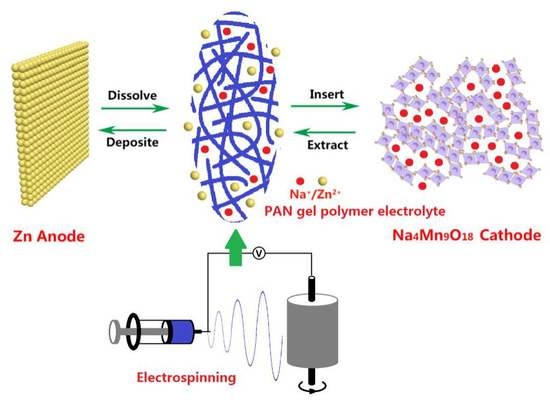
Polyacrylonitrile-Nanofiber-Based Gel Polymer Electrolyte for Novel Aqueous Sodium-Ion Battery Based on a Na4Mn9O18 Cathode and Zn Metal Anode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Physical Characterization
2.3. Electrochemical Characterization
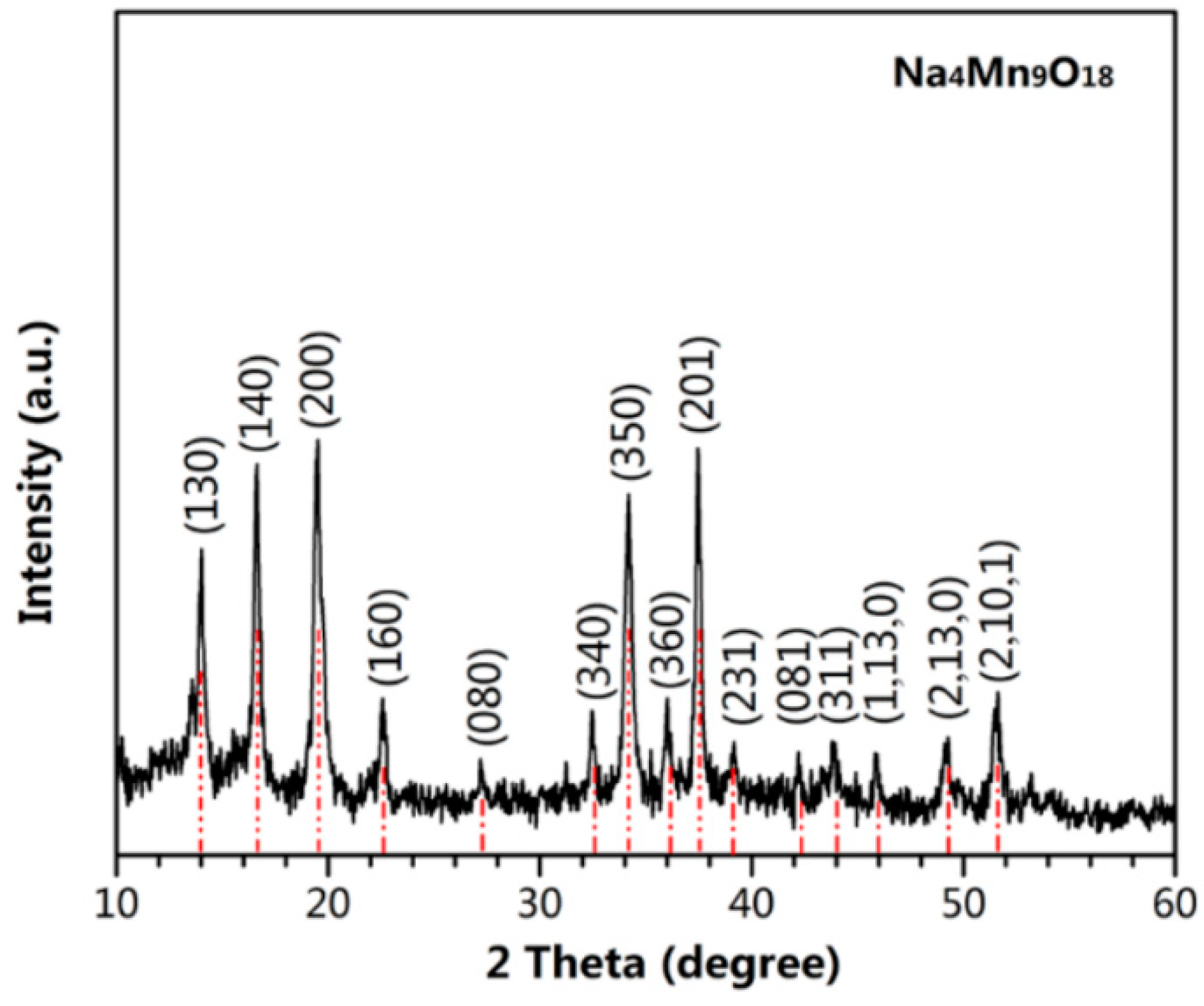
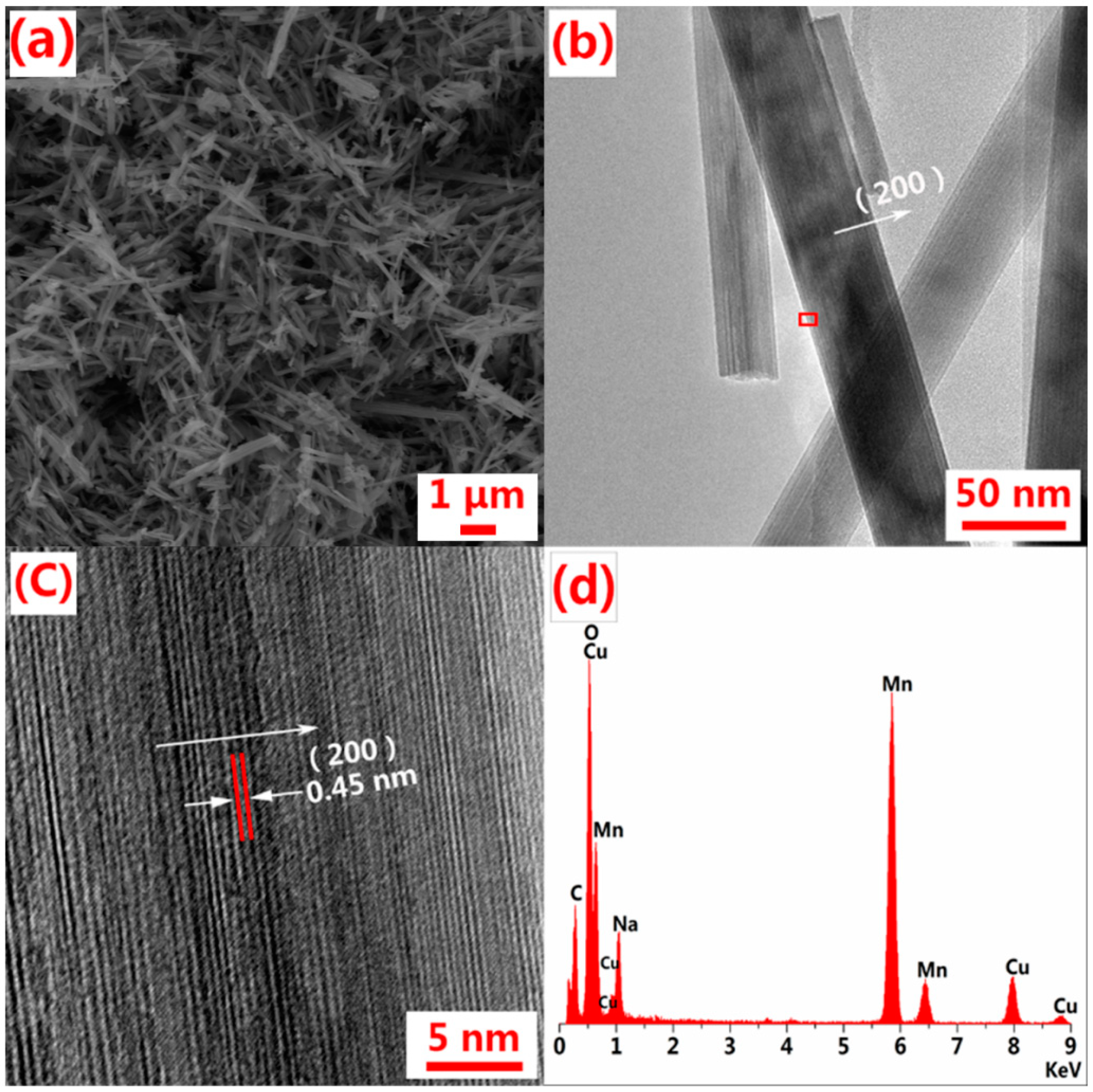
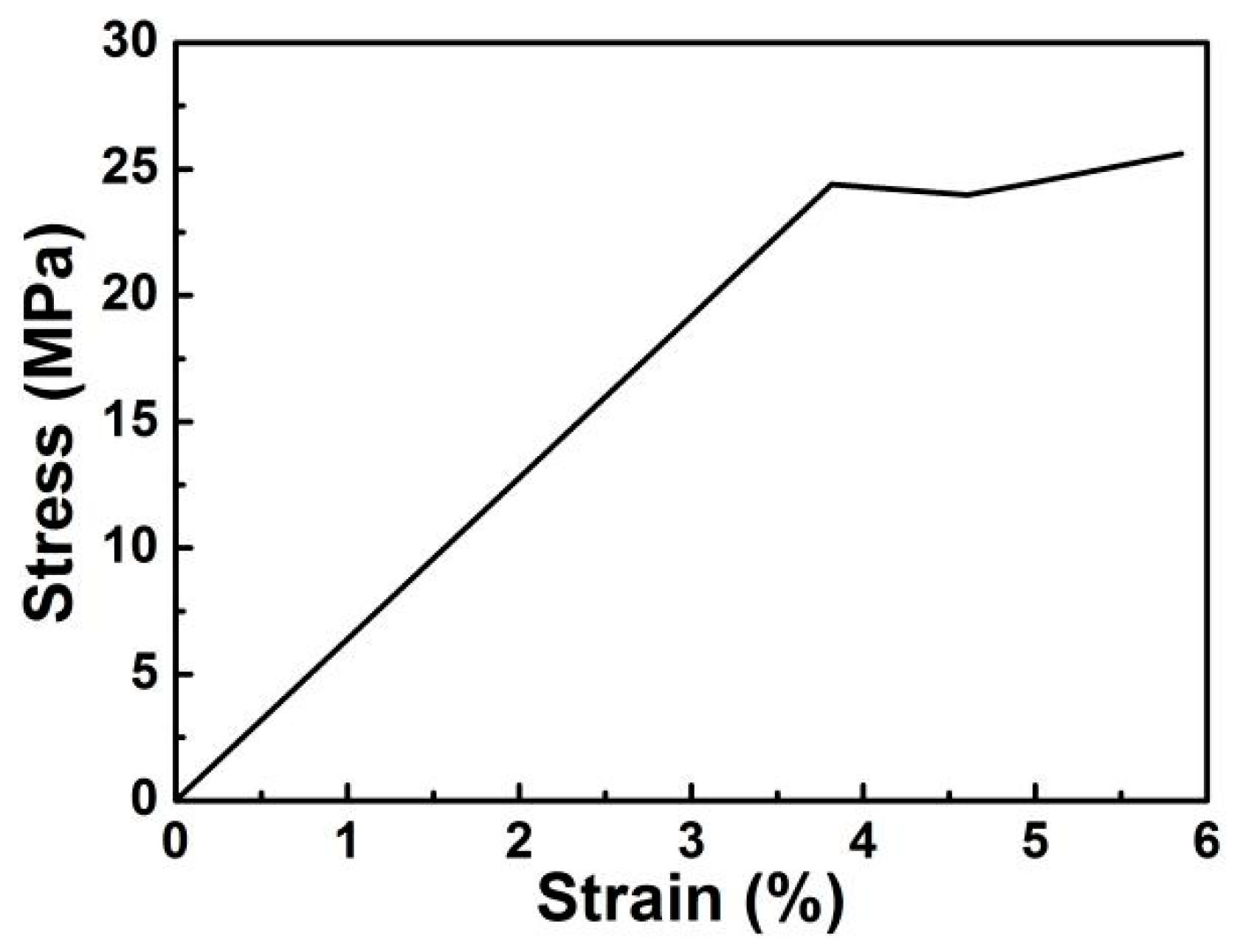
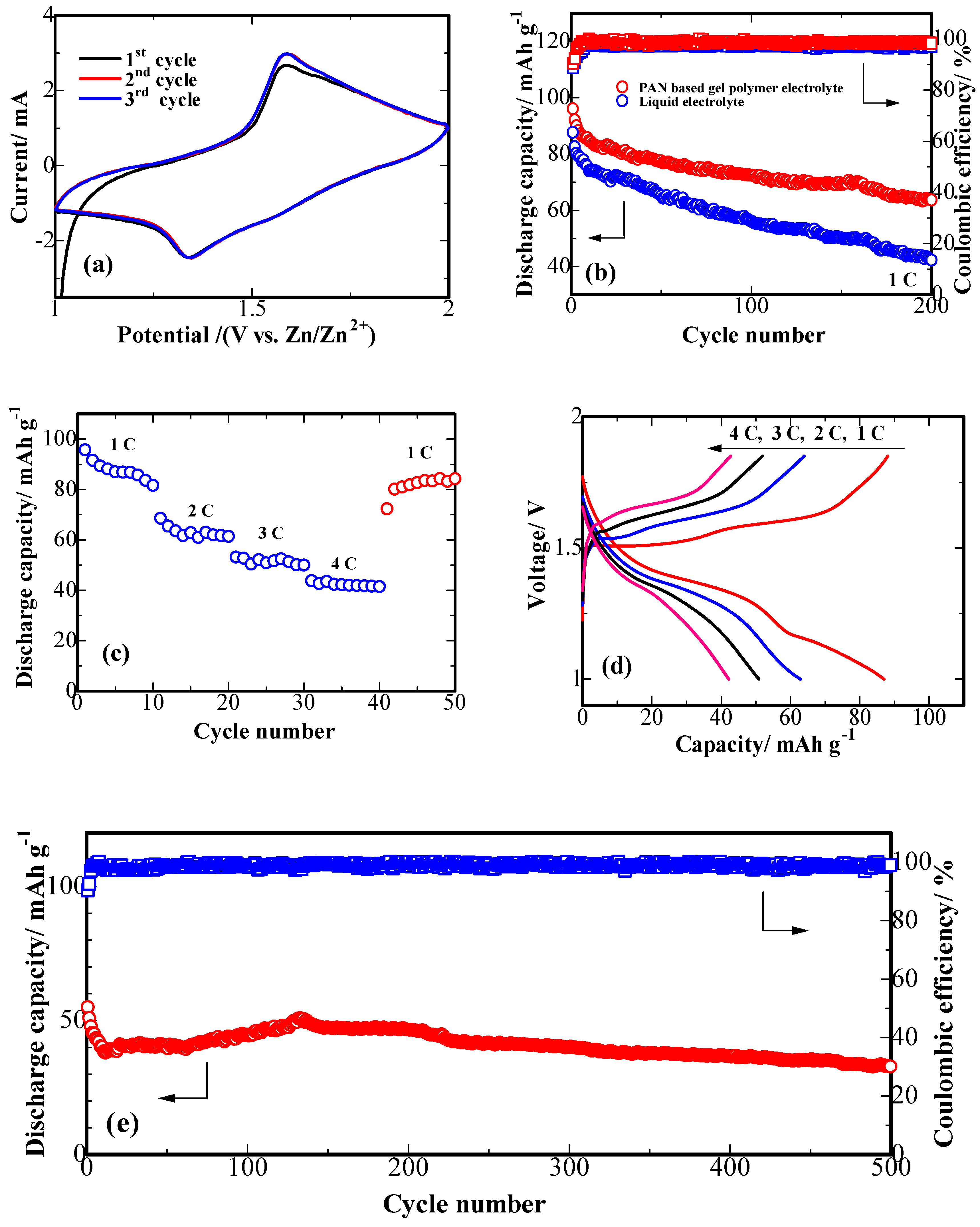
3. Results and Discussion
- The reaction at cathode:Na4Mn9O18 ⇔ Na4−xMn9O18 + xNa+ + xe−
- The reaction at anode:Zn2+ + 2e− ⇔ Zn
- The total reaction:2Na4Mn9O18 + xZn2+ ⇔ 2Na4−xMn9O18 + 2xNa+ + xZn
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dong, Y.C.; Md, K.; Chui, Y.S.; Xia, Y.; Cao, C.; Lee, J.M.; Zapien, J.A. Synthesis of CNT@Fe3O4-C hybrid nanocables as anode materials with enhanced electrochemical performance for lithium ion batteries. Electrochim. Acta 2015, 176, 1332–1337. [Google Scholar] [CrossRef]
- Li, H.P.; Zhang, Y.G.; Zhang, C.W.; Wang, G.K.; Zhao, Y.; Yin, F.X.; Bakenov, Z. Synthesis of hierarchical MoS2 microspheres composed of nanosheets assembled via facile hydrothermal method as anode material for lithium-ion batteries. J. Nanopart. Res. 2016, 18, 1–9. [Google Scholar]
