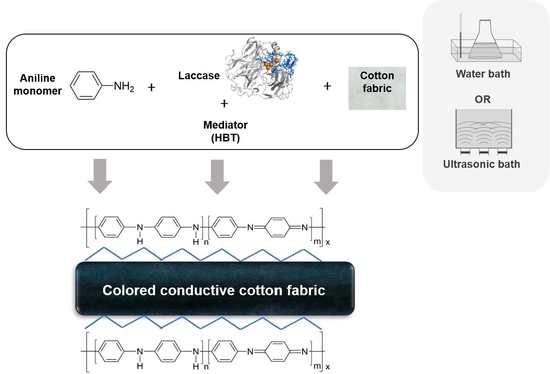
Conductive Cotton by In Situ Laccase-Polymerization of Aniline
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experiments
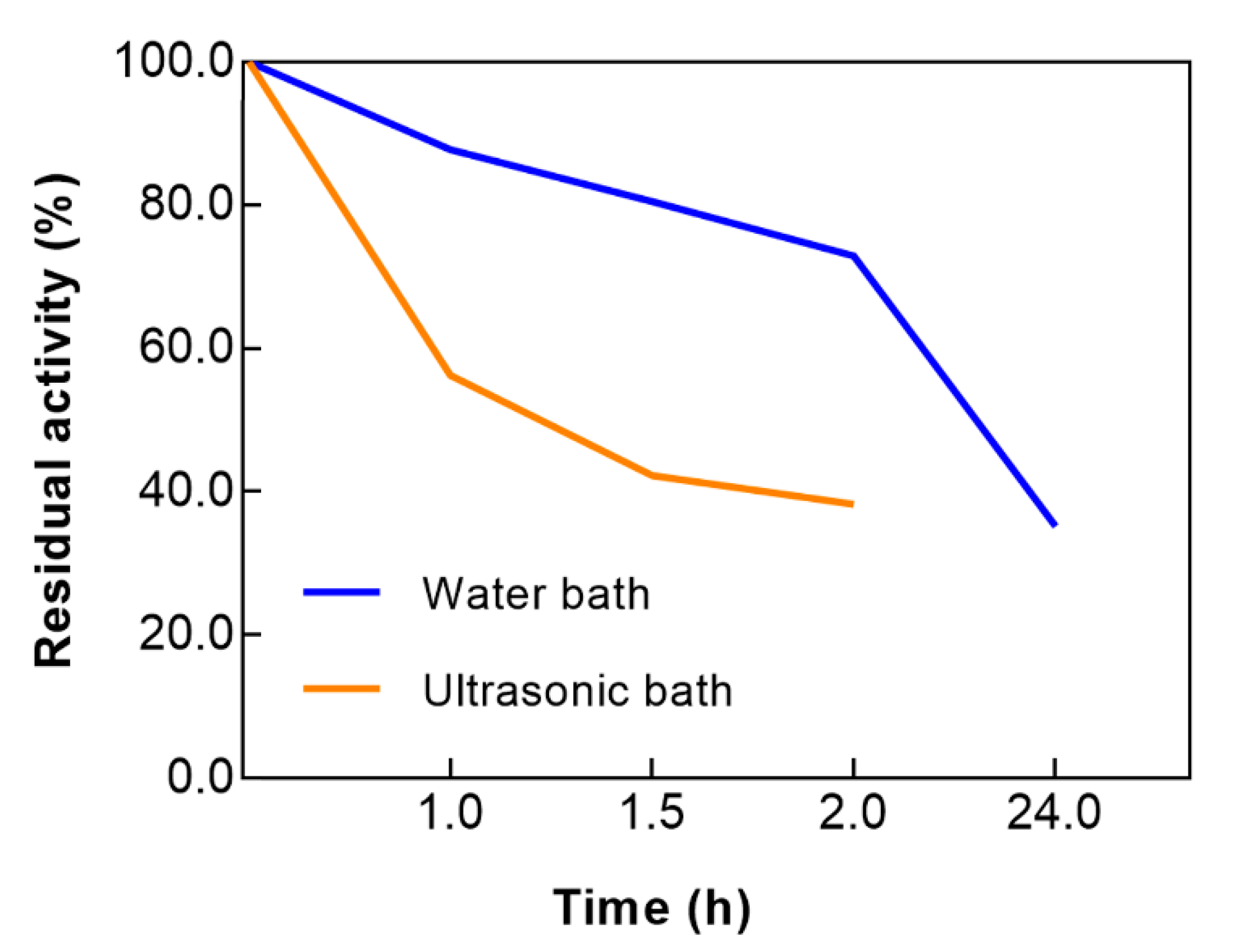
2.2.1. Evaluation of Enzyme Activity and Stability
2.2.2. In Situ Laccase Polymerization of Aniline on Cotton
2.2.3. UV-Visible-NIR (Ultraviolet–Visible-Near-Infrared) Spectroscopy Evaluation
2.2.4. 1H NMR Spectra
2.2.5. MALDI-TOF (Matrix-Assisted Laser Desorption/Ionization with Time-of-Flight) Spectroscopy Analysis
2.2.6. Scanning Electron Microscopy
2.2.7. Conductivity of Coated Cotton Fabric
2.2.8. K/S Evaluation of Coated Cotton Fabrics
3. Results and Discussion
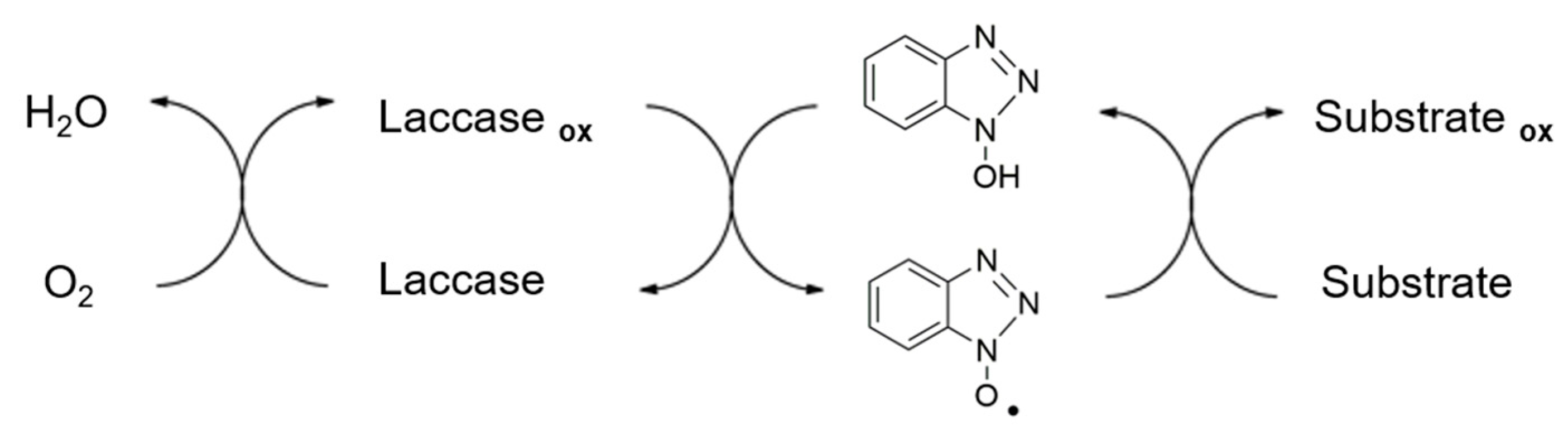
3.1. Evaluation of Laccase Activity and Stability
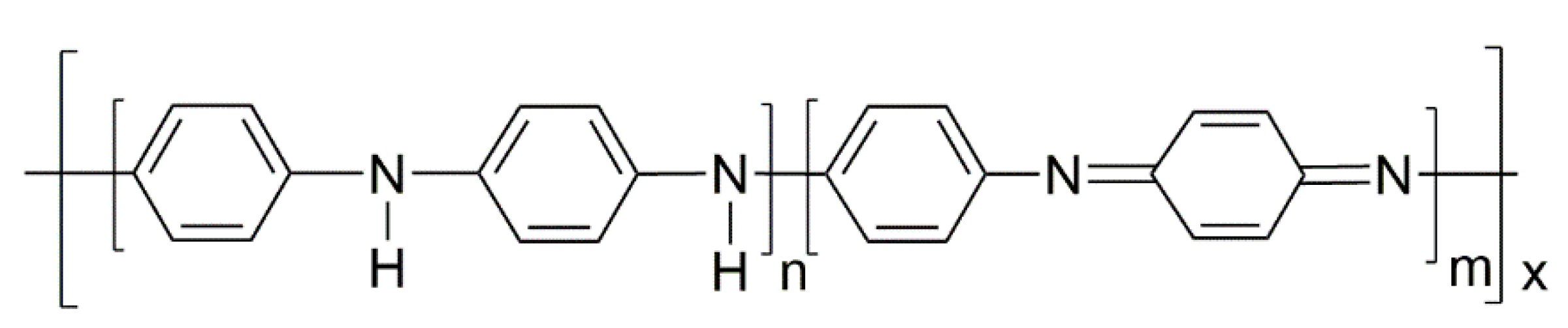
3.2. In Situ Polymerization of Polyaniline and Characterization
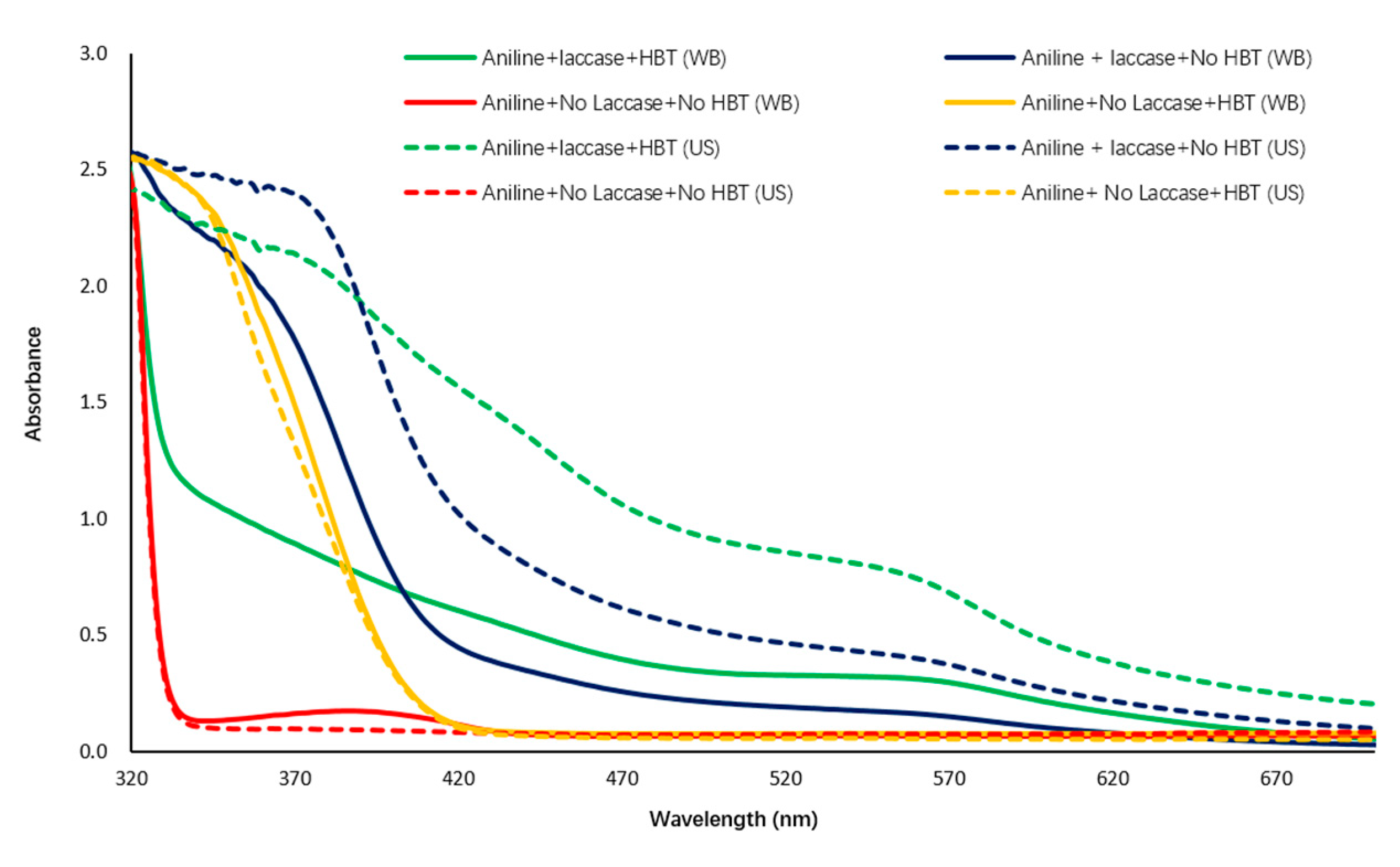
3.2.1. UV-Vis-NIR Spectroscopy Observation
3.2.2. 1H NMR Spectroscopy
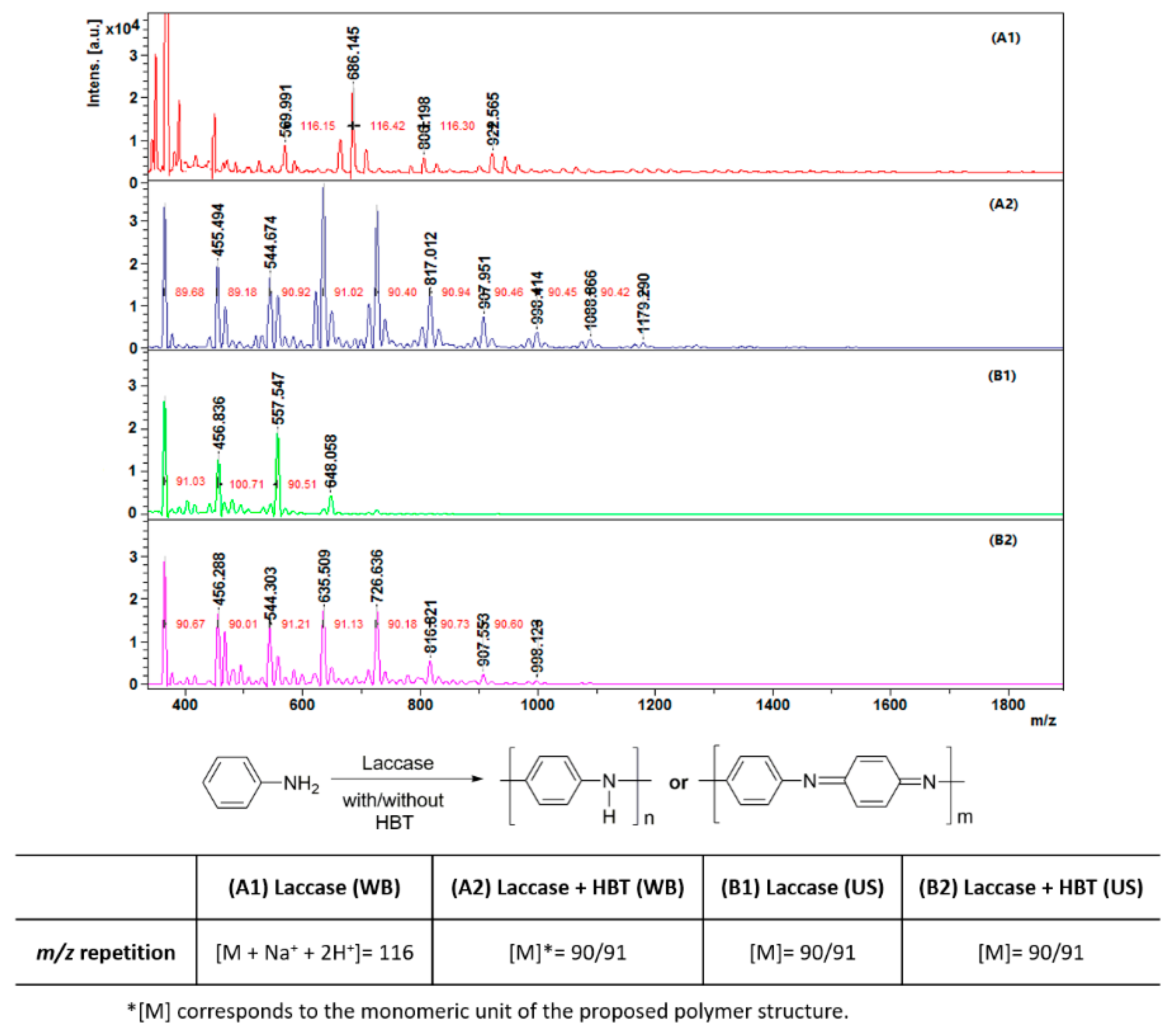
3.2.3. MALDI-TOF Mass Spectrometry
3.3. Characterization of the Coated Fabrics
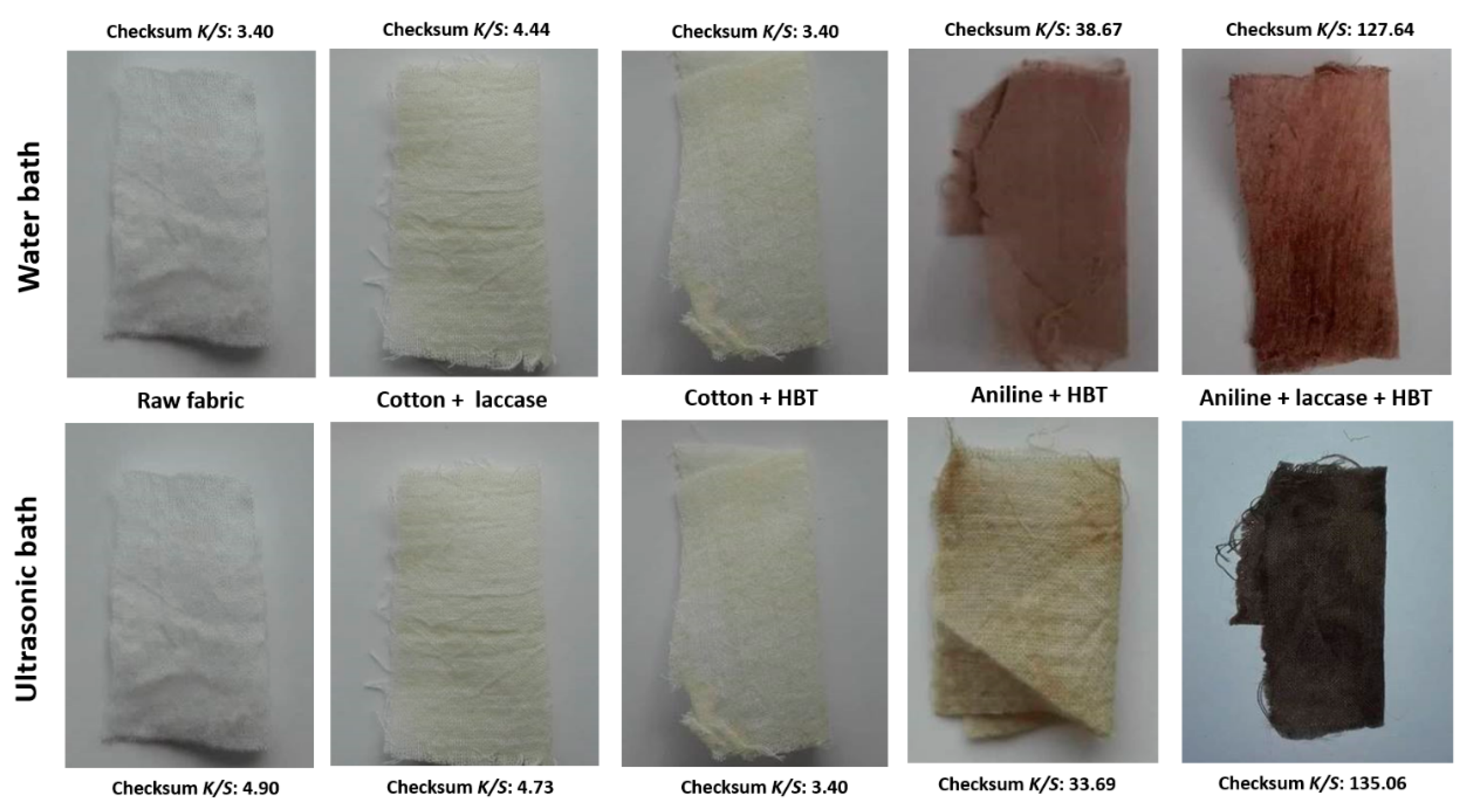
3.3.1. Colour Evaluation of Polyaniline Coated Fabrics
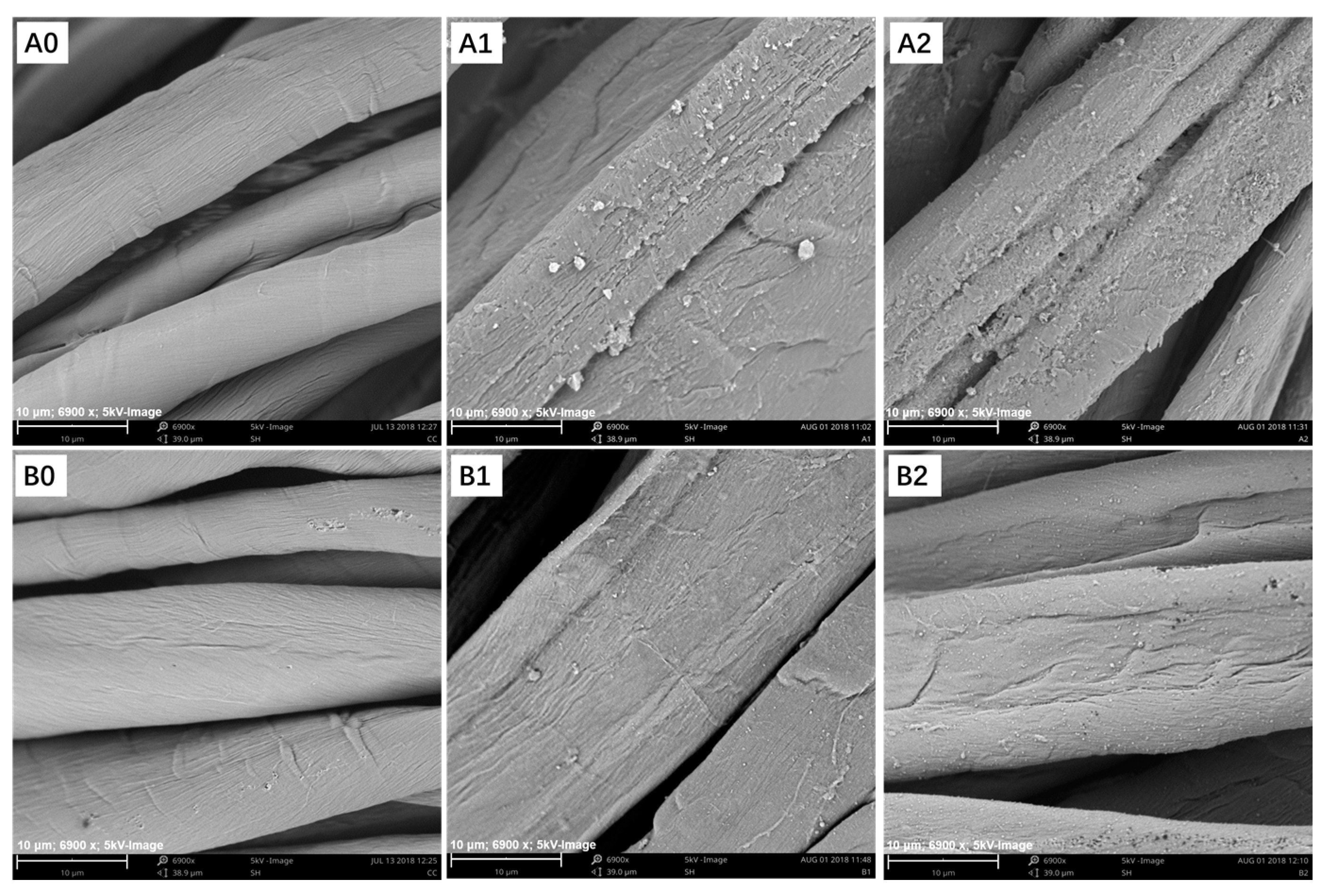
3.3.2. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
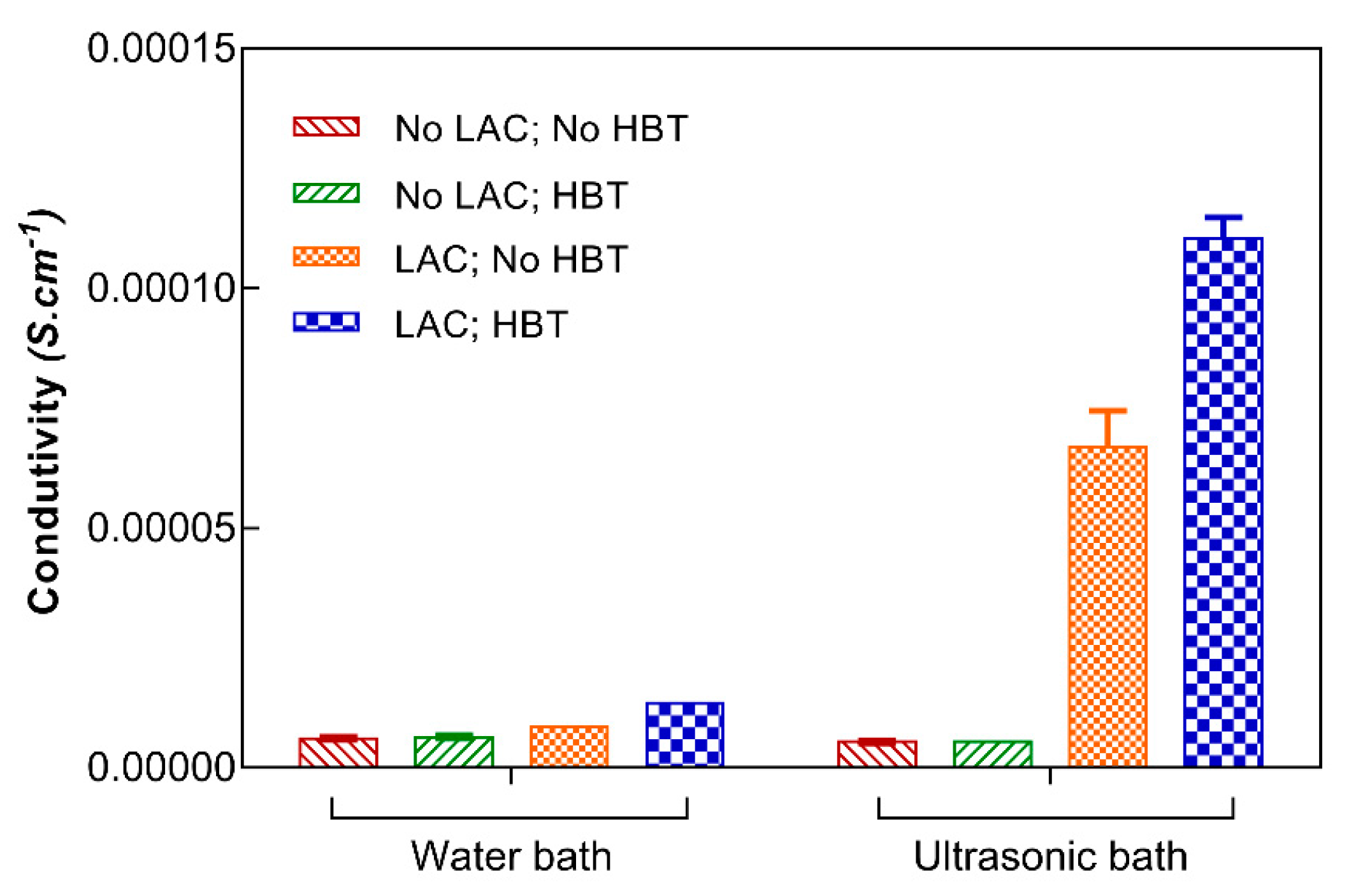
3.3.3. Electric Conductivity of Fabrics Coated with Polyaniline
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lux, F. Properties of electronically conductive polyaniline: A comparison between well-known literature data and some recent experimental findings. Polymer 1994, 35, 2915–2936. [Google Scholar] [CrossRef]
