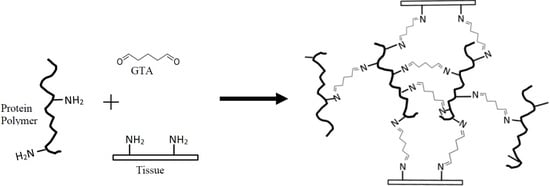
Use of Whey Protein as a Natural Polymer for Tissue Adhesive: Preliminary Formulation and Evaluation In Vitro
Abstract
:1. Introduction
2. Materials and Methods
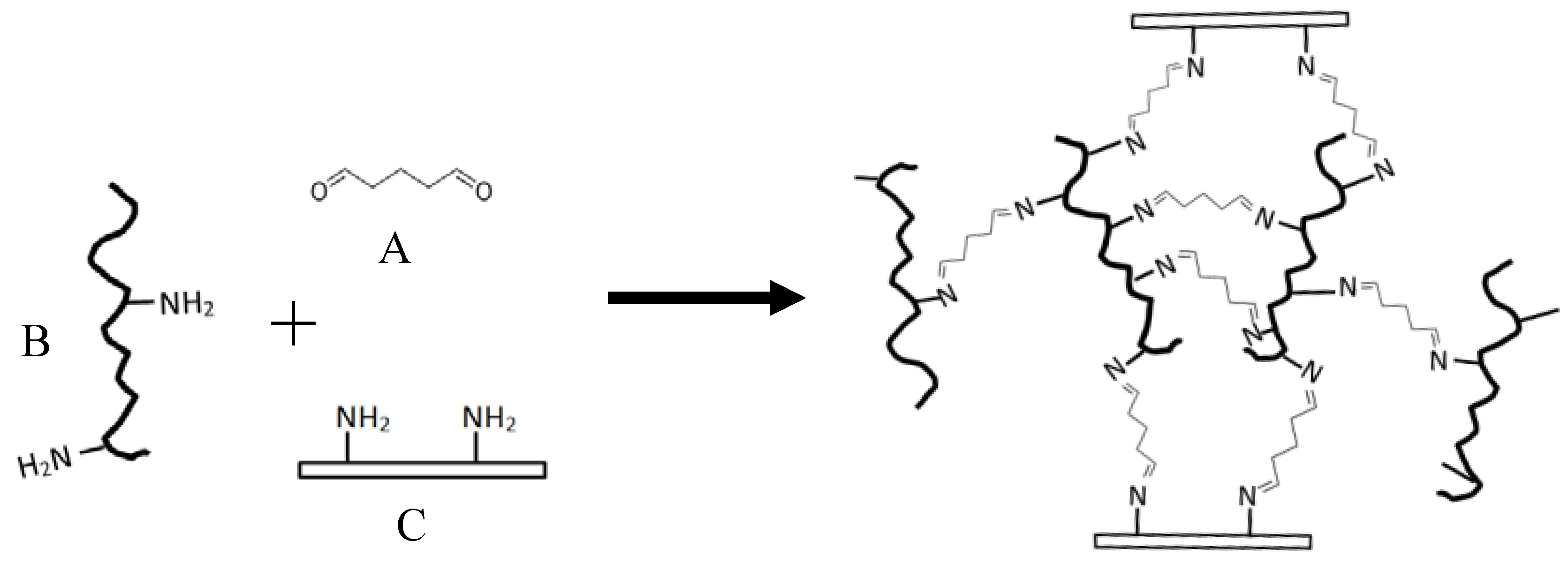
2.1. Glue Components Preparation
2.2. Gelation Time
2.3. Lap-Shear Bonding Strength
2.4. Bonding Curve
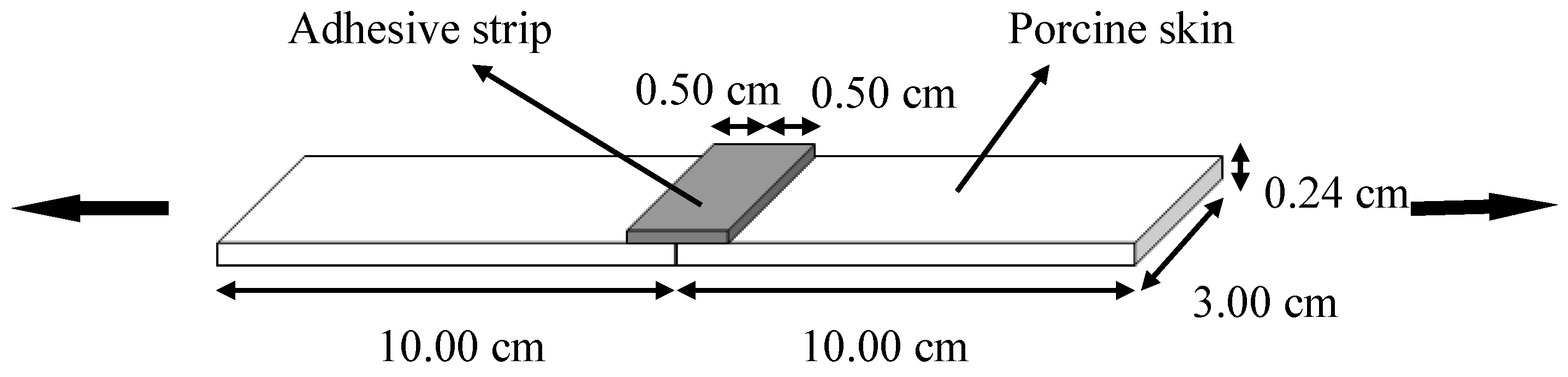
2.5. Wound Closure Strength
2.6. Statistical Analysis
3. Results and Discussion
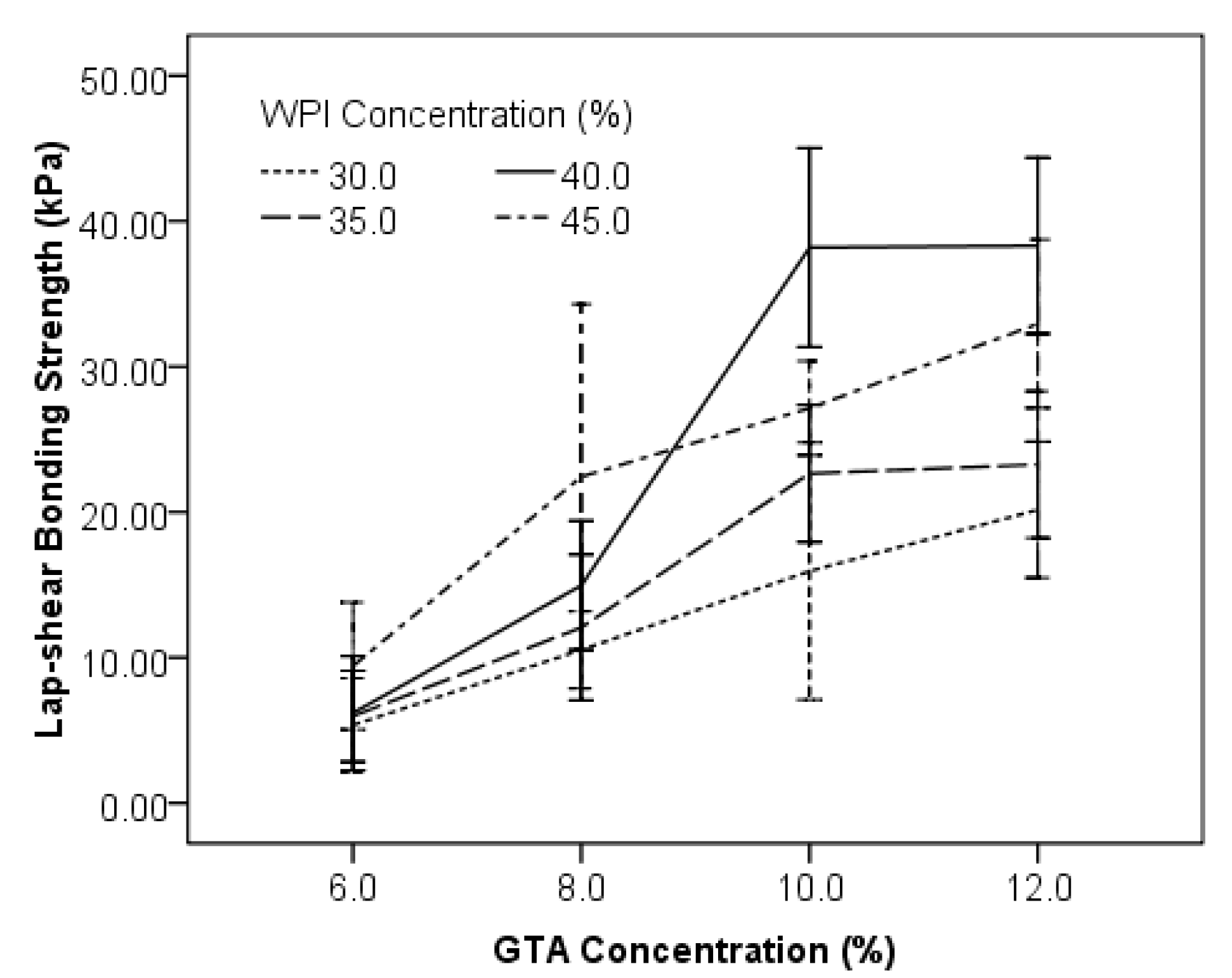
3.1. Effects of Concentrations of WPI and GTA on the Lap-Shear Bonding Strength
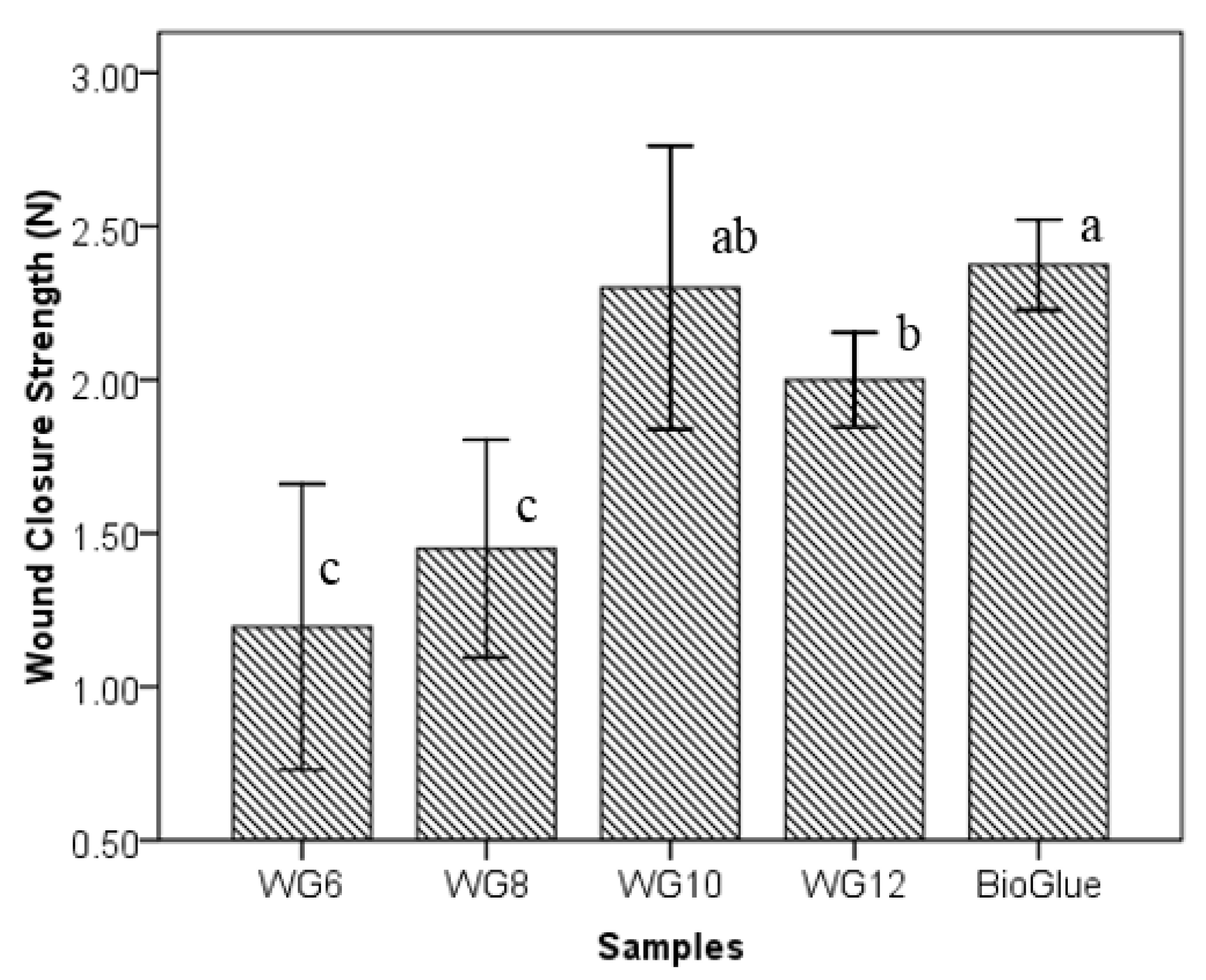
3.2. Effects of GTA Concentration on Wound Closure Strength
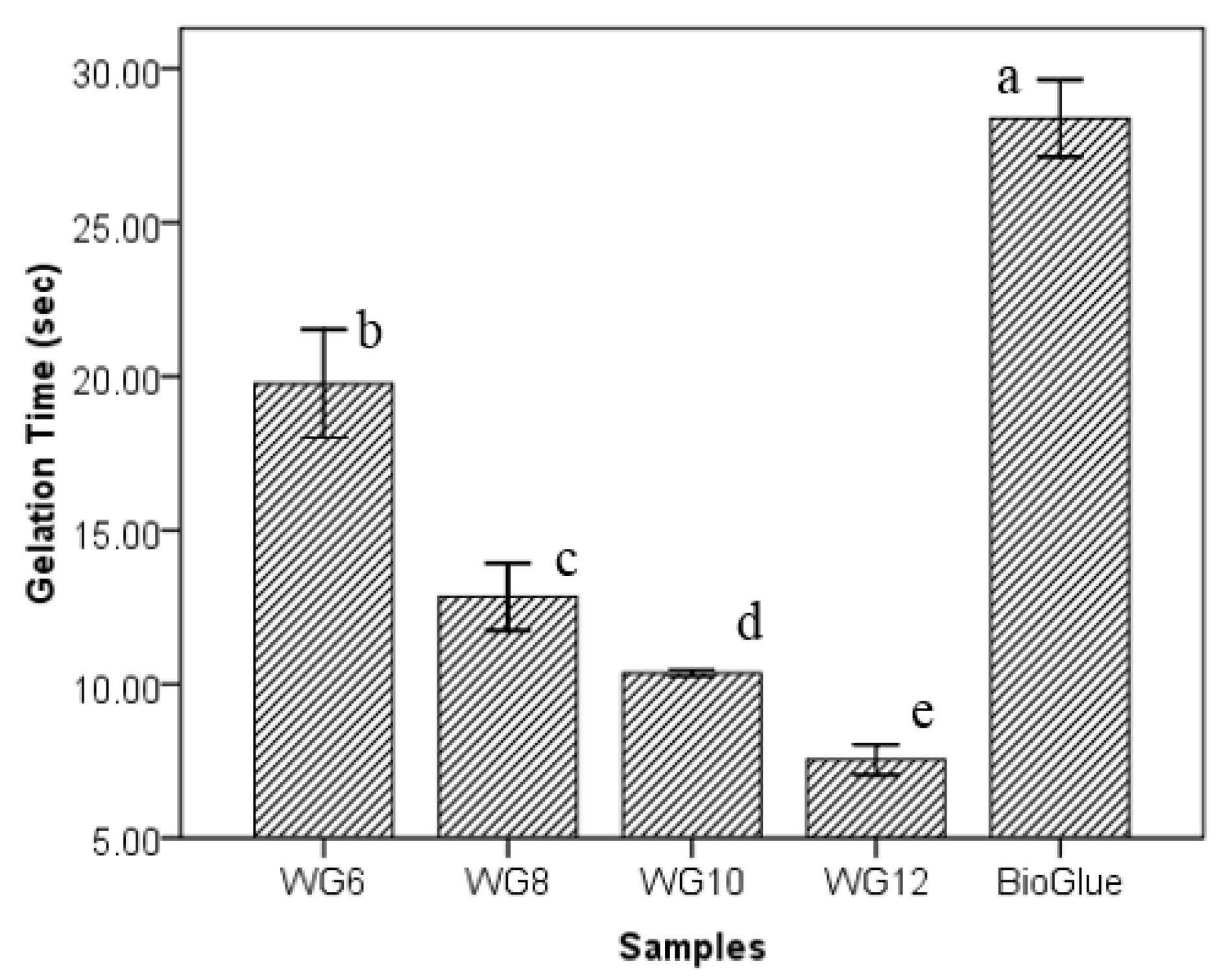
3.3. Effects of GTA Concentration on Gelation Time
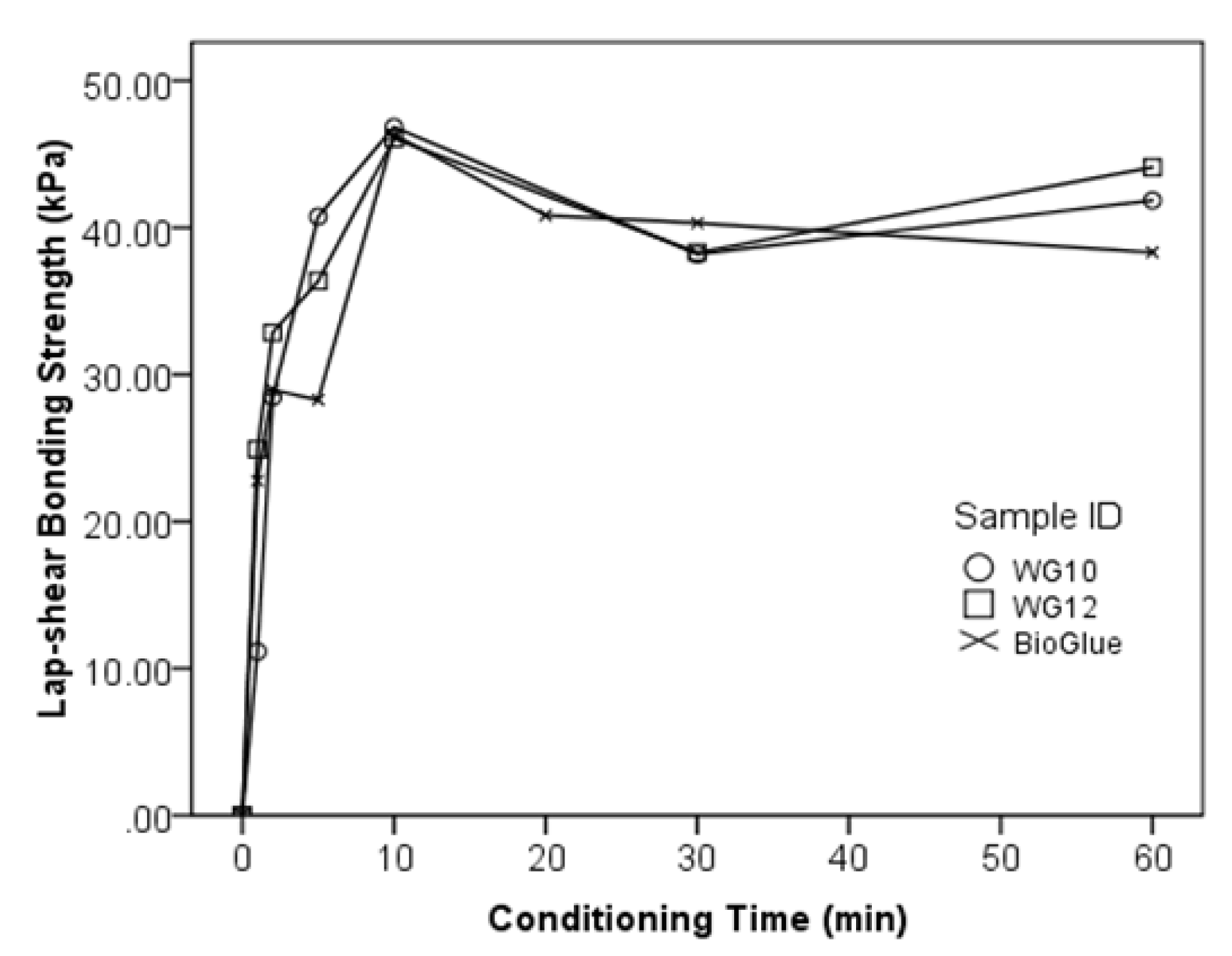
3.4. Bonding Curves of the WPI/GTA Glue
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Quinn, J.V. Tissue Adhesives in Clinical Medicine; BC Decker Inc.: Hamilton, ON, Canada, 2005. [Google Scholar]
- Lauto, A.; Mawad, D.; Foster, L.J.R. Adhesive biomaterials for tissue reconstruction. J. Chem. Technol. Biotechnol. 2008, 83, 464–472. [Google Scholar] [CrossRef]