- Wang, X.; Huang, L.Y.; Zhang, Y.G.; Yin, F.X.; Bakenov, Z.; Umirov, N.; Jin, M.L.; Zhou, G.F. Novel silicon nanowire film on copper foil as high performance anode for lithium-ion batteries. Ionics 2018, 24, 373–378. [Google Scholar] [CrossRef]
- Zhou, H.S. New energy storage devices for post lithium-ion batteries. Energy Environ. Sci. 2013, 6, 2256. [Google Scholar] [CrossRef]
- Zhao, W.M.; Fei, P.Y.; Zhang, X.M.; Zhang, Y.G.; Qin, C.L.; Wang, Z.F. Porous TiO2/Fe2O3 nanoplate composites prepared by de-alloying method for Li-ion batteries. Mater. Lett. 2018, 211, 254–257. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhao, Y.; Bakenov, Z.; Gosselink, D.; Chen, P. Poly(vinylidene fluoride-co-hexafluoropropylene)/poly(methylmethacrylate)/nanoclay composite gel polymer electrolyte for lithium/sulfur batteries. J. Solid State Electrochem. 2014, 18, 1111–1116. [Google Scholar] [CrossRef]
- Kim, T.; Kang, I.J.; Cho, G.; Park, K.P. New method for the preparation of solid polymer electrolyte based on poly(vinylidene fluoride-co-hexafluoropropylene). Korean J. Chem. Eng. 2005, 22, 234–237. [Google Scholar] [CrossRef]
