High Pressure Treatment for Improving Water Vapour Barrier Properties of Poly(lactic acid)/Ag Nanocomposite Films
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of PLA/AgNPs Composite Films
2.3. Microstructure
2.4. Water Vapour Permeability (WVP) Measurement
2.5. Thermal Analysis
2.6. Mechanical Property
2.7. Migration Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. Microstructure
3.2. WVP of PLA and Its AgNPs Nanocomposite Films
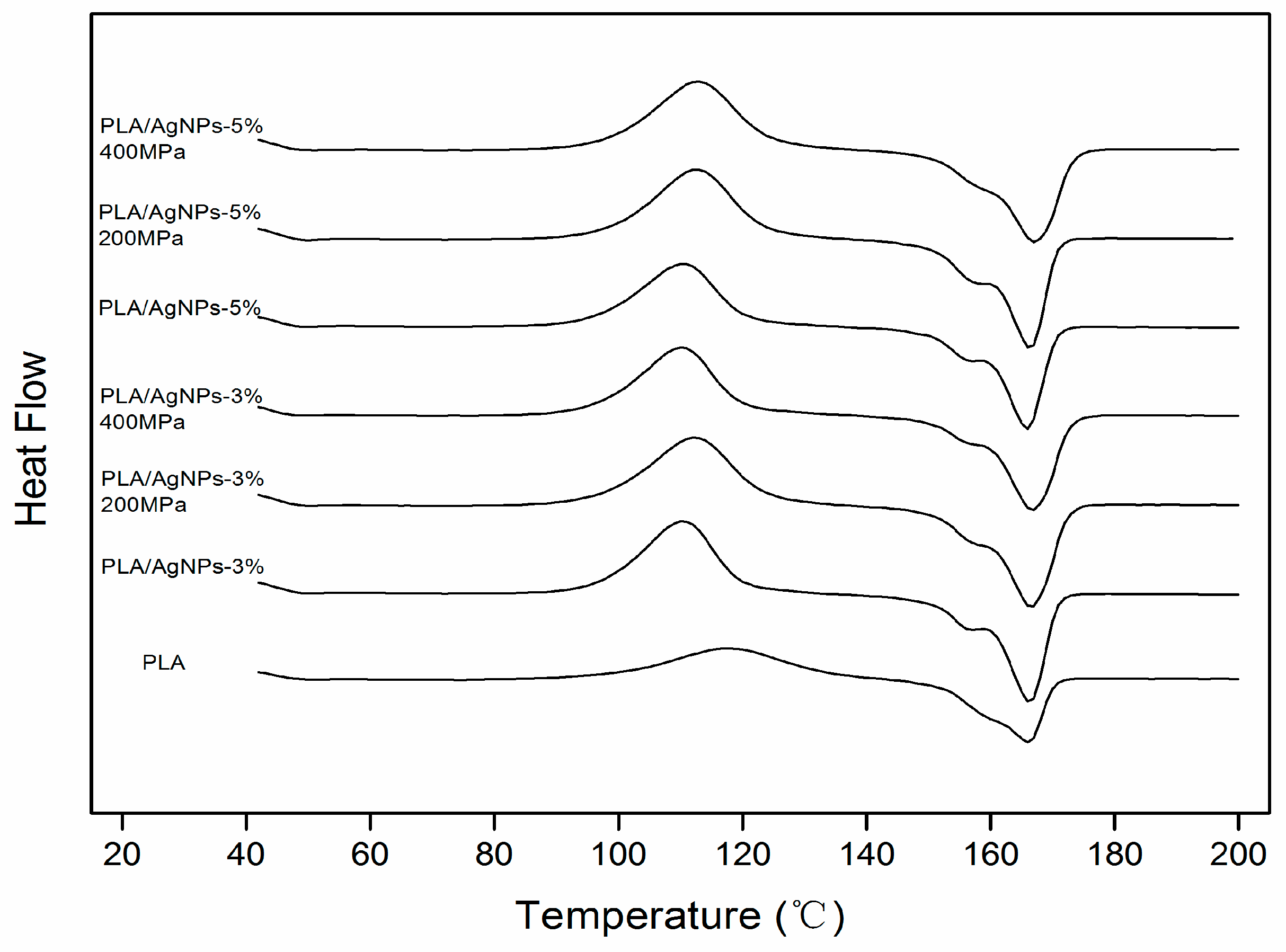
3.3. Thermal Analysis
3.4. Mechanical Property
3.5. Migration Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García-Campo, M.J.; Boronat, T.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Manufacturing and characterization of toughened poly(lactic acid) (pla) formulations by ternary blends with biopolyesters. Polymers 2018, 10, 3. [Google Scholar] [CrossRef]
- Meriçer, Ç.; Minelli, M.; Angelis, M.G.D.; Baschetti, M.G.; Stancampiano, A.; Laurita, R.; Gherardi, M.; Colombo, V.; Trifol, J.; Szabo, P.; et al. Atmospheric plasma assisted pla/microfibrillated cellulose (mfc) multilayer biocomposite for sustainable barrier application. Ind. Crops Prod. 2016, 93, 235–243. [Google Scholar] [CrossRef]
- Bendahou, D.; Bendahou, A.; Grohens, Y.; Kaddami, H. New nanocomposite design from zeolite and poly(lactic acid). Ind. Crops Prod. 2015, 72, 107–118. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Stark, N.M. Spin-coating: A new approach for improving dispersion of cellulose nanocrystals and mechanical properties of poly (lactic acid) composites. Carbohydr. Polym. 2018, 190, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.; Cran, M.J.; Nerín, C.; Bigger, S.W. Development and characterisation of hpmc films containing pla nanoparticles loaded with green tea extract for food packaging applications. Carbohydr. Polym. 2017, 156, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2016, 103, 76–82. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Tang, C.H.; Yin, S.W.; Yang, X.Q. Development and characterization of novel antimicrobial bilayer films based on polylactic acid (pla)/pickering emulsions. Carbohydr. Polym. 2018, 181, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Pantani, R.; Gorrasi, G.; Vigliotta, G.; Murariu, M.; Dubois, P. Pla-zno nanocomposite films: Water vapor barrier properties and specific end-use characteristics. Eur. Polym. J. 2013, 49, 3471–3482. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Jiménez, A.; Kenny, J.M. Combined effects of cellulose nanocrystals and silver nanoparticles on the barrier and migration properties of pla nano-biocomposites. J. Food Eng. 2013, 118, 117–124. [Google Scholar] [CrossRef]
