Influence of Two Polymer-Based Superplasticizers (Poly-naphthalene Sulfonate, PNS, and Lignosulfonate, LS) on Compressive and Flexural Strength, Freeze-Thaw, and Sulphate Attack Resistance of Lime-Metakaolin Grouts
Abstract
1. Introduction
2. Materials and Methods
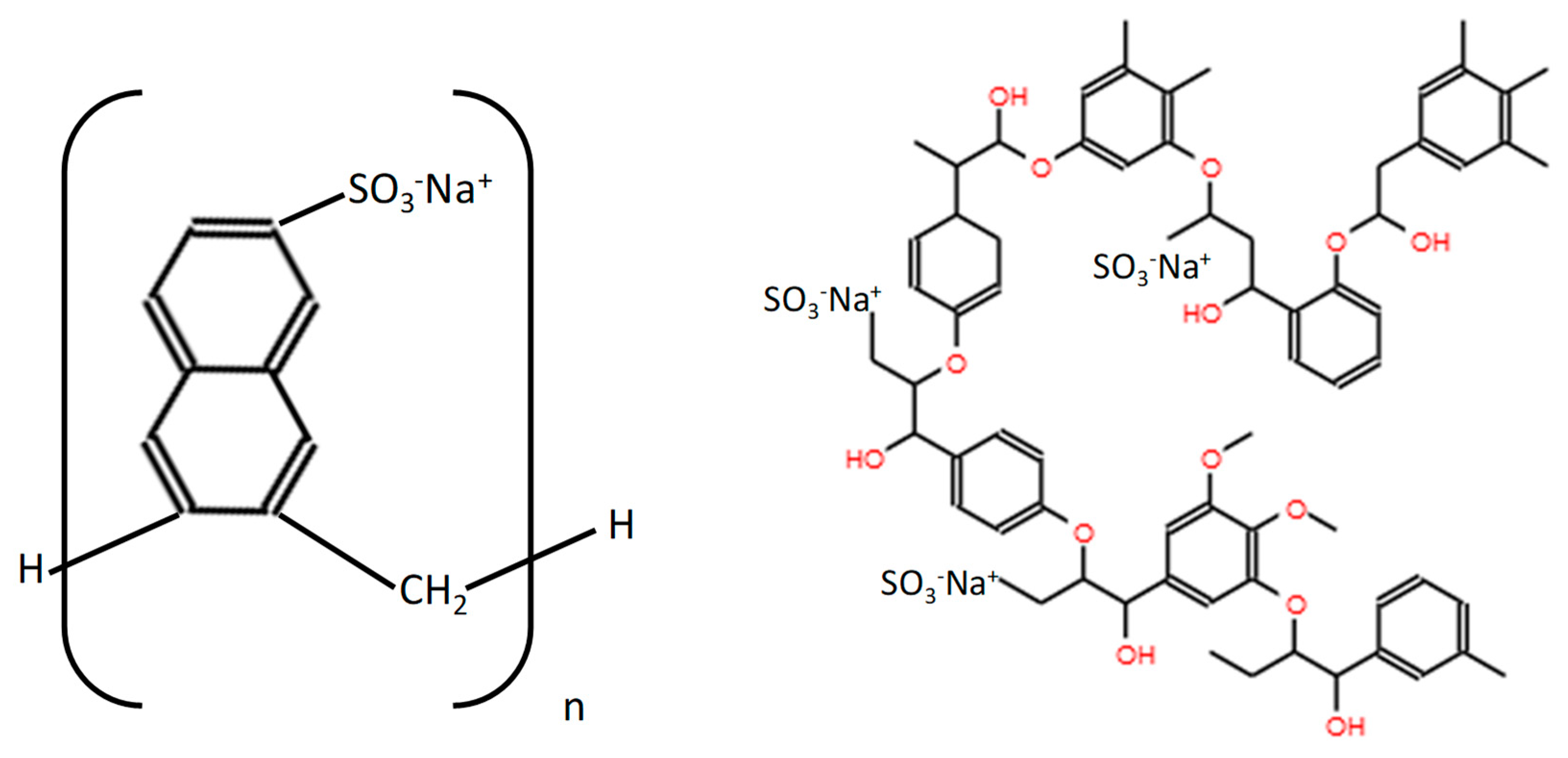
2.1. Materials
2.2. Experimental Methods
- (a)
- Frost resistance was determined by means of freezing-thawing cycles. The cycles consisted of water immersion of the samples for 24 h and subsequently freezing at −10 °C for 24 h. For these experiments, a CARAVELL 521-102 freezer was used.
