Effect of Intercalation Structure of Organo-Modified Montmorillonite/Polylactic Acid on Wheat Straw Fiber/Polylactic Acid Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Preparation
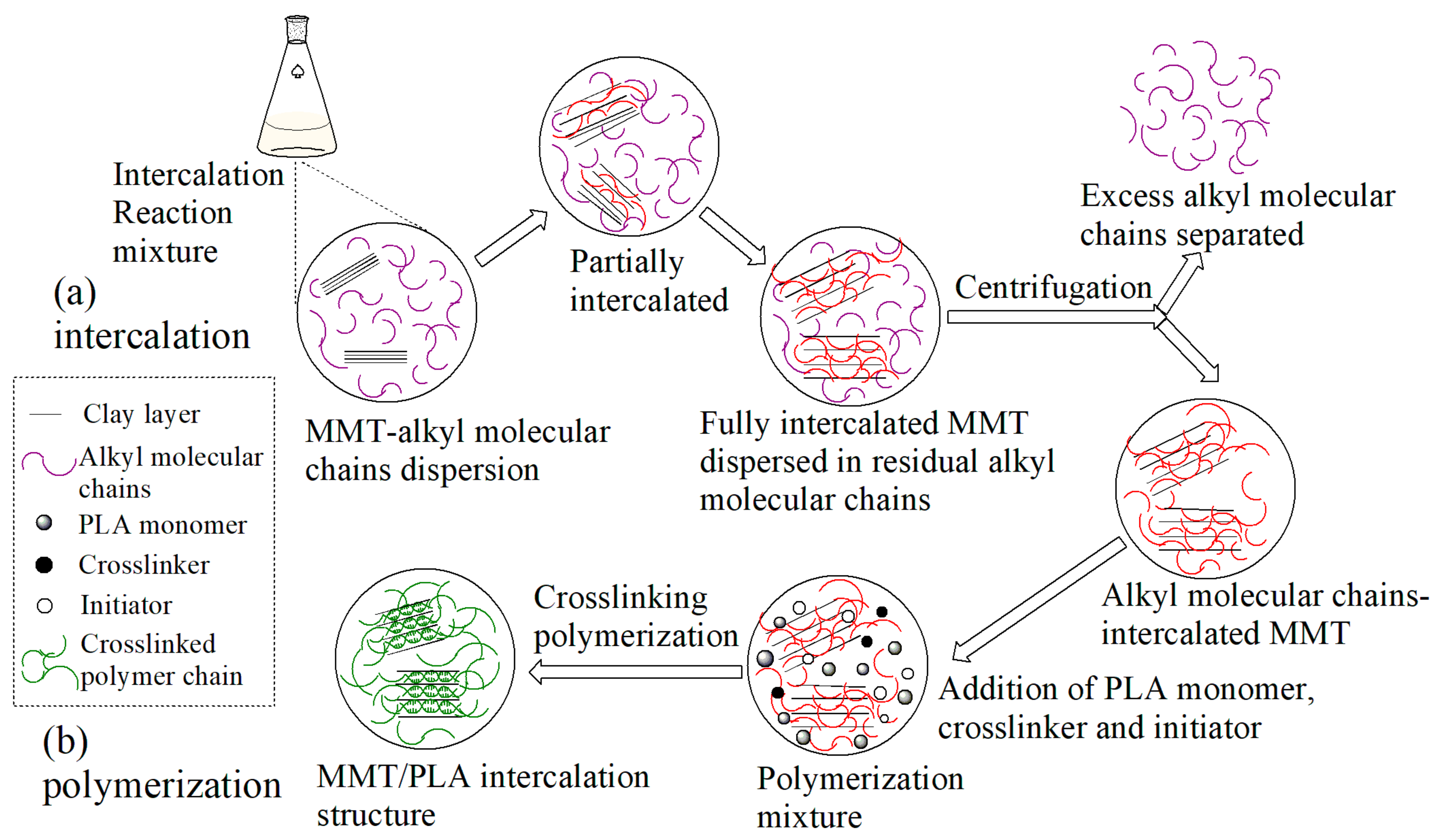
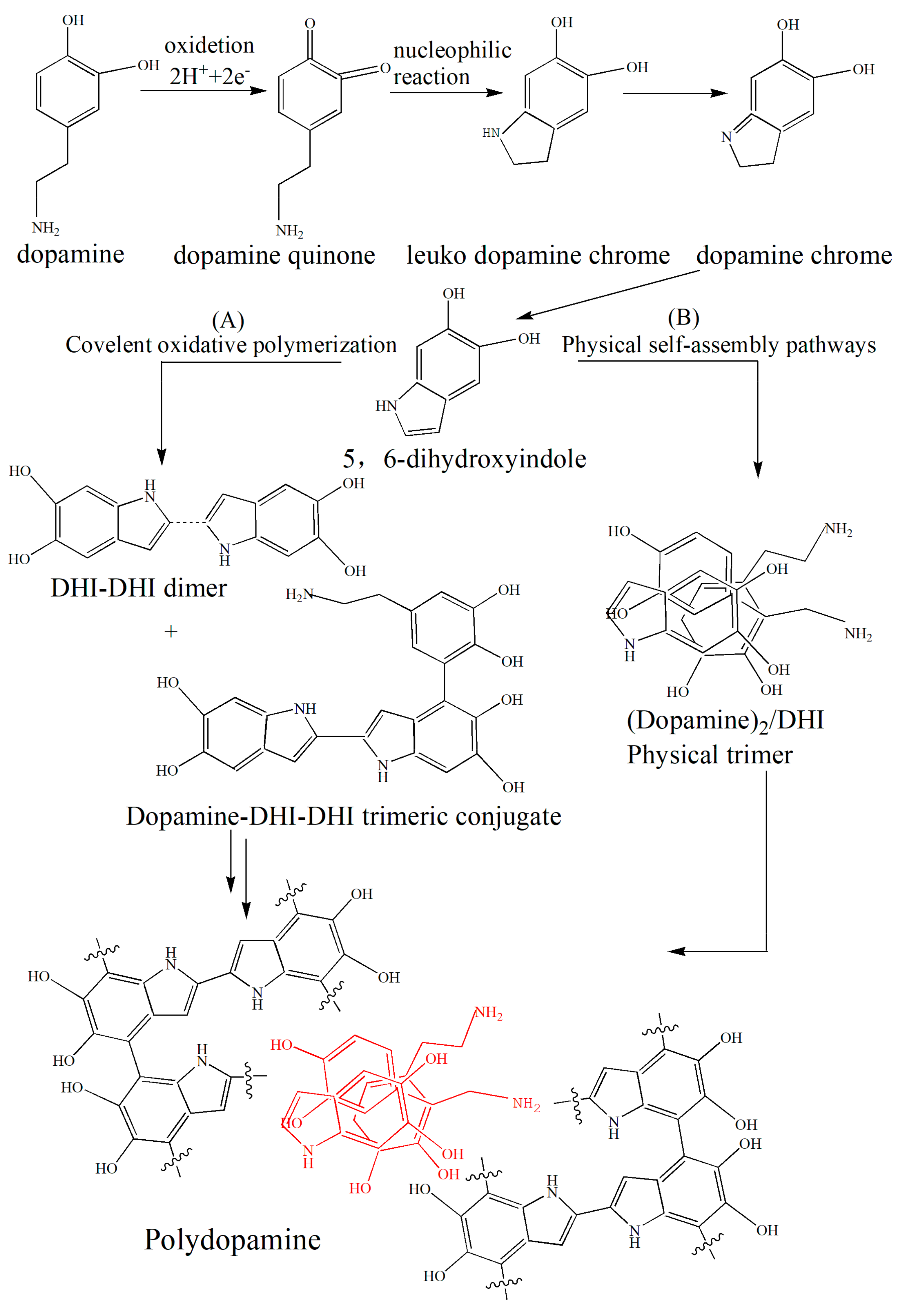
2.2. Modification of Montmorillonite
2.3. Preparation of Wheat Straw Fiber
2.4. Preparation of Composites
2.5. Material Characterization
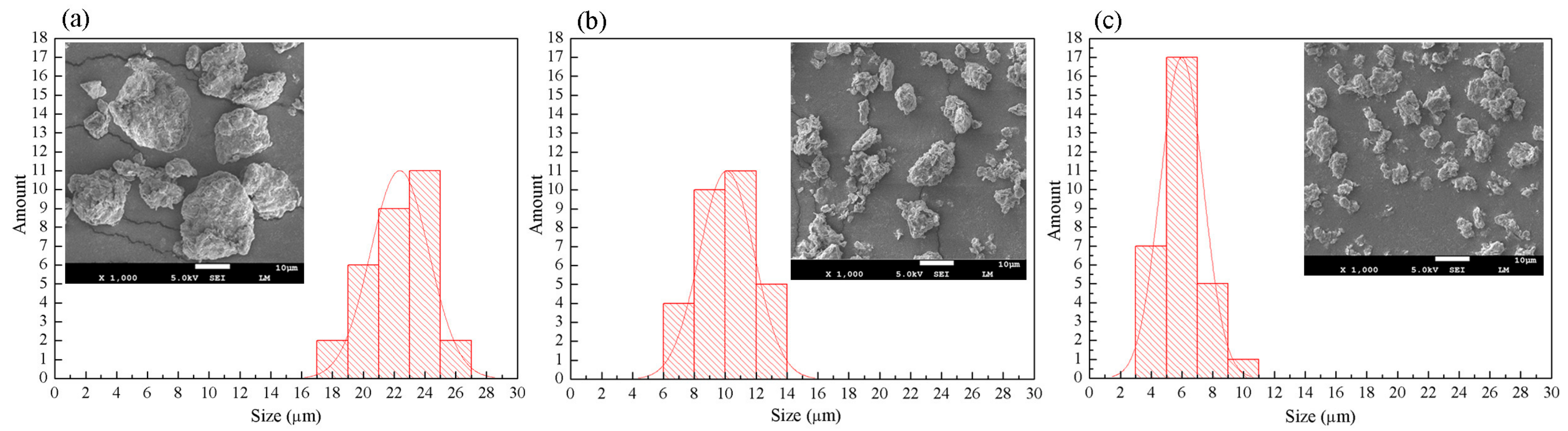
2.5.1. Characterization Using SEM
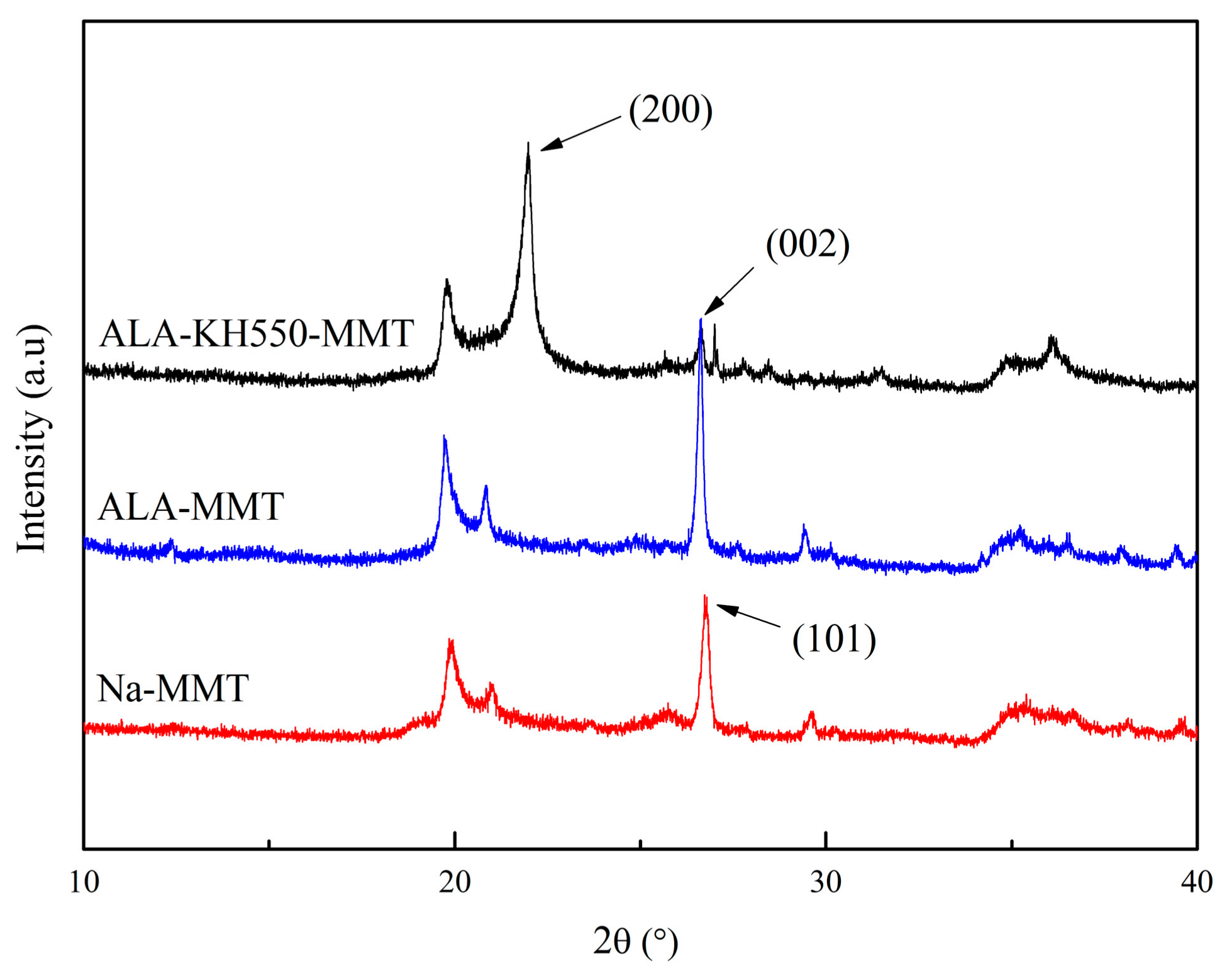
2.5.2. XRD Analysis
2.5.3. FTIR Analysis
2.5.4. Thermogravimetric Analysis
2.5.5. Mechanical Properties of the WSF/PLA Composites
3. Results and Discussion
3.1. SEM Analysis
3.2. XRD Analysis
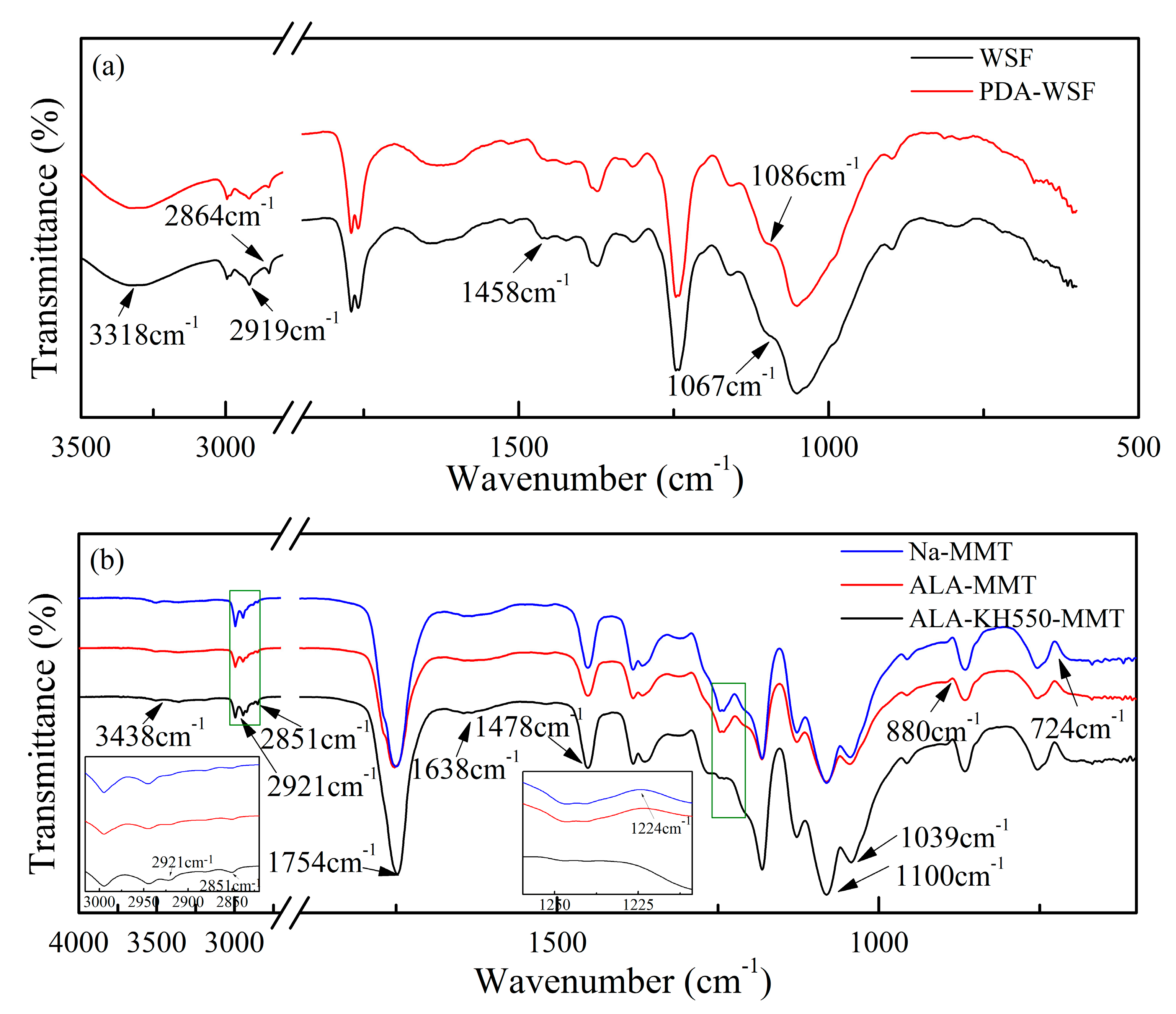
3.3. FTIR Analysis
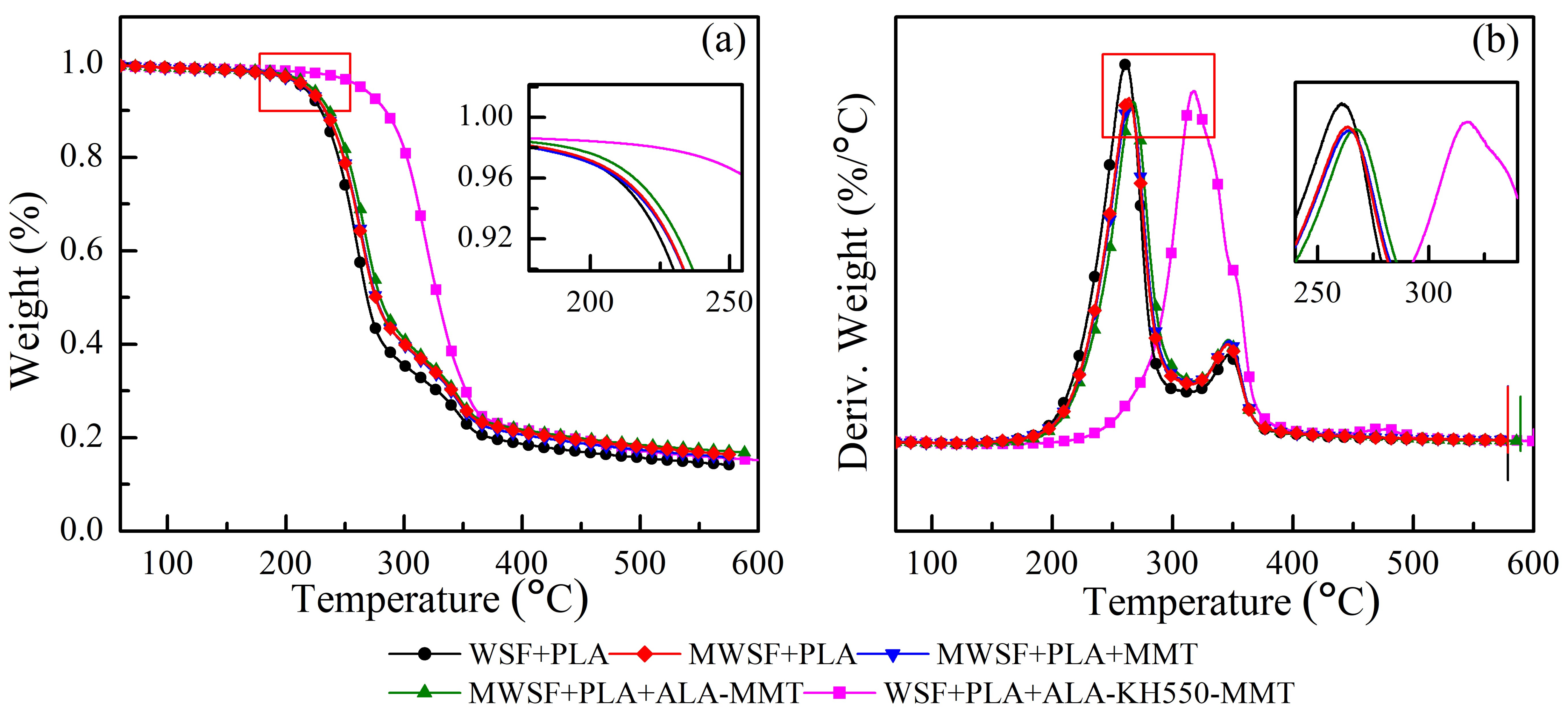
3.4. Thermogravimetric Analysis
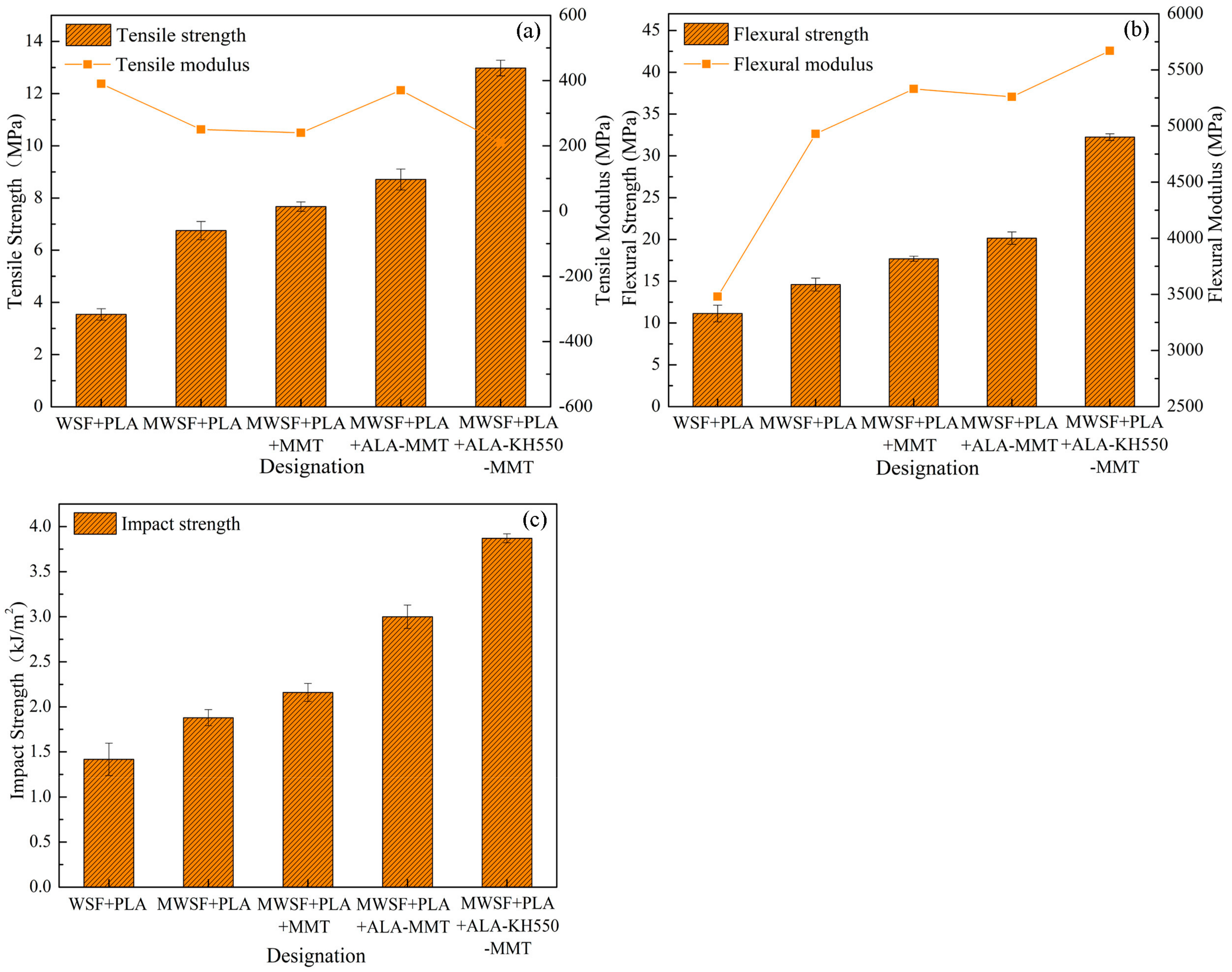
3.5. Study of Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ogami, A.; Ueda, K. New polylactide/layered silicate nanocomposites. 3. High-performance biodegradable materials. Chem. Mater. 2003, 15, 1456–1465. [Google Scholar]
- Wypych, F.; Satyanarayana, K.G. Functionalization of single layers and nanofibers: A new strategy to produce polymer nanocomposites with optimized properties. J. Colloid. Interf. Sci. 2005, 285, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Mistra, M.; Drzal, L.T. Sustainable bio-composites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–25. [Google Scholar] [CrossRef]