- Sun, J.; Shen, J.; Chen, S.; Cooper, M.; Fu, H.; Wu, D.; Yang, Z. Nanofiller reinforced biodegradable pla/pha composites: Current status and future trends. Polymers 2018, 10, 505. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W.; Won, K. Preparation of poly(lactide)/lignin/silver nanoparticles composite films with uv light barrier and antibacterial properties. Int. J. Biol. Macromol. 2018, 107, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, V.E.; Solonin, A.N.; Urzhumtsev, O.D.; Schielling, R.; Tavitov, A.G. Strength of pla components fabricated with fused deposition technology using a desktop 3d printer as a function of geometrical parameters of the process. Polymers 2018, 10. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Wang, L.J.; Li, D.; Wei, Q.; Adhikari, B. Effects of high-pressure homogenization on the properties of starch-plasticizer dispersions and their films. Carbohydr. Polym. 2011, 86, 202–207. [Google Scholar] [CrossRef]
- Molinaro, S.; Cruz-Romero, M.; Sensidoni, A.; Morris, M.; Lagazio, C.; Kerry, J.P. Combination of high-pressure treatment, mild heating and holding time effects as a means of improving the barrier properties of gelatin-based packaging films using response surface modeling. Innov. Food Sci. Emerg. Technol. 2015, 30, 15–23. [Google Scholar] [CrossRef]
- Lian, Z.; Zhang, Y.; Zhao, Y. Nano-tio2 particles and high hydrostatic pressure treatment for improving functionality of polyvinyl alcohol and chitosan composite films and nano-tio2 migration from film matrix in food simulants. Innov. Food Sci. Emerg. Technol. 2016, 33, 145–153. [Google Scholar] [CrossRef]
- Kang, H.J.; Min, S. Potato peel-based biopolymer film development using high-pressure homogenization, irradiation, and ultrasound. LWT Food Sci. Technol. 2010, 43, 903–909. [Google Scholar] [CrossRef]
- Simões, C.L.; Viana, J.C.; Cunha, A.M. Mechanical properties of poly(ε-caprolactone) and poly(lactic acid) blends. J. Appl. Polym. Sci. 2010, 112, 345–352. [Google Scholar] [CrossRef]
- Fortunati, E.; Rinaldi, S.; Peltzer, M.; Bloise, N.; Visai, L.; Armentano, I.; Jiménez, A.; Latterini, L.; Kenny, J.M. Nano-biocomposite films with modified cellulose nanocrystals and synthesized silver nanoparticles. Carbohydr. Polym. 2014, 101, 1122–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yang, S.G.; Ding, J.X.; Li, Z.M. Tailor-made poly(l-lactide)/poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds prepared via high-pressure compression molding/salt leaching. RSC Adv. 2016, 6, 47418–47426. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, uv-light barrier, and antibacterial properties of pla-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef]
- Monteiro, M.; Oliveira, V.; Santos, F.; Barros, E.N.; Leite, R.; Aroucha, E.; Silva, R.R.; Silva, K. Incorporation of bentonite clay in cassava starch films for the reduction of water vapor permeability. Food Res. Int. 2018, 105, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Fang, X.; Chen, H.; Qin, Y.; Xu, F.; Jin, T.Z. Physiochemical properties and food application of antimicrobial pla film. Food Control 2016, 73, 1522–1531. [Google Scholar] [CrossRef]
- Rhim, J.W.; Wang, L.F.; Hong, S.I. Preparation and characterization of agar/silver nanoparticles composite films with antimicrobial activity. Food Hydrocolloids 2013, 33, 327–335. [Google Scholar] [CrossRef]