- Bhandari, S. Polyaniline: Structure and Properties Relationship. In Polyaniline Blends, Composites, and Nanocomposites; Elsevier: New York, NY, USA, 2018; pp. 23–60. [Google Scholar]
- Sapurina, I.; Stejskal, J. The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures. Polym. Int. 2008, 57, 1295–1325. [Google Scholar] [CrossRef]
- Letheby, H. XXIX—On the production of a blue substance by the electrolysis of sulphate of aniline. J. Chem. Soc. 1862, 15, 161–163. [Google Scholar] [CrossRef]
- Costolo, M.; Heeger, A.J. Anisotropic conductivity in polyaniline and image processing applications. Synth. Met. 2000, 114, 85–90. [Google Scholar] [CrossRef]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. JACS 2003, 125, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Macdiarmid, A.G.; Chiang, J.C.; Richter, A.F.; Epstein, A.J. Polyaniline: A new concept in conducting polymers. Synth. Met. 1987, 18, 285–290. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Yoon, H. Recent advances in nanostructured conducting polymers: From synthesis to practical applications. Polymers 2016, 8, 118. [Google Scholar] [CrossRef]
- Zhang, X.; Manohar, S.K. Polyaniline nanofibers: Chemical synthesis using surfactants. Chem. Commun. 2004, 20, 2360–2361. [Google Scholar] [CrossRef] [PubMed]