- (b)
- Sulphate attack resistance: the monolithic samples were completely submerged in a MgSO4 saturated aqueous solution at 20 °C and 95% HR for 24 h. After this process, the samples were dried in an oven at 65 °C for 24 h and submerged in water for 24 h at 20 °C and 95%HR. To conclude the cycle, the specimens were again dried as described above. The cycles were continuously repeated until the total destruction of the specimens.
3. Results and Discussion
3.1. Fresh State Properties
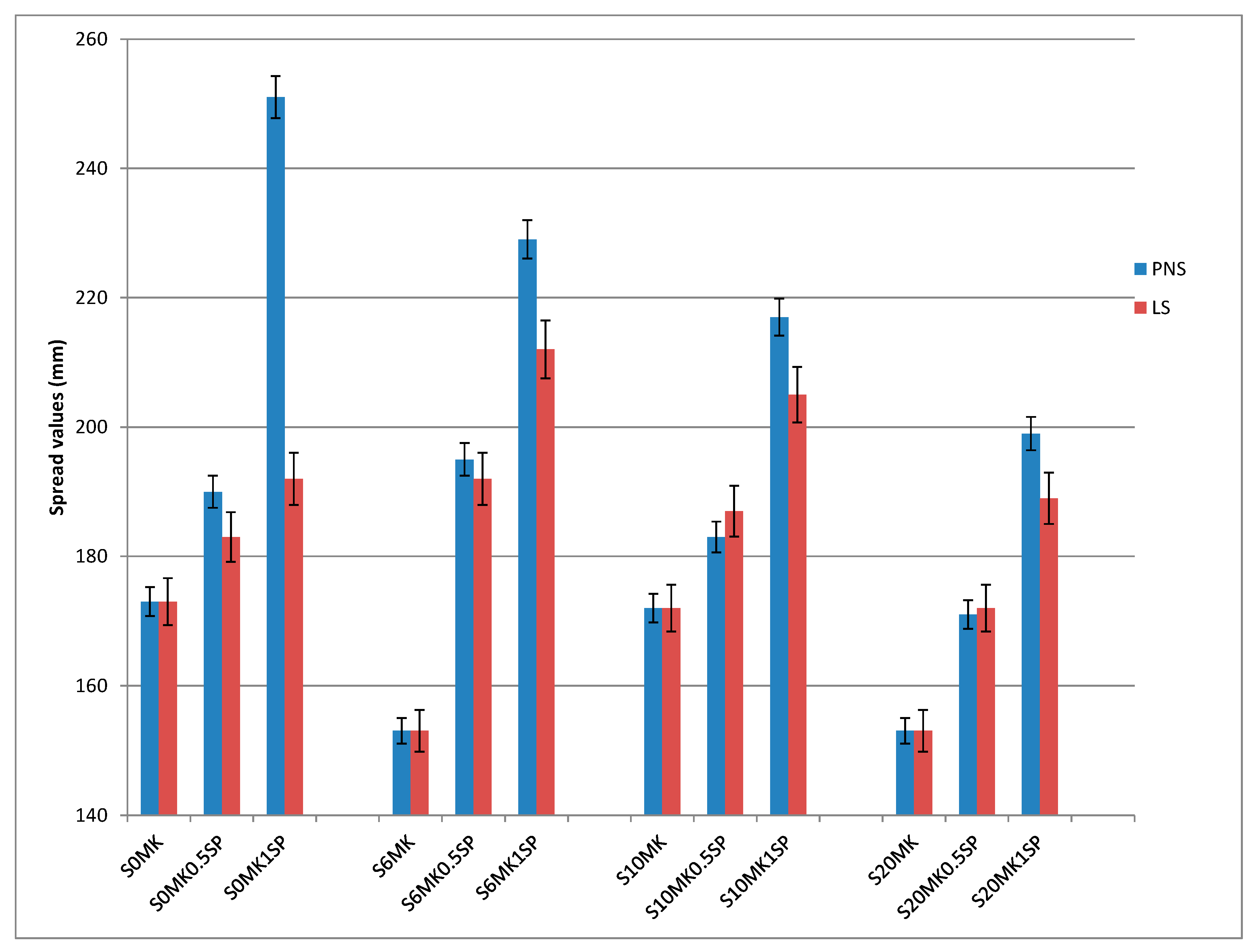
3.1.1. Fluidity, Density, and Air Content