- Baillie, C. Eco-composites. Compos. Sci. Technol. 2003, 63, 1223–1224. [Google Scholar] [CrossRef]
- Lee, S.H.; Wang, S. Biodegradable polymers/bamboo fiber biocomposite with bio-based coupling agent. Compos. Part A-Appl. S. 2006, 37, 80–91. [Google Scholar] [CrossRef]
- Takatani, M.; Ikeda, K.; Sakamoto, K.; Okamoto, T. Cellulose esters as compatibilizers in wood/poly (lactic acid) composite. J. Wood Sci. 2008, 54, 54–61. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E. Crystallization, structure and properties of plasticized poly(l-lactide). Polymer 2005, 46, 10290–10300. [Google Scholar] [CrossRef]
- Niu, X.F.; Wang, Y.L.; Luo, Y.F.; Pan, J.; Shang, J.F.; Guo, L.X. Synthesis of the biomimetic polymer: Aliphatic diamine and RGDS modified poly(d,l-lactic acid). Chin. Chem. Lett. 2005, 16, 1035–1038. [Google Scholar]
- Kolstad, J.J. Crystallization kinetics of poly(l-lactide-co-meso-lactide). J. Appl. Polym. Sci. 2015, 62, 1079–1091. [Google Scholar] [CrossRef]
- Paul, M.; Alexandre, M.; Degée, P.; Calberg, C.; Jérôme, R.; Dubois, P. Exfoliated Polylactide/Clay Nanocomposites by In-Situ Coordination–Insertion Polymerization. Macromol. Rapid Commun. 2010, 24, 561–566. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Ogami, A.; Okamoto, M.; Ueda, K. New Polylactide/Layered Silicate Nanocomposite: Nanoscale Control over Multiple Properties. Macromol. Rapid Commun. 2002, 23, 943–947. [Google Scholar] [CrossRef]
- Lewitus, D.; McCarthy, O.A.; Kenig, S. The effect of nanoclays on the properties of PLLA-modified polymers part 1: Mechanical and thermal properties. J. Polym. Environ. 2006, 14, 171–177. [Google Scholar] [CrossRef]
- Elsaied, H.; Basta, A.H.; Hassanen, M.E.; Korte, H.; Helal, A. Behaviour of Rice-Byproducts and Optimizing the Conditions for Production of High Performance Natural Fiber Polymer Composites. J. Polym. Environ. 2012, 20, 838–847. [Google Scholar] [CrossRef]
- El-Shekeil, Y.A.; Sapuan, S.M.; Abdan, K.; Zainudin, E.S. Influence of fiber content on the mechanical and thermal properties of Kenaf fiber reinforced thermoplastic polyurethane composites. Mater. Des. 2012, 40, 299–303. [Google Scholar] [CrossRef]
- Liu, D.; Song, J.; Anderson, D.P.; Chang, P.R.; Hua, Y. Bamboo fiber and its reinforced composites: Structure and properties. Cellulose 2012, 19, 1449–1480. [Google Scholar] [CrossRef]
- Nyambo, C.; Mohanty, A.K.; Misra, M. Effect of Maleated Compatibilizer on Performance of PLA/Wheat Straw-Based Green Composites. Macromol. Mater. Eng. 2011, 296, 710–718. [Google Scholar] [CrossRef]