- Shumakovich, G.; Kurova, V.; Vasileva, I.; Pankratov, D.; Otrokhov, G.; Morozova, O.; Yaropolov, A. Laccase-mediated synthesis of conducting polyaniline. J. Mol. Catal. B Enzym. 2012, 77, 105–110. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Hassan, H.K.; Atta, N.F.; Galal, A. Electropolymerization of aniline over chemically converted graphene-systematic study and effect of dopant. Int. J. Electrochem. Soc. 2012, 7, 11161–11181. [Google Scholar]
- Zhang, Y.; Dong, A.; Wang, Q.; Fan, X.; Cavaco-Paulo, A.; Zhang, Y. Conductive cotton prepared by polyaniline in situ polymerization using laccase. Appl. Biochem. Biotechnol. 2014, 174, 820–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streltsov, A.V.; Morozova, O.V.; Arkharova, N.A.; Klechkovskaya, V.V.; Staroverova, I.N.; Shumakovich, G.P.; Yaropolov, A.I. Synthesis and characterization of conducting polyaniline prepared by laccase-catalyzed method in sodium dodecylbenzenesulfonate micellar solutions. J. Appl. Polym. Sci. 2009, 114, 928–934. [Google Scholar] [CrossRef]
- Liu, W.; Kumar, J.; Tripathy, S.; Senecal, K.J.; Samuelson, L. Enzymatically synthesized conducting polyaniline. JACS 1999, 121, 71–78. [Google Scholar] [CrossRef]
- Alvarez, S.; Manolache, S.; Denes, F. Synthesis of polyaniline using horseradish peroxidase immobilized on plasma-functionalized polyethylene surfaces as initiator. J. Appl. Polym. Sci. 2003, 88, 369–379. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Nagarajan, R.; Roy, S.; Samuelson, L.A.; Kumar, J.; Cholli, A.L. An enzymatically synthesized polyaniline: A solid-state nmr study. Macromolecules 2004, 37, 4130–4138. [Google Scholar] [CrossRef]
