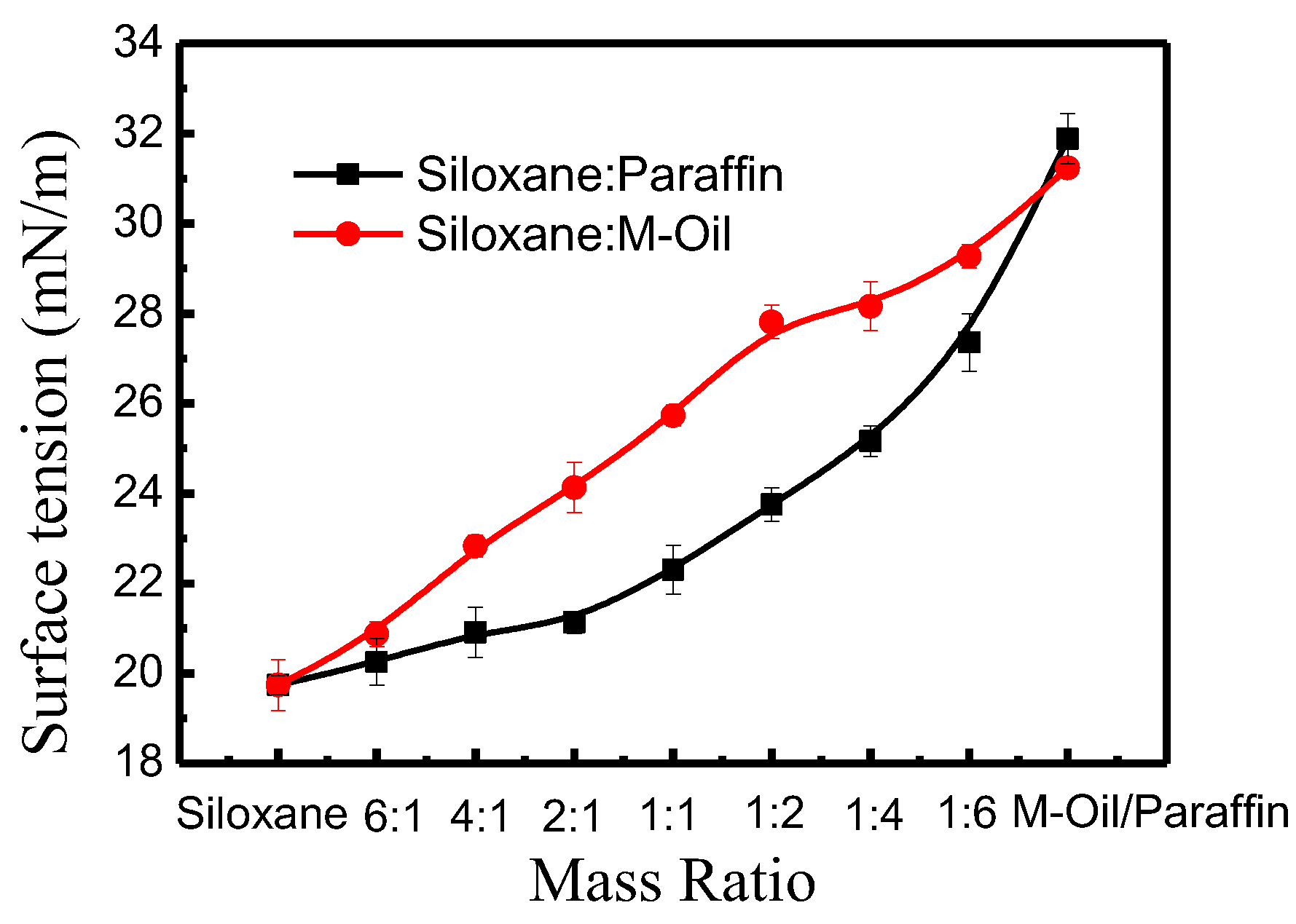
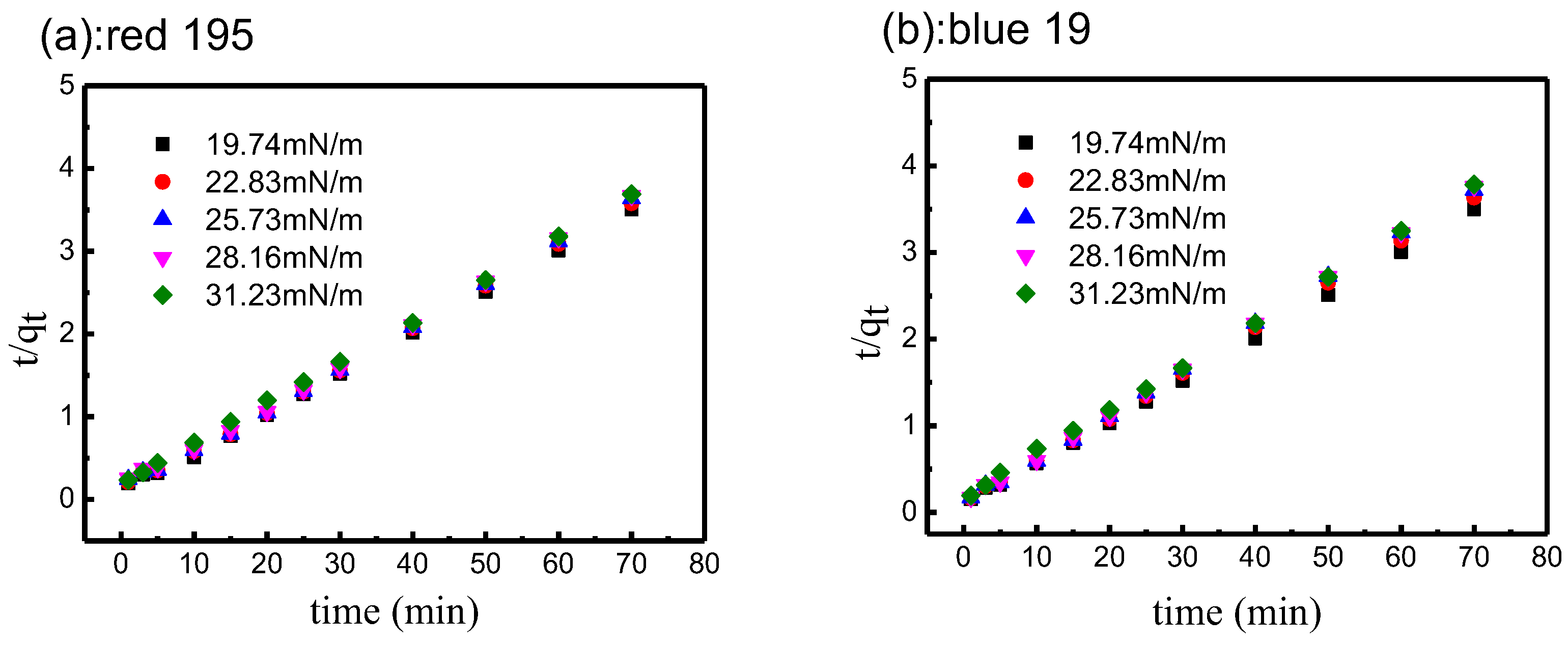
3.4. Influence of Non-Aqueous Media Surface Tension on Dye Adsorption Kinetics
3.4.1. Non-Aqueous Media with Different Surface Tension
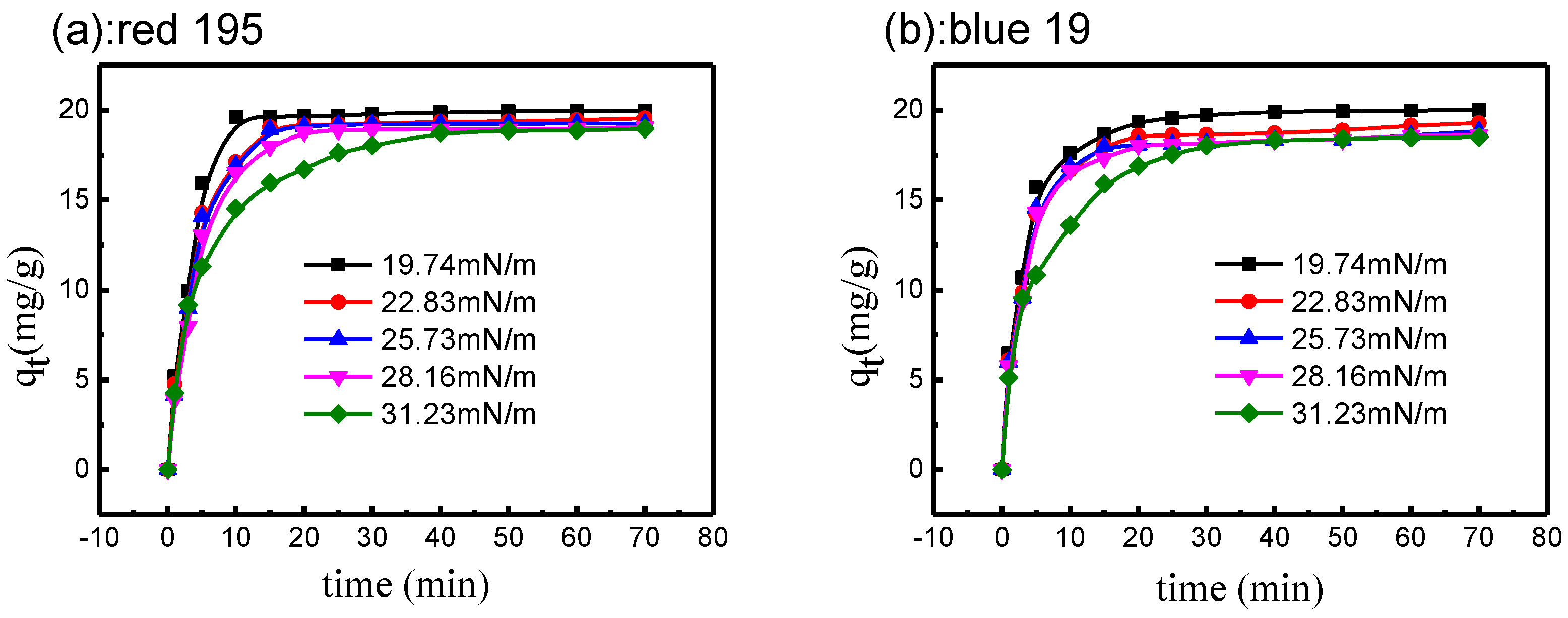
3.4.2. Effect of Surface Tension on Reactive Dye Adsorption
3.4.3. Fitting of Pseudo-First-Order Kinetic Model
3.4.4. Fitting of Pseudo-Second-Order Kinetic Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Almasian, A.; Olya, M.E.; Mahmoodi, N.M. Synthesis of polyacrylonitrile/polyamidoamine composite nanofibers using electrospinning technique and their dye removal capacity. J. Taiwan Inst. Chem. Eng. 2015, 49, 119–128. [Google Scholar] [CrossRef]
- Mao, X.; Zhong, Y.; Xu, H.; Zhang, L.; Sui, X.; Mao, Z. A novel low add-on technology of dyeing cotton fabric with reactive dyestuff. Text. Res. J. 2018, 88, 1345–1355. [Google Scholar] [CrossRef]
- Ali, S.; Khatri, Z.; Khatri, A.; Tanwari, A. Integrated desizing-bleaching-reactive dyeing process for cotton towel using glucose oxidase enzyme. J. Clean. Prod. 2014, 66, 562–567. [Google Scholar] [CrossRef]
- Banchero, M. Supercritical fluid dyeing of synthetic and natural textiles—A review. Color. Technol. 2013, 129, 2–17. [Google Scholar] [CrossRef]
- Cid, M.V.F.; Spronsen, J.V.; Kraan, M.V.D.; Veugeler, W.J.T.; Woerlee, G.F.; Witkamp, G.J. A significant approach to dye cotton in supercritical carbon dioxide with fluorotriazine reactive dyes. J. Supercrit. Fluid. 2007, 40, 477–484. [Google Scholar]
- Tang, Y.L.; Lee, C.H.; Wang, Y.; Kan, C.W. Octane-assisted reverse micellar dyeing of cotton with reactive dyes. Polymers 2017, 9, 678. [Google Scholar] [CrossRef]
- Banchero, M.; Ferri, A. Simulation of aqueous and supercritical fluid dyeing of a spool of yarn. J. Supercrit. Fluid. 2005, 35, 157–166. [Google Scholar] [CrossRef]
- Fang, K.; Shu, D.; Liu, X.; Cai, Y.; An, F.; Zhang, X. Reactive pad-steam dyeing of cotton fabric modified with cationic p(st-ba-vbt) nanospheres. Polymers 2018, 10, 564. [Google Scholar] [CrossRef]