- Zhong, Y.; Poloso, T.; Hetzer, M.; Kee, D. Enhancement of wood/polyethylene composites via compatibilization and incorporation of organoclay particles. Polym. Eng. Sci. 2010, 47, 797–803. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Cao, J. Morphological, thermal and dynamic mechanical properties of Cathay poplar/organoclay composites prepared by in situ process. Mater. Des. 2014, 59, 233–240. [Google Scholar] [CrossRef]
- Liu, R.; Cao, J.; Luo, S.; Wang, X. Effects of two types of clay on physical and mechanical properties of poly (lactic acid)/wood flour composites at various wood flour contents. J. Appl. Polym. Sci. 2012, 127, 2566–2573. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, F.; Xue, L. Under seawater superoleophobic PVDF membrane inspired by polydopamine for efficient oil/seawater separation. J. Membr. Sci. 2015, 476, 321–329. [Google Scholar] [CrossRef]
- Huang, L.; Arena, J.T.; Manickam, S.S.; Jiang, X.; Willis, B.G.; McCutcheon, J.R. Improved mechanical properties and hydrophilicity of electrospun nanofiber membranes for filtration applications by dopamine modification. J. Membr. Sci. 2014, 460, 241–249. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Duan, S.; Shan, D.; Wu, Z.; Cai, Q.; Yang, X. Electrospun biodegradable polyorganophosphazene fibrous matrix with poly(dopamine) coating for bone regeneration. J. Biomed. Mater. Res. A 2015, 102, 3894–3902. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.Y.; Liu, T.Y.; Kuo, W.H.; Wang, M.J.; Tsai, W.B. Dopamine-assisted immobilization of hydroxyapatite nanoparticles and RGD peptides to improve the osteoconductivity of titanium. J. Biomed. Mater. Res. A 2013, 101, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.P.; Yu, J.Z.; Xu, Y.Y.; Xi, Z.Y.; Zhu, B.K. Surface modification of PVDF porous membranes via poly(DOPA) coating and heparin immobilization. Colloid. Surface B 2009, 69, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Mccloskey, B.D.; Choi, T.H.; Lee, C.; Kim, M.; Freeman, B.D.; Park, H.B. Oxygen concentration control of dopamine-induced high uniformity surface coating chemistry. ACS Appl. Mater. Inter. 2013, 5, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Karmarkar, A.; Chauhan, S.S.; Modak, J.M.; Chanda, M. Mechanical properties of wood–fiber reinforced polypropylene composites: Effect of a novel compatibilizer with isocyanate functional group. Compos. Part A-Appl. Sci. Manuf. 2007, 38, 227–233. [Google Scholar] [CrossRef]
- Xuan, L.; Han, G.; Wang, D.; Cheng, W.; Gao, X.; Chen, F.; Li, Q. Effect of surface-modified TiO2 nanoparticles on the anti-ultraviolet aging performance of foamed wheat straw fiber/polypropylene composites. Materials 