3.1.2. Setting Times
3.1.3. Adsorption
3.2. Hardened State Properties
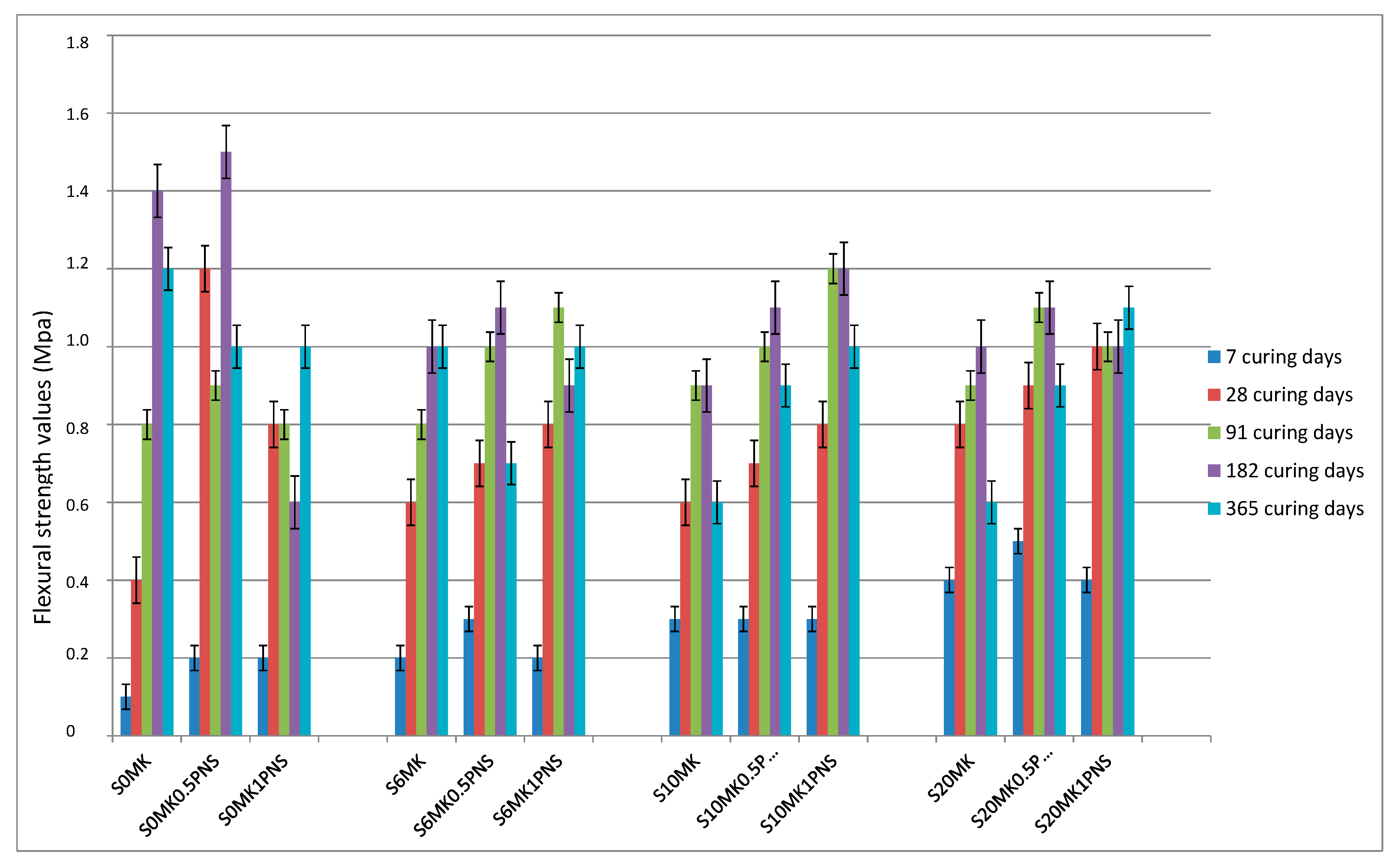
3.2.1. Mechanical Strength