- Tomihata, K.; Suzuki, M.; Oka, T.; Ikada, Y. A new resorbable monofilament suture. Polym. Degrad. Stab. 1998, 59, 13–18. [Google Scholar] [CrossRef]
- Singer, A.J.; Quinn, J.V.; Hollander, J.E. The cyanoacrylate topical skin adhesives. Am. J. Emerg. Med. 2008, 26, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.; Maw, J.; Ramotar, K.; Wenckebach, G.; Wells, G. Octylcyanoacrylate tissue adhesive versus suture wound repair in a contaminated wound model. Surgery 1997, 122, 69–72. [Google Scholar] [CrossRef]
- Quinn, J.; Wells, G.; Sutcliffe, T.; Jarmuske, M.; Maw, J.; Stiell, I.; Johns, P. Tissue adhesive versus suture wound repair at 1 year: Randomized clinical trial correlating early, 3-month, and 1-year cosmetic outcome. Ann. Emerg. Med. 1998, 32, 645–649. [Google Scholar] [CrossRef]
- Webster, J.; Alghamdi, A. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst. Rev. 2013, 1, CD006353-1. [Google Scholar]
- LeMaire, S.A.; Schmittling, Z.C.; Coselli, J.S.; Undar, A.; Deady, B.A.; Clubb, F.J.; Fraser, C.D. Bioglue surgical adhesive impairs aortic growth and causes anastomotic strictures. Ann. Thorac. Surg. 2002, 73, 1500–1505. [Google Scholar] [CrossRef]