2017, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.X.; Liu, H.W.; Zhong, Z.L.; Xu, P. Effect of silane couping agent treatment on tensile properties of single basalt filament. J. Tianjin Polytech. Univ. 2010, 29, 19–22. [Google Scholar]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stabil. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Rana, D.; Lee, C.H.; Cho, K.; Lee, B.H.; Choe, S. Thermal and mechanical properties for binary blends of metallocene polyethylene with conventional polyolefins. J. Appl. Polym. Sci. 1998, 69, 2441–2450. [Google Scholar] [CrossRef]
- Rana, D.; Cho, K.; Woo, T.; Lee, B.H.; Choe, S. Blends of ethylene 1-octene copolymer synthesized by Ziegler–Natta and metallocene catalysts. I. Thermal and mechanical properties. J. Appl. Polym. Sci. 2015, 74, 1169–1177. [Google Scholar] [CrossRef]
- Rana, D.; Bag, K.; Bhattacharyya, S.N.; Mandal, B.M. Miscibility of poly(styrene-co-butyl acrylate) with poly(ethyl methacrylate): Existence of both UCST and LCST. J. Polym. Sci. Polym. Phys. 2015, 38, 369–375. [Google Scholar] [CrossRef]
- Rana, D.; And, B.M.; Bhattacharyya, S.N. Analogue Calorimetric Studies of Blends of Poly (vinyl ester)s and Polyacrylates. Macromolecules 1996, 29, 1579–1583. [Google Scholar] [CrossRef]
- Rana, D.; Mandal, B.M.; Bhattacharyya, S.N. Analogue calorimetry of polymer blends: Poly(styrene-co-acrylonitrile) and poly(phenyl acrylate) or poly(vinyl benzoate). Polymer 1996, 37, 2439–2443. [Google Scholar] [CrossRef]