- Chen, L.; Wang, B.; Ruan, X.; Chen, J.; Yang, Y. Hydrolysis-free and fully recyclable reactive dyeing of cotton in green, non-nucleophilic solvents for a sustainable textile industry. J. Clean. Prod. 2015, 107, 550–556. [Google Scholar] [CrossRef]
- Xie, K.; Cheng, F.; Zhao, W.; Xu, L. Micelle dyeing with low liquor ratio for reactive dyes using dialkyl maleic acid ester surfactants. J. Clean. Prod. 2011, 19, 332–336. [Google Scholar] [CrossRef]
- Radei, S.F.; Javier, C.F.; Ardanuy, M.; José, M.C. Kinetics of low temperature polyester dyeing with high molecular weight disperse dyes by solvent microemulsion and agrosourced auxiliaries. Polymers 2018, 10, 200. [Google Scholar] [CrossRef]
- Yi, S.; Dong, Y.; Li, B.; Ding, Z.; Huang, X.; Xue, L. Adsorption and fixation behaviour of ci reactive red 195 on cotton woven fabric in a nonionic surfactant triton X-100 reverse micelle. Colo. Technol. 2012, 128, 306–314. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, L.; Xu, D.; Li, M.; Zhang, Y.; Fu, S. Preparation of reactive nanoscale carbon black dispersion for pad coloration of cotton fabric. Color. Technol. 2017, 134, 92–99. [Google Scholar] [CrossRef]
- Wang, B.; Ruan, X.; Chen, L.; Chen, J.; Yang, Y. Heterogeneous chemical modification of cotton cellulose with vinyl sulfone dyes in non-nucleophilic organic solvents. Ind. Eng. Chem. Res. 2015, 53, 15802–15810. [Google Scholar] [CrossRef]
- Fu, C.; Wang, J.; Shao, J.; Pu, D.; Chen, J.; Liu, J. A non-aqueous dyeing process of reactive dye on cotton. J. Tex. Inst. 2015, 106, 152–161. [Google Scholar] [CrossRef]
- Pei, L.; Liu, J.; Cai, G.; Wang, J. Study of hydrolytic kinetics of vinyl sulfone reactive dye in siloxane reverse micro-emulsion. Text. Res. J. 2017, 87, 2368–2378. [Google Scholar] [CrossRef]
- Pei, L.; Liu, J.; Wang, J. Study of Dichlorotriazine Reactive Dye Hydrolysis in Siloxane Reverse Micro-emulsion. J. Clean. Prod. 2017, 165, 994–1004. [Google Scholar] [CrossRef]
- Pei, L.; Wu, P.; Liu, J.; Wang, J. Effect of nonionic surfactant on the micro-emulsifying water in silicone media. J. Surfactants Deterg. 2017, 20, 247–254. [Google Scholar] [CrossRef]
- Miao, H.; Liu, J.; Li, Y.; Fu, C.; Zhang, Y. Study on hydrolysis kinetics of reactive dyes in dye/D5 suspending system. J. Tex. Res. 2013, 34, 77–82. [Google Scholar]
- Burns-Naas, L.A.; Mast, R.W.; Meeks, R.G.; Mann, P.C.; Thevenaz, P. Inhalation toxicology of decamethylcyclopentasiloxane (D5) following a 3-monthnose-only exposure in Fischer 344 rats. Toxicol. Sci. 1998, 43, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.B.; Looney, R.J.; Utell, M.J.; Plotzke, K.P.; Andersen, M.Z. Modeling of human dermalabsorption of octamethylcyclotetrasiloxane (D4) anddecamethylcyclopentasiloxane (D5). Toxicol. Sci. 2007, 99, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.L.; McMahon, J.M.; McNett, D.A.; Tobin, J.M.; Plotzke, K.P. In vitro and in vivo percutaneousabsorption of 14 C-octamethylcyclotetrasiloxane (14C-D4) and 14C-decamethylcyclopentasiloxane (14C-D5). Regul. Toxicol.Pharmacol. 2008, 50, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y.; Liu, J.Q.; Li, Y.Q. The impact of D5-washing on the fabric performance. J. Zhejiang Sci.-Tech. Univ. 2007, 24, 6–10. [Google Scholar]
- Ahmed, N.S.E. The use of sodium edate in the dyeing of cotton with reactive dyes. Dyes Pigment. 2005, 65, 221–225. [Google Scholar] [CrossRef]
- Gök, Ö.; Özcan, A.S.; Özcan, A. Adsorption behavior of a textile dye of Reactive Blue 19 from aqueous solutions onto modified bentonite. Appl. Surf. Sci. 2010, 256, 5439–5443. [Google Scholar] [CrossRef]
- Gong, J.; Ren, Y.; Fu, R.; Li, Z.; Zhang, J. Ph-mediated antibacterial dyeing of cotton with prodigiosins nanomicelles produced by microbial fermentation. Polymers 2017, 9, 468. [Google Scholar] [CrossRef]
- Renfrew, A.H.M. Reactive Dyes for Cellulose: Replacement of Anthraquinone Blues by Triphenodioxazines. Color. Technol. 2010, 15, 15–20. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Chiou, M.S.; Li, H.Y. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 2003, 50, 1095–1105. [Google Scholar] [CrossRef]
- Vanaamudan, A.; Sudhakar, P.P. Equilibrium, kinetics and thermodynamic study on adsorption of reactive blue-21 and reactive red-141 by chitosan-organically modified nanoclay (Cloisite 30B) nano-bio composite. Taiwan Inst. Chem. Eng. 2015, 55, 145–151. [Google Scholar] [CrossRef]
- Bhavsar, P.S.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Mossotti, R.; Giansetti, M.; Rovero, G.; Maier, S.S.; Muresan, A.; Tonin, C. Superheated water hydrolyzed keratin: A new application as a foaming agent in foam dyeing of cotton and wool fabrics. ACS Sustain. Chem. Eng. 2017, 5, 9150–9159. [Google Scholar] [CrossRef]
- Annadurai, G.; Ling, L.Y.; Lee, J.F. Adsorption of reactive dye from an aqueous solution by chitosan: Isotherm, kinetic and thermodynamic analysis. J. Hazard. Mater. 2008, 152, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Ueda, M. Dyeing of protein fiber in a reverse micellar system. Dyes Pigment. 2003, 58, 99–103. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Comparative sorption kinetic studies of dyes and aromatic compounds onto flyash. J. Environ. Sci. Health. Part A 1999, 34, 1179–1204. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Wang, L.J. Adsorption of Reactive blue 21 onto functionalized cellulose under ultrasonic pretreatment: Kinetic and equilibrium study. J. Taiwan Inst. Chem. Eng. 2015, 50, 229–235. [Google Scholar] [CrossRef]
- Demir, H.; Top, A.; Balköse, D.; Ulkü, S. Dye adsorption behavior of luffacylindrica fibers. J. Hazard. Mater. 2008, 153, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chan, K.; Shen, J.; Liao, C.; Yeung, K.; Tjong, S. Polyetheretherketone hybrid composites with bioactive nanohydroxyapatite and multiwalled carbon nanotube fillers. Polymers 2016, 8, 425. [Google Scholar] [CrossRef]
- Blanco, S.P.D.M.; Scheufele, F.B.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Marin, P.; Kroumov, A.D.; Borba, C.E. Kinetic, equilibrium and thermodynamic phenomenological modeling of reactive dye adsorption onto polymeric adsorbent. Chem. Eng. J. 2016, 307, 466–475. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Wang, X.; Huang, K.; Chen, Y.; Liu, J.; Chen, S.; Cao, J.; Mei, S.; Zhou, Y.; Jing, T. Preparation of dumbbell manganese dioxide/gelatin composites and their application in the removal of lead and cadmium ions. J. Hazard. Mater. 2018, 350, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001, 35, 613–618. [Google Scholar] [CrossRef]
- Arslan-Alaton, I. The effect of pre-ozonation on the biocompatibility of reactive dye hydrolysates. Chemosphere 2003, 51, 825–833. [Google Scholar] [CrossRef]
- Mane, V.S.; Mall, I.D.; Srivastava, V.C. Kinetic and equilibrium isotherm studies for the adsorptive removal of Brilliant Green dye from aqueous solution by rice husk ash. J. Environ. Mang. 2007, 84, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.F.; Lewis, D.M.; Broadbent, P.J. Design and application of a multifunctional reactive dye capable of high fixation efficiency on cellulose. Color. Technol. 2008, 124, 186–194. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Y.; Li, J.; Wang, H.; Wen, S. Investigation on the nanomechanics of liposome adsorption on titanium alloys: Temperature and loading effects. Polymers 2018, 10, 383. [Google Scholar] [CrossRef]
- McKay, G.; Otterburn, M.S.; Sweeney, A.G. The removal of color from effluent using various adsorbents III. Silica: Rate processes. Water Res. 1980, 14, 15–20. [Google Scholar] [CrossRef]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.T.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye remazol brilliant blue. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Adsorption of, C.I. Reactive Red 228 dye from aqueous solution by modified cellulose from flax shive: Kinetics, equilibrium, an thermodynamics. Ind. Crop. Prod. 2013, 42, 153–158. [Google Scholar] [CrossRef]
- Marin, P.; Borba, C.E.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Oliveira, S.P.D.D.; Kroumov, A.D. Determination of the mass transfer limiting step of dye adsorption onto commercial adsorbent by using mathematical models. Environ. Technol. 2014, 35, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Moradi, O.; Gupta, V.K.; Agarwal, S.; Tyagi, I.; Asif, M.; Makhlouf, A.S.H.; Sadegh, H. Characteristics and electrical conductivity of graphene and graphene oxide for adsorption of cationic dyes from liquids: Kinetic and thermodynamic study. J. Ind. Eng. Chem. 2015, 28, 294–301. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad, A.L.; Hameed, B.H. Adsorption of reactive dye onto cross-linked chitosan/oil palm ash composite beads. Chem. Eng. J. 2008, 136, 164–172. [Google Scholar] [CrossRef]
- Castaldo, R.; Gentile, G.; Avella, M.; Carfagna, C.; Ambrogi, V. Microporous hyper-crosslinked polystyrenes and nanocomposites with high adsorption properties: A review. Polymers 2017, 9, 651. [Google Scholar] [CrossRef]




| Medium | Surface Tension (dyn/cm) | Boiling Point (°C) | Viscosity (mm2/s) |
|---|---|---|---|
| Siloxane | 18.13 | 210 | 5.63 |
| Paraffin | 19.45 | 330 | 16.34 |
| C8H18 | 23.34 | 126 | 0.77 |
| M-Oil | 31.23 | 200 | 4.20 |
| H2O | 72.04 | 100 | 1.00 |
| Dye | Media | ||||
|---|---|---|---|---|---|
| Siloxane | Paraffin | C8H18 | M-Oil | H2O | |
| Red-195 | 0.757 | 0.819 | 0.825 | 0.916 | 0.696 |
| Blue-19 | 0.961 | 0.830 | 0.929 | 0.942 | 0.655 |
| Medium Dye | Parameter | Siloxane | Paraffin | C8H18 | M-Oil | H2O |
|---|---|---|---|---|---|---|
| Red-195 | k2×10−2 (g/mg·min)−2(min−1) | 2.88 | 1.08 | 1.16 | 1.52 | 0.46 |
| qe,exp (mg/g) | 19.97 | 18.81 | 19.08 | 18.97 | 12.86 | |
| qe,cal (mg/g) | 20.59 | 20.47 | 20.37 | 10.11 | 14.61 | |
| t0.5 (min) | 1.57 | 4.94 | 4.52 | 3.48 | 17.02 | |
| R2 | 0.999 | 0.998 | 0.998 | 0.999 | 0.997 | |
| Blue-19 | k2×10−2 (g/mg·min)−2(min−1) | 3.18 | 1.62 | 1.82 | 1.74 | 0.63 |
| qe,exp (mg/g) | 19.99 | 18.35 | 18.83 | 18.50 | 8.72 | |
| qe,cal (mg/g) | 20.64 | 19.33 | 19.80 | 19.54 | 11.30 | |
| t0.5 (min) | 1.74 | 3.38 | 2.92 | 3.11 | 18.19 | |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.991 |
| Dye | Siloxane:W-Oil | R2 |
|---|---|---|
| Reactive red 195 | 1:0 | 0.852 |
| 4:1 | 0.847 | |
| 1:1 | 0.918 | |
| 1:4 | 0.838 | |
| 0:1 | 0.916 | |
| Reactive blue 19 | 1:0 | 0.962 |
| 4:1 | 0.782 | |
| 1:1 | 0.744 | |
| 1:4 | 0.868 | |
| 0:1 | 0.942 |
| Dye | Surface Tension (mN/m) | qe,exp (g/mg) | Second-Order Kinetic Model | |||
|---|---|---|---|---|---|---|
| k2×10−2 (g/mg·min) | qe,cal (mg/g) | t0.5 (min) | R2 | |||
| Red 195 | 19.74 | 19.97 | 3.18 | 20.59 | 1.57 | 0.998 |
| 22.83 | 19.55 | 2.47 | 20.19 | 2.07 | 0.999 | |
| 25.73 | 19.26 | 2.29 | 20.11 | 2.27 | 0.998 | |
| 28.16 | 19.06 | 2.11 | 20.00 | 2.49 | 0.998 | |
| 31.23 | 18.97 | 1.52 | 20.11 | 3.48 | 0.999 | |
| Blue19 | 19.74 | 19.98 | 2.88 | 20.64 | 1.74 | 0.999 |
| 22.83 | 19.28 | 2.62 | 19.82 | 1.98 | 0.999 | |
| 25.73 | 18.83 | 2.24 | 19.25 | 2.37 | 0.999 | |
| 28.16 | 18.61 | 2.14 | 19.19 | 2.51 | 0.999 | |
| 31.23 | 18.50 | 1.74 | 19.54 | 3.11 | 0.999 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Gao, Y.; Zhu, L.; Gu, X.; Dou, H.; Pei, L. Dyeing Property and Adsorption Kinetics of Reactive Dyes for Cotton Textiles in Salt-Free Non-Aqueous Dyeing Systems. Polymers 2018, 10, 1030. https://doi.org/10.3390/polym10091030
Wang J, Gao Y, Zhu L, Gu X, Dou H, Pei L. Dyeing Property and Adsorption Kinetics of Reactive Dyes for Cotton Textiles in Salt-Free Non-Aqueous Dyeing Systems. Polymers. 2018; 10(9):1030. https://doi.org/10.3390/polym10091030
Chicago/Turabian StyleWang, Jiping, Yuanyuan Gao, Lei Zhu, Xiaomin Gu, Huashu Dou, and Liujun Pei. 2018. "Dyeing Property and Adsorption Kinetics of Reactive Dyes for Cotton Textiles in Salt-Free Non-Aqueous Dyeing Systems" Polymers 10, no. 9: 1030. https://doi.org/10.3390/polym10091030