- Yeon, S.H.; Kim, K.S.; Choi, S.; Cha, J.H.; Lee, H.; Oh, J.; Lee, B.B. Poly(vinylidenefluoride)- hexafluoropropylene gel electrolytes based on N-(2-hydroxyethyl)-N-methyl morpholinium ionic liquids. Korean J. Chem. Eng. 2006, 23, 940–947. [Google Scholar] [CrossRef]
- Feuillade, G.; Perche, P. Ion-conductive macromolecular gels and membranes for solid lithium cells. J. Appl. Electrochem. 1975, 5, 63–69. [Google Scholar] [CrossRef]
- Xue, R.; Huang, H.; Menetrier, M.; Chen, L. Impedance study for the interface and whole battery with PAN-based polymer electrolyte. J. Power Sources 1993, 44, 431–438. [Google Scholar] [CrossRef]
- Yang, H.; Bang, H.; Amine, K.; Prakash, J. Investigations of the exothermic reactions of natural graphite anode for Li-ion batteries during thermal runaway. J. Electrochem. Soc. 2005, 152, A73–A79. [Google Scholar] [CrossRef]
- Dahn, J.R.; Fuller, E.W. Thermal stability of LixCoO2, LixNiO2 and λ-MnO2 and consequences for the safety of Li-ion cells. Solid State Ion. 1994, 69, 265–270. [Google Scholar] [CrossRef]
- Seo, J.; Sankarasubramanian, S.; Kim, C.S.; Hovington, P.; Prakash, J.; Zaghib, K. Thermal characterization of Li/sulfur, Li/S-LiFePO4 and Li/S-LiV3O8 cells using isothermal micro-calorimetry and accelerating rate calorimetry. J. Power Sources 2015, 289, 1–7. [Google Scholar] [CrossRef]