- Rana, D.; Mandal, B.M.; Bhattacharyya, S.N. Miscibility and phase diagrams of poly(phenyl acrylate) and poly(styrene-co-acrylonitrile) blends. Polymer 1993, 34, 1454–1459. [Google Scholar] [CrossRef]
- Santra, R.N.; Mukunda, P.G.; Nando, G.B.; Chaki, T.K. Thermogravimetric studies on miscible blends of ethylene-methyl acrylate copolymer (EMA) and polydimethylsiloxane rubber (PDMS). Thermochim. Acta. 1993, 219, 283–292. [Google Scholar] [CrossRef]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Fujimoto, Y.; Ogami, A.; Ueda, K. New polylactide/layered silicate nanocomposites. 5. Designing of materials with desired properties. Polymer 2003, 44, 6633–6646. [Google Scholar] [Green Version]
- Lee, S.R.; Park, H.M.; Lim, H.; Kang, Y.; Li, Y.; Cho, W.; Ha, C. Microstructure, tensile properties, and biodegradability of aliphatic polyester/clay nanocomposites. Polymer 2002, 43, 2495–2500. [Google Scholar] [CrossRef]
- Meng, Q.K.; Hetzer, M.; De Kee, D. PLA/clay/wood nanocomposites: Nanoclay effects on mechanical and thermal properties. J. Compos. Mater. 2011, 45, 1145–1158. [Google Scholar] [CrossRef]

| Code | Modified MMT (wt%) | Silane coupling agent (wt%) | Wheat straw fiber (wt%) | PLA (wt%) | Dopamine (wt%) | ZnO (wt%) | Paraffin (wt%) |