- Seo, K.-D.; Lee, K.-P.; Gopalan, A.I.; Chung, S.J.; Lim, Y.T.; Choi, S.-H. Horseradish peroxidase (hrp) immobilized poly (aniline-co-m-aminophenol) film electrodes–fabrication and evaluation as hydrogen peroxide sensor. Sensors 2007, 7, 719–729. [Google Scholar] [CrossRef]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Salas, F.; Pardo, I.; Salavagione, H.J.; Aza, P.; Amougi, E.; Vind, J.; Martínez, A.T.; Camarero, S. Advanced synthesis of conductive polyaniline using laccase as biocatalyst. PLoS ONE 2016, 11, e0164958. [Google Scholar] [CrossRef] [PubMed]
- Streltsov, A.V.; Shumakovich, G.P.; Morozova, O.V.; Gorbacheva, M.A.; Yaropolov, A.I. Micellar laccase-catalyzed synthesis of electroconductive polyaniline. Appl. Biochem. Microbiol. 2008, 44, 264–270. [Google Scholar] [CrossRef]
- Vasil’eva, I.S.; Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Sakharov, I.Y.; Yaropolov, A.I. Laccase-catalyzed synthesis of optically active polyaniline. Synth. Met. 2007, 157, 684–689. [Google Scholar] [CrossRef]
- Crestini, C.; Argyropoulos, D.S. On the role of 1-hydroxybenzotriazole as mediator in laccase oxidation of residual kraft lignin. In Oxidative Delignification Chemistry; American Chemical Society: Montreal, QC, Canada, 2001; Volume 785, pp. 373–390. [Google Scholar]
- Moilanen, U.; Kellock, M.; Várnai, A.; Andberg, M.; Viikari, L. Mechanisms of laccase-mediator treatments improving the enzymatic hydrolysis of pre-treated spruce. Biotechnol. Biofuels 2014, 7, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbrini, M.; Galli, C.; Gentili, P. Comparing the catalytic efficiency of some mediators of laccase. J. Mol. Catal. B Enzym. 2002, 16, 231–240. [Google Scholar] [CrossRef]