- Chao, H.H.; Torchiana, D.F. Bioglue: Albumin/glutaraldehyde sealant in cardiac surgery. J. Card. Surg. 2003, 18, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Nadler, R.B.; Loeb, S.; Rubenstein, R.A.; Vardi, I.Y. Use of bioglue in laparoscopic partial nephrectomy. Urology 2006, 68, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Hidas, G.; Kastin, A.; Mullerad, M.; Shental, J.; Moskovitz, B.; Nativ, O. Sutureless nephron-sparing surgery: Use of albumin glutaraldehyde tissue adhesive (bioglue). Urology 2006, 67, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Gaberel, T.; Borgey, F.; Thibon, P.; Lesteven, C.; Lecoutour, X.; Emery, E. Surgical site infection associated with the use of bovine serum albumine-glutaraldehyde surgical adhesive (Bioglue®) in cranial surgery: A case–control study. Acta Neurochir. 2011, 153, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ranu, H.; Gatheral, T.; Sheth, A.; Smith, E.E.J.; Madden, B.P. Successful endobronchial seal of surgical bronchopleural fistulas using bioglue. Ann. Thorac. Surg. 2009, 88, 1691–1692. [Google Scholar] [CrossRef] [PubMed]
- Bahouth, Z.; Moskovitz, B.; Halachmi, S.; Nativ, O. Bovine serum albumin–glutaraldehyde (bioglue®) tissue adhesive versus standard renorrhaphy following renal mass enucleation: A retrospective comparison. Ther. Adv. Urol. 2017, 9, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Onwulata, C.; Huth, P.J. (Eds.) Whey Processing, Functionality and Health Benefits; Wiley-Blackwell: Ames, IA, USA, 2008. [Google Scholar]
- Schmidt, R.H.; Packard, V.S.; Morris, H.A. Effect of processing on whey protein functionality. J. Dairy Sci. 1984, 67, 2723–2733. [Google Scholar] [CrossRef]
- Zydney, A.L. Protein separations using membrane filtration: New opportunities for whey fractionation. Int. Dairy J. 1998, 8, 243–250. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Doultani, S.; Turhan, K.; Etzel, M. Whey protein isolate and glyco-macropeptide recovery from whey using ion exchange chromatography. J. Food Sci. 2003, 68, 1389–1395. [Google Scholar] [CrossRef]
- Tunick, M.H. Whey protein production and utilization: A brief history. In Whey Processing: Functionality and Health Benefits; Wiley-Blackwell: Ames, IA, USA, 2008; pp. 1–13. [Google Scholar]