- Lee, C.W.; Venkatachalapathy, R.; Prakash, J. A novel flame-retardant additive for lithium batteries. Electrochem. Solid-State Lett. 2000, 3, 63–65. [Google Scholar] [CrossRef]
- Jeddi, K.; Zhao, Y.; Zhang, Y.G.; Konarov, A.; Chen, P. Fabrication and characterization of an effective polymer nanocomposite electrolyte membrane for high performance lithium/sulfur batteries. J. Electrochem. Soc. 2013, 160, A1052–A1060. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.G.; Bakenov, Z.; Chen, P. Electrochemical performance of lithium gel polymer battery with nanostructured sulfur/carbon composite cathode. Solid State Ion. 2013, 234, 40–45. [Google Scholar] [CrossRef]
- Li, H.; Li, M.J.; Hussain, S.H.; Zhu, M.; Lan, J.L.; Sui, G.; Yu, Y.H.; Zhong, W.H.; Yang, X.P. A sandwich structure polymer/polymer-ceramics/polymer gel electrolytes for the safe, stable cycling of lithium metal batteries. J. Membr. Sci. 2018, 555, 169–176. [Google Scholar] [CrossRef]
- Yan, J.; Wang, J.; Liu, H.; Bakenov, Z.; Gosselink, D.; Chen, P. Rechargeable hybrid aqueous batteries. J. Power Sources 2012, 216, 222–226. [Google Scholar] [CrossRef]