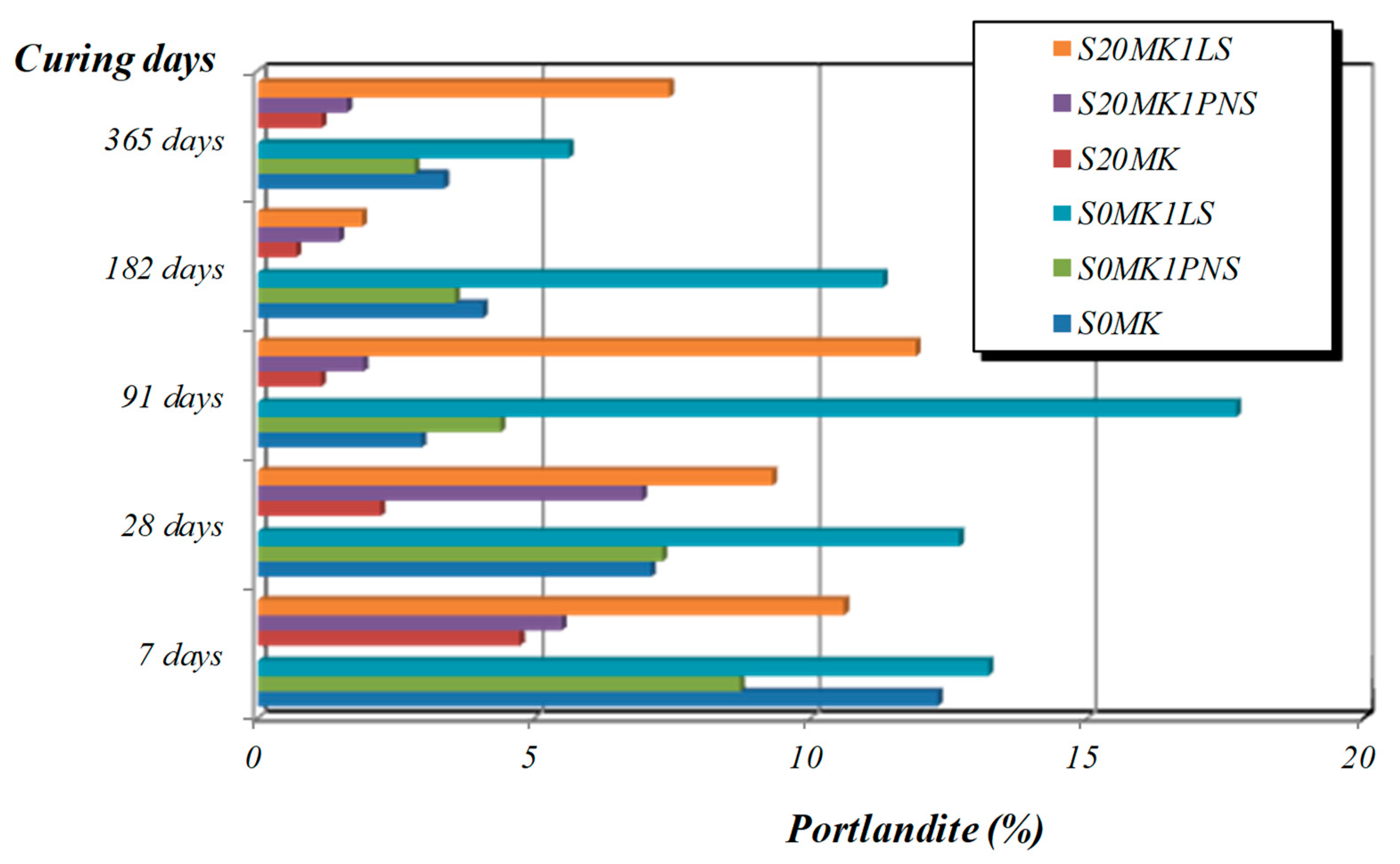
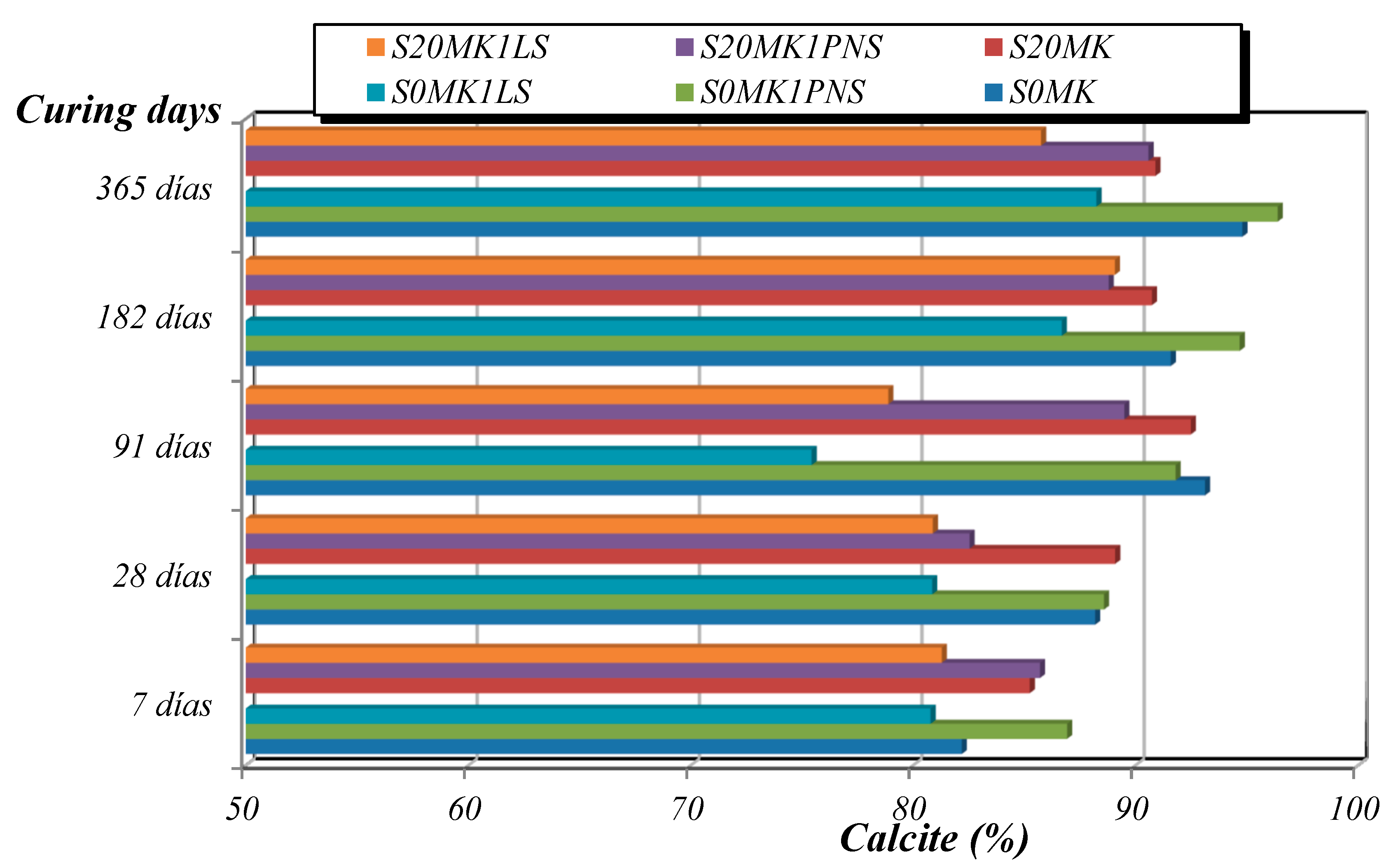
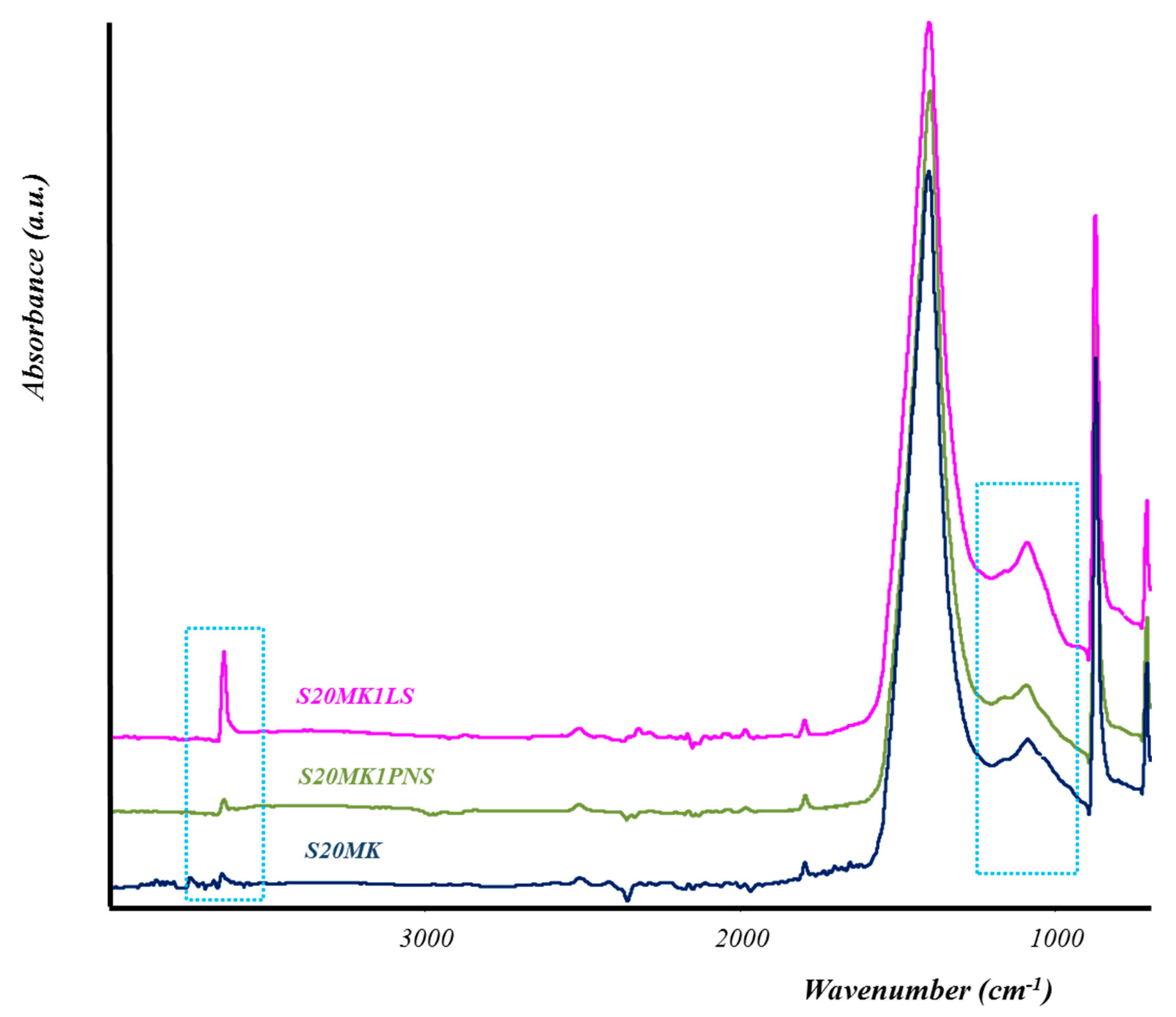
3.2.2. TG-DTA, FTIR-ATR, and XRD Studies