- Díezpascual, A.M.; Díezvicente, A.L. Zno-reinforced poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bionanocomposites with antimicrobial function for food packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Kim, S.W.; Ho, C.K. The effect of high pressure processing on the morphology of polyethylene films tested by differential scanning calorimetry and x-ray diffraction and its influence on the permeability of the polymer. J. Appl. Polym. Sci. 2010, 112, 107–113. [Google Scholar] [CrossRef]
- Aulin, C.; Gällstedt, M.; Lindström, T. Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 2010, 17, 559–574. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Jimenez, A.; Kenny, J.M.; Garrigós, M.C. Characterization and disintegrability under composting conditions of pla-based nanocomposite films with thymol and silver nanoparticles. Polym. Degrad. Stab. 2016, 132, 2–10. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, J.; Yuan, M.; Xue, J.; Chao, J.; Wu, Y.; Yuan, M. Mechanical, barrier, and thermal properties of poly(lactic acid)/poly(trimethylene carbonate)/talc composite films. J. Appl. Polym. Sci. 2014, 131, 596–602. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Biodegradable electrospun bionanocomposite fibers based on plasticized pla–phb blends reinforced with cellulose nanocrystals. Ind. Crops Prod. 2016, 93, 290–301. [Google Scholar] [CrossRef]
- Sonseca, Á.; Camarero-Espinosa, S.; Peponi, L.; Weder, C.; Foster, E.J.; Kenny, J.M.; Giménez, E. Mechanical and shape-memory properties of poly(mannitol sebacate)/cellulose nanocrystal nanocomposites. J. Polym. Sci. Part A Polym. Chem. 2015, 52, 3123–3133. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Zhang, H.; Yuan, M.; Qin, Y. Evaluation of pla nanocomposite films on physicochemical and microbiological properties of refrigerated cottage cheese. J. Food Process. Preserv. 2018, 42, e13362. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.; Bing, X.; Gao, C.; Wang, T.; Yuan, B. Nanosilver migrated into food-simulating solutions from commercially available food fresh containers. Packag. Technol. Sci. 2011, 24, 291–297. [Google Scholar] [CrossRef]
- Girdthep, S.; Worajittiphon, P.; Molloy, R.; Lumyong, S.; Leejarkpai, T.; Punyodom, W. Biodegradable nanocomposite blown films based on poly(lactic acid) containing silver-loaded kaolinite: A route to controlling moisture barrier property and silver ion release with a prediction of extended shelf life of dried longan. Polymer 2014, 55, 6776–6788. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J.M. Multifunctional bionanocomposite films of poly(lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Xiang, C.; Taylor, A.G.; Hinestroza, J.P.; Frey, M.W. Controlled release of nonionic compounds from poly(lactic acid)/cellulose nanocrystal nanocomposite fibers. J. Appl. Polym. Sci. 2013, 127, 79–86. [Google Scholar] [CrossRef]
- Andersson, S.R.; Hakkarainen, M.; Albertsson, A.C. Long-term properties and migration of low molecular mass compounds from modified plla materials during accelerated ageing. Polym. Degrad. Stab. 2012, 97, 914–920. [Google Scholar] [CrossRef]