- Su, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Laccase: A green catalyst for the biosynthesis of poly-phenols. Crit. Rev. Biotechnol. 2018, 38, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Noro, J.; Loureiro, A.; Martins, M.; Azoia, N.G.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Pegylation greatly enhances laccase polymerase activity. ChemCatChem 2017, 9, 3888–3894. [Google Scholar] [CrossRef]
- Su, J.; Castro, T.G.; Noro, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. The effect of high-energy environments on the structure of laccase-polymerized poly(catechol). Ultrason. Sonochem. 2018, 48, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, C.; Zhang, X.; Carey, T.; Chen, K.; Yin, Y.; Torrisi, F. Environmentally-friendly conductive cotton fabric as flexible strain sensor based on hot press reduced graphene oxide. Carbon 2017, 111, 622–630. [Google Scholar] [CrossRef]
- Hu, L.; Pasta, M.; La Mantia, F.; Cui, L.; Jeong, S.; Deshazer, H.D.; Choi, J.W.; Han, S.M.; Cui, Y. Stretchable, porous, and conductive energy textiles. Nano Lett. 2010, 10, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Childs, R.E.; Bardsley, W.G. The steady-state kinetics of peroxidase with 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem J. 1975, 145, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Povedano, M.M.; Luque de Castro, M.D. A review on enzyme and ultrasound: A controversial but fruitful relationship. Anal. Chim. Acta 2015, 889, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Rokhina, E.V.; Lens, P.; Virkutyte, J. Low-frequency ultrasound in biotechnology: State of the art. Trends Biotechnol. 2009, 27, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. Ultrasound assisted intensification of enzyme activity and its properties: A mini-review. World J. Microbiol. Biotechnol. 2017, 33, 170. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Shibata, H.; Kawai, S.; Nishida, T. Role of 1-hydroxybenzotriazole in oxidation by laccase from trametes versicolor. Kinetic analysis of the laccase-1-hydroxybenzotriazole couple. FEMS Microbiol. Lett. 2006, 265, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Davin, L.B.; Bedgar, D.L.; Katayama, T.; Lewis, N.G. On the stereoselective synthesis of (+)-pinoresinol in forsythia suspensa from its achiral precursor, coniferyl alcohol. Phytochemistry 1992, 31, 3869–3874. [Google Scholar] [CrossRef]
- Goncalves, I.; Silva, C.; Cavaco-Paulo, A. Ultrasound enhanced laccase applications. Green Chem. 2015, 17, 1362–1374. [Google Scholar] [CrossRef]
- Kim, S.; Lopez, C.; Guebitz, G.; Cavaco-Paulo, A. Biological coloration of flax fabrics with flavonoids using laccase from trametes hirsuta. Eng. Life Sci. 2008, 8, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Guebitz, G.; Cavaco-Paulo, A. “In situ” enzymatically prepared polymers for wool coloration. Macromol. Mater. Eng. 2001, 286, 691–694. [Google Scholar] [CrossRef]