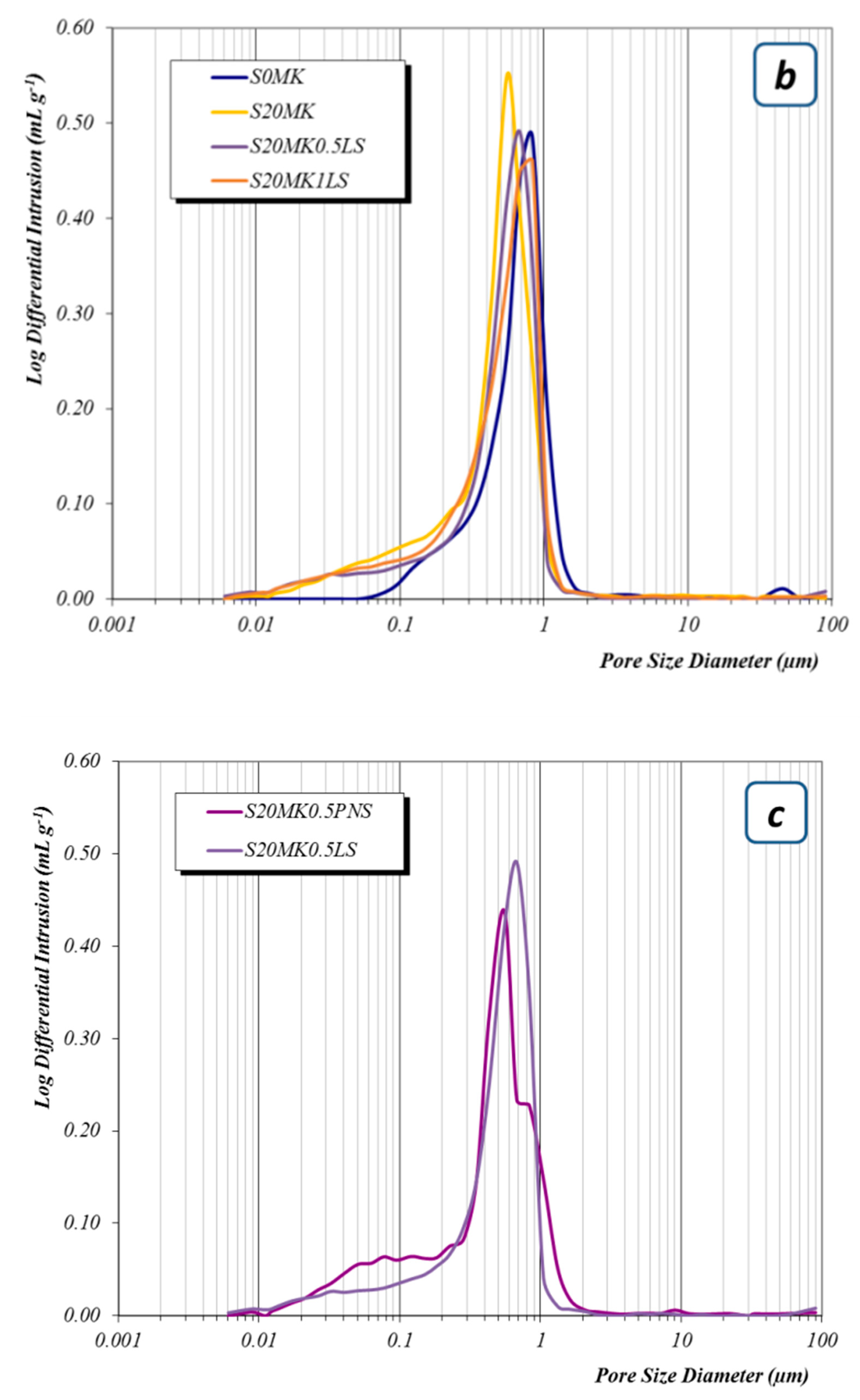
3.2.3. Porosity Measurements
3.3. Durability Experiments
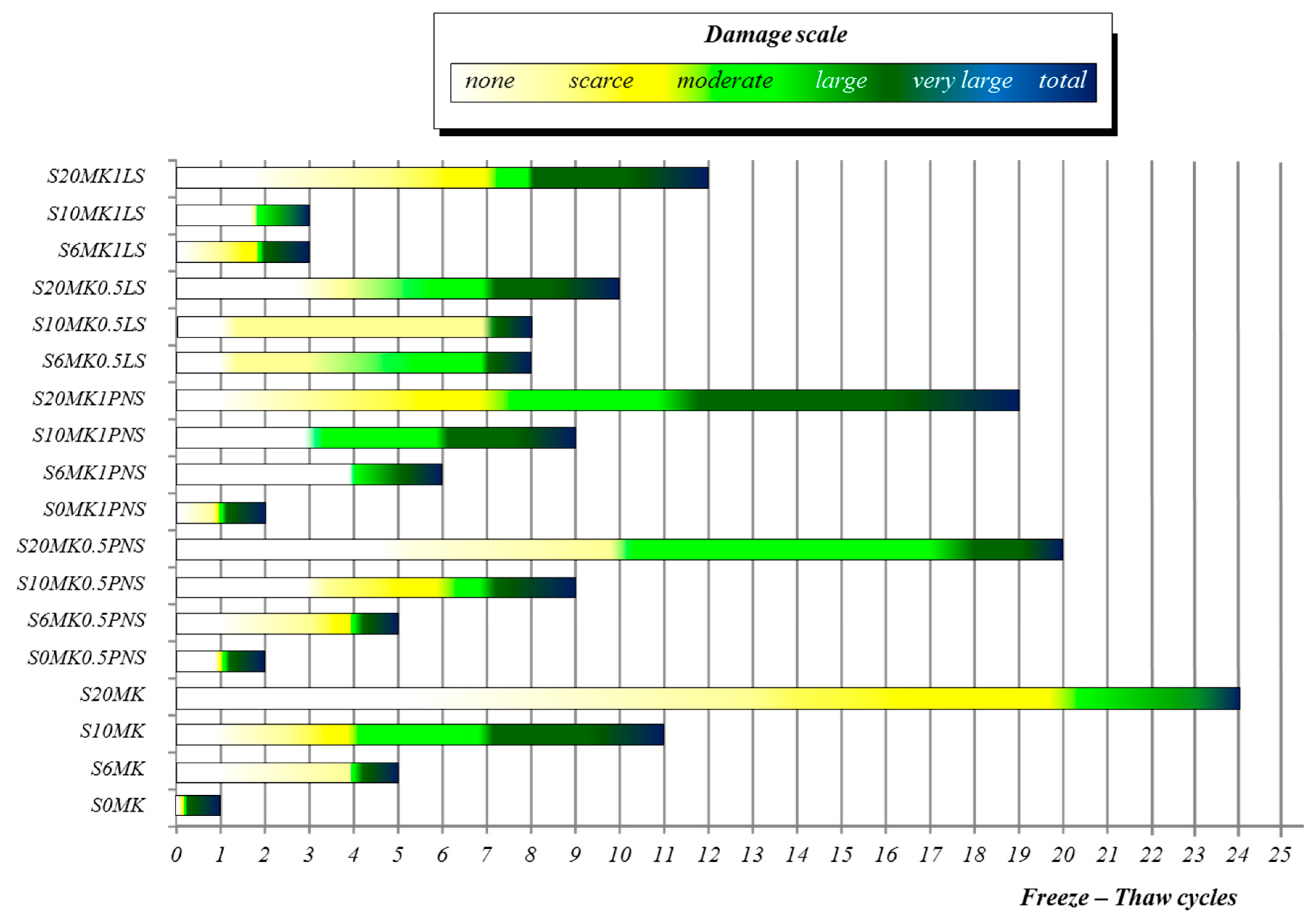