- Yesibolati, N.; Umirov, N.; Koishybay, A.; Omarova, M.; Kurmanbayeva, I.; Zhang, Y.G.; Zhao, Y.; Bakenov, Z. High performance Zn/LiFePO4 aqueous rechargeable battery for large scale applications. Electrochim. Acta 2015, 152, 505–511. [Google Scholar] [CrossRef]
- Yin, F.; Liu, Z.; Yang, S.; Shan, Z.Z.; Zhao, Y.; Feng, Y.; Zhang, C.; Bakenov, Z. Na4Mn9O18/Carbon Nanotube Composite as a High Electrochemical Performance Material for Aqueous Sodium-ion Batteries. Nanoscale Res. Lett. 2017, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Zhou, X.; Wang, Y. High-rate performance electrospun Na0.44MnO2 nanofibers as cathode material for sodium-ion batteries. J. Power Sources 2016, 310, 102–108. [Google Scholar] [CrossRef]
- Rajendran, S.; Kannan, R.; Mahendran, O. An electrochemical investigation on PMMA/PVDF blend-based polymer electrolytes. Mater. Lett. 2001, 49, 172–179. [Google Scholar] [CrossRef]
- Hodge, R.M.; Edward, G.H.; Simon, G.P. Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 1996, 37, 1371–1376. [Google Scholar] [CrossRef]