- Fu, J.; Nyanhongo, G.S.; Gübitz, G.M.; Cavaco-Paulo, A.; Kim, S. Enzymatic colouration with laccase and peroxidases: Recent progress. Biocatal. Biotransform. 2012, 30, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Gregory, R.V.; Kimbrell, W.C.; Kuhn, H.H. Conductive textiles. Synth. Met. 1989, 28, 823–835. [Google Scholar] [CrossRef]
- Dhawan, S.K.; Singh, N.; Venkatachalam, S. Shielding behaviour of conducting polymer-coated fabrics in x-band, w-band and radio frequency range. Synth. Met. 2002, 129, 261–267. [Google Scholar] [CrossRef]
- Dhawan, S.K.; Singh, N.; Venkatachalam, S. Shielding effectiveness of conducting polyaniline coated fabrics at 101 ghz. Synth. Met. 2002, 125, 389–393. [Google Scholar] [CrossRef]
- Kim, B.; Koncar, V.; Dufour, C. Polyaniline-coated pet conductive yarns: Study of electrical, mechanical, and electro-mechanical properties. J. Appl. Polym. Sci. 2006, 101, 1252–1256. [Google Scholar] [CrossRef]
- Hirase, R.; Shikata, T.; Shirai, M. Selective formation of polyaniline on wool by chemical polymerization, using potassium iodate. Synth. Met. 2004, 146, 73–77. [Google Scholar] [CrossRef]
| Water Bath Reactor (24 h) | Ultrasonic Bath Reactor (2 h) | |||
|---|---|---|---|---|
| Laccase | Laccase + HBT | Laccase | Laccase + HBT | |
| m/z(max) | 922 | 1179 | 648 | 998 |
| DP | 5.5 | 7.5 | 5.5 | 7.0 |
| Mw | 507 | 675 | 511 | 626 |
| Mn | 455 | 614 | 484 | 572 |
| PDI | 1.11 | 1.09 | 1.05 | 1.09 |