- Song, H.; Li, B.; Lin, Q.B.; Wu, H.J.; Chen, Y. Migration of silver from nanosilver–polyethylene composite packaging into food simulants. Food Addit. Contam. Part. A 2011, 28, 1758–1762. [Google Scholar] [CrossRef] [PubMed]

| Samples AgNPs (wt.%) | Pressure (MPa) | WVP × 10−10 (g·m/m2·s·Pa) | Reduction in WVP (%) |
|---|---|---|---|
| 0 | 0 | 5.8 ± 0.1 a | - |
| 3 | 0 | 5.3 ± 0.2 b | 7.8 |
| 3 | 200 | 3.4 ± 0.2 de | 41.2 |
| 3 | 400 | 3.6 ± 0.1 d | 37.9 |
| 5 | 0 | 4.3 ± 0.3 c | 26.1 |
| 5 | 200 | 2.8 ± 0.1 f | 51.5 |
| 5 | 400 | 3.2 ± 0.2 e | 44.3 |
| SamplesAgNPs (wt.%) | Pressure (MPa) | Tg (°C) | Tc (°C) | Tm (°C) | Xc (%) |
|---|---|---|---|---|---|
| 0 | 0 | 49.9 ± 0.3 c | 116.6 ± 0.2 a | 166.7 ± 0.4 a | 12.9 ± 0.3 g |
| 3 | 0 | 50.5 ± 0.4 bc | 112.7 ± 0.8 b | 166.3 ± 0.4 a | 14.3 ± 0.4 f |
| 3 | 200 | 50.5 ± 0.1 bc | 112.2 ± 0.3 b | 166.5 ± 0.2 a | 25.5 ± 0.5 b |
| 3 | 400 | 50.3 ± 0.1 bc | 110.2 ± 0.2 c | 166.8 ± 0.1 a | 28.7 ± 0.7 a |
| 5 | 0 | 50.1 ± 0.2 c | 110.4 ± 0.4 c | 165.9 ± 0.2 a | 15.8 ± 0.6 e |
| 5 | 200 | 50.9 ± 0.5 ab | 112.4 ± 0.6 b | 166.5 ± 0.8 a | 20.5 ± 0.6 d |
| 5 | 400 | 51.9 ± 0.2 a | 112.9 ± 0.5 b | 167.0 ± 0.3 a | 23.9 ± 0.4 c |
| Samples AgNPs (wt.%) | Pressure (MPa) | σ (MPa) | E (MPa) | ε (%) |
|---|---|---|---|---|
| 0 | 0 | 30 ± 3 d | 1083 ± 89 d | 194 ± 6 a |
| 3 | 0 | 33 ± 2 bc | 1123 ± 74 d | 179 ± 13 b |
| 3 | 200 | 34 ± 2 ab | 1664 ± 101 b | 159 ± 10 c |
| 3 | 400 | 36 ± 2 a | 1930 ± 70 a | 129 ± 9 d |
| 5 | 0 | 34 ± 2 ab | 1142 ± 95 d | 170 ± 8 b |
| 5 | 200 | 35 ± 2 ab | 1369 ± 62 c | 161 ± 14 c |
| 5 | 400 | 36 ± 2 a | 1479 ± 89 c | 119 ± 14 d |
| Samples AgNPs (wt.%) | Pressure (MPa) | 5d (μg/kg) | 10d (μg/kg) | 20d (μg/kg) | 30d (μg/kg) | 40d (μg/kg) |
|---|---|---|---|---|---|---|
| 3 | 0 | 138 ± 17 aBC | 223 ± 15 bAB | 348 ± 16 cABC | 357 ± 25 cBC | 369 ± 20 cAB |
| 3 | 200 | 82 ± 9 aA | 181 ± 11 bA | 302 ± 27 cA | 288 ± 14 cA | 312 ± 18 cA |
| 3 | 400 | 99 ± 11 aAB | 182 ± 17 bA | 330 ± 25 cAB | 339 ± 11 cAC | 341 ± 21 cAB |
| 5 | 0 | 169 ± 12 aC | 262 ± 22 bB | 412 ± 17 cC | 424 ± 18 cD | 434 ± 13 cC |
| 5 | 200 | 128 ± 23 aABC | 229 ± 13 bAB | 333 ± 13 cAB | 341 ± 16 cCB | 354 ± 10 cAB |
| 5 | 400 | 131 ± 12 aABC | 235 ± 18 bAB | 385 ± 15 cBC | 395 ± 18 cAD | 409 ± 16 cAC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, H.; Xue, J.; Zhang, C.; Chen, H.; Li, L.; Qin, Y. High Pressure Treatment for Improving Water Vapour Barrier Properties of Poly(lactic acid)/Ag Nanocomposite Films. Polymers 2018, 10, 1011. https://doi.org/10.3390/polym10091011
Chi H, Xue J, Zhang C, Chen H, Li L, Qin Y. High Pressure Treatment for Improving Water Vapour Barrier Properties of Poly(lactic acid)/Ag Nanocomposite Films. Polymers. 2018; 10(9):1011. https://doi.org/10.3390/polym10091011
Chicago/Turabian StyleChi, Hai, Jing Xue, Cheng Zhang, Haiyan Chen, Lin Li, and Yuyue Qin. 2018. "High Pressure Treatment for Improving Water Vapour Barrier Properties of Poly(lactic acid)/Ag Nanocomposite Films" Polymers 10, no. 9: 1011. https://doi.org/10.3390/polym10091011