|---|---|---|---|---|---|---|---|
| A | 0 | 2 | 40 | 54 | 0 | 1 | 1 |
| B | 0 | 2 | 40 | 54 | 1 | 1 | 1 |
| C | 1(Na–) | 2 | 40 | 54 | 1 | 1 | 1 |
| D | 1(ALA–) | 2 | 40 | 54 | 1 | 1 | 1 |
| E | 1(ALA–KH550–) | 2 | 40 | 54 | 1 | 1 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Q.; Han, G.; Cheng, W.; Tian, H.; Wang, D.; Xuan, L. Effect of Intercalation Structure of Organo-Modified Montmorillonite/Polylactic Acid on Wheat Straw Fiber/Polylactic Acid Composites. Polymers 2018, 10, 896. https://doi.org/10.3390/polym10080896
Fan Q, Han G, Cheng W, Tian H, Wang D, Xuan L. Effect of Intercalation Structure of Organo-Modified Montmorillonite/Polylactic Acid on Wheat Straw Fiber/Polylactic Acid Composites. Polymers. 2018; 10(8):896. https://doi.org/10.3390/polym10080896
Chicago/Turabian StyleFan, Qiqi, Guangping Han, Wanli Cheng, Huafeng Tian, Dong Wang, and Lihui Xuan. 2018. "Effect of Intercalation Structure of Organo-Modified Montmorillonite/Polylactic Acid on Wheat Straw Fiber/Polylactic Acid Composites" Polymers 10, no. 8: 896. https://doi.org/10.3390/polym10080896
APA StyleFan, Q., Han, G., Cheng, W., Tian, H., Wang, D., & Xuan, L. (2018). Effect of Intercalation Structure of Organo-Modified Montmorillonite/Polylactic Acid on Wheat Straw Fiber/Polylactic Acid Composites. Polymers, 10(8), 896. https://doi.org/10.3390/polym10080896