- Sathiya, A.R.; Subramania, A.; Jung, Y.S.; Kim, K.J. High-performance quasi-solid-state solar cell based on an electrospun PVdF-HFP membrane electrolyte. Langmuir 2008, 24, 9816. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, D.W. Photovoltaic performance of dye-sensitized solar cells assembled with hybrid composite membrane based on polypropylene non-woven matrix. Bull. Korean Chem. Soc. 2011, 32, 605. [Google Scholar] [CrossRef]
- Wu, X.W.; Li, Y.H.; Xiang, Y.H.; Liu, Z.X.; He, Z.Q.; Wu, X.M.; Li, Y.J.; Xiong, L.Z.; Li, C.C.; Chen, J. The electrochemical performance of aqueous rechargeable battery of Zn/Na0.44MnO2 based on hybrid electrolyte. J. Power Sources 2016, 336, 35–39. [Google Scholar] [CrossRef]
- Gao, H.C.; Zhou, W.D.; Park, K.B.; Goodenough, J. A sodium-ion battery with a low-cost cross-linked gel-polymer electrolyte. Adv. Energy Mater. 2016, 6, 1600467. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Zhao, Y.H.; Feng, X.M.; Chen, W.H.; Zhao, Y.F. Novel safer phosphonate-based gel polymer electrolytes for sodium-ion batteries with excellent cycling performance. J. Mater. Chem. A 2018, 6, 6559–6564. [Google Scholar] [CrossRef]
- Che, H.Y.; Chen, S.L.; Xie, Y.Y.; Wang, H.; Amine, K.; Liao, X.Z.; Ma, Z.F. Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1075–1101. [Google Scholar] [CrossRef]




| Material | Cycle Number | Capacity Remaining (mAh g−1) | Current Density | Reference |
|---|---|---|---|---|
| PMMA-based gel polymer electrolyte | 100th | 68 | 1 C | [27] |
| Phosphonate-based gel polymer electrolyte | 35th | 117.8 | 1 C | [28] |
| Glass-fiber-based gel polymer electrolyte | 100th | 70 | 1 C | [29] |
| PAN-based-based gel polymer electrolyte | 100th | 74 | 1 C | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Bakenov, Z.; Tan, T.; Huang, J. Polyacrylonitrile-Nanofiber-Based Gel Polymer Electrolyte for Novel Aqueous Sodium-Ion Battery Based on a Na4Mn9O18 Cathode and Zn Metal Anode. Polymers 2018, 10, 853. https://doi.org/10.3390/polym10080853
Zhang Y, Bakenov Z, Tan T, Huang J. Polyacrylonitrile-Nanofiber-Based Gel Polymer Electrolyte for Novel Aqueous Sodium-Ion Battery Based on a Na4Mn9O18 Cathode and Zn Metal Anode. Polymers. 2018; 10(8):853. https://doi.org/10.3390/polym10080853
Chicago/Turabian StyleZhang, Yongguang, Zhumabay Bakenov, Taizhe Tan, and Jin Huang. 2018. "Polyacrylonitrile-Nanofiber-Based Gel Polymer Electrolyte for Novel Aqueous Sodium-Ion Battery Based on a Na4Mn9O18 Cathode and Zn Metal Anode" Polymers 10, no. 8: 853. https://doi.org/10.3390/polym10080853