- Havea, P.; Singh, H.; Creamer, L.K. Characterization of heat-induced aggregates of β-lactoglobulin, α-lactalbumin and bovine serum albumin in a whey protein concentrate environment. J. Dairy Res. 2001, 68, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, T.; Ahmad, S.; Cheng, J.; Guo, M. Physicochemical and adhesive properties, microstructure and storage stability of whey protein-based paper glue. Int. J Adhes. Adhes. 2013, 41, 198–205. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, G.; Bao, Y.; Guo, M. Whey-protein based environmentally friendly wood adhesives. Pigm. Resin Technol. 2011, 40, 42–48. [Google Scholar] [CrossRef]
- Guo, M.; Wang, G. Whey protein polymerisation and its applications in environmentally safe adhesives. Int. J. Dairy Technol. 2016, 69, 481–488. [Google Scholar] [CrossRef]
- Audic, J.-L.; Chaufer, B.; Daufin, G. Non-food applications of milk components and dairy co-products: A review. Lait 2003, 83, 417–438. [Google Scholar] [CrossRef] [Green Version]
- Foegeding, E.A.; Davis, J.P.; Doucet, D.; McGuffey, M.K. Advances in modifying and understanding whey protein functionality. Trends Food Sci. Technol. 2002, 13, 151–159. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, J.; Zhang, L.; Guo, M. Physicochemical and functional properties, microstructure, and storage stability of whey protein/polyvinylpyrrolidone based glue sticks. BioResources 2012, 7, 5422–5434. [Google Scholar] [CrossRef]
- Poutrel, B.; Caffin, J.; Rainard, P. Physiological and pathological factors influencing bovine serum albumin content of milk. J. Dairy Sci. 1983, 66, 535–541. [Google Scholar] [CrossRef]
- Brew, K.; Vanaman, T.C.; Hill, R.L. Comparison of the amino acid sequence of bovine α-lactalbumin and hens egg white lysozyme. J. Biol. Chem. 1967, 242, 3747–3748. [Google Scholar] [PubMed]
- Hirayama, K.; Akashi, S.; Furuya, M.; Fukuhara, K.-I. Rapid confirmation and revision of the primary structure of bovine serum albumin by ESIMS and Frit-FAB LC/MS. Biochem. Biophys. Res. Commun. 1990, 173, 639–646. [Google Scholar] [CrossRef]
- O’Mahony, J.A.; Fox, P.F. Milk Proteins: Introduction and Historical Aspects. In Advanced Dairy Chemistry, Volume 1: Proteins, Part A, 4rd ed.; McSweeney, P.L.H., Fox, P.F., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Mo, X.; Iwata, H.; Ikada, Y. A tissue adhsives evaluated in vitro and in vivo analysis. J. Biomed. Mater. Res. A 2010, 94A, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Kull, S.; Martinelli, I.; Briganti, E.; Losi, P.; Spiller, D.; Tonlorenzi, S.; Soldani, G. Glubran2 surgical glue: In vitro evaluation of adhesive and mechanical properties. J. Surg. Res. 2009, 157, e15–e21. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.J.; Dinsmore, R.C.; North, J.H., Jr. Tensile strength of wound closure with cyanoacrylate glue. Am. Surg. 2001, 67, 1113–1115. [Google Scholar] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Liu, N.; Guo, M. Use of Whey Protein as a Natural Polymer for Tissue Adhesive: Preliminary Formulation and Evaluation In Vitro. Polymers 2018, 10, 843. https://doi.org/10.3390/polym10080843
Wang G, Liu N, Guo M. Use of Whey Protein as a Natural Polymer for Tissue Adhesive: Preliminary Formulation and Evaluation In Vitro. Polymers. 2018; 10(8):843. https://doi.org/10.3390/polym10080843
Chicago/Turabian StyleWang, Guorong, Ning Liu, and Mingruo Guo. 2018. "Use of Whey Protein as a Natural Polymer for Tissue Adhesive: Preliminary Formulation and Evaluation In Vitro" Polymers 10, no. 8: 843. https://doi.org/10.3390/polym10080843