| Element conc. (%) | Element | Control Fabric | Water Bath Reactor (24 h) | Ultrasonic Bath Reactor (2 h) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Laccase | Laccase + HBT | Laccase | Laccase + HBT | ||||||||
| Spot 1 | Spot 2 | Spot 1 | Spot 2 | Spot 1 | Spot 2 | Spot 1 | Spot 2 | Spot 1 | Spot 2 | ||
| Atomic conc. | Carbon | 80.23 | 78.53 | 59.67 | 59.47 | 66.83 | 74.11 | 74.43 | 79.63 | 64.05 | 67.62 |
| Oxygen | 19.77 | 21.47 | 34.36 | 32.62 | 22.54 | 13.00 | 22.85 | 18.51 | 29.63 | 26.60 | |
| Nitrogen | 0 | 0 | 5.97 | 7.91 | 10.64 | 12.89 | 2.72 | 1.86 | 6.32 | 5.78 | |
| Weight conc. | Carbon | 75.29 | 73.30 | 53.08 | 52.31 | 61.17 | 69.62 | 68.89 | 74.80 | 57.76 | 63.14 |
| Oxygen | 24.71 | 26.70 | 40.72 | 39.58 | 27.48 | 16.26 | 28.17 | 23.16 | 35.59 | 30.90 | |
| Nitrogen | 0 | 0 | 6.20 | 8.11 | 11.35 | 14.12 | 2.94 | 2.04 | 6.65 | 5.96 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Shim, E.; Noro, J.; Fu, J.; Wang, Q.; Kim, H.R.; Silva, C.; Cavaco-Paulo, A. Conductive Cotton by In Situ Laccase-Polymerization of Aniline. Polymers 2018, 10, 1023. https://doi.org/10.3390/polym10091023
Su J, Shim E, Noro J, Fu J, Wang Q, Kim HR, Silva C, Cavaco-Paulo A. Conductive Cotton by In Situ Laccase-Polymerization of Aniline. Polymers. 2018; 10(9):1023. https://doi.org/10.3390/polym10091023
Chicago/Turabian StyleSu, Jing, Euijin Shim, Jennifer Noro, Jiajia Fu, Qiang Wang, Hye Rim Kim, Carla Silva, and Artur Cavaco-Paulo. 2018. "Conductive Cotton by In Situ Laccase-Polymerization of Aniline" Polymers 10, no. 9: 1023. https://doi.org/10.3390/polym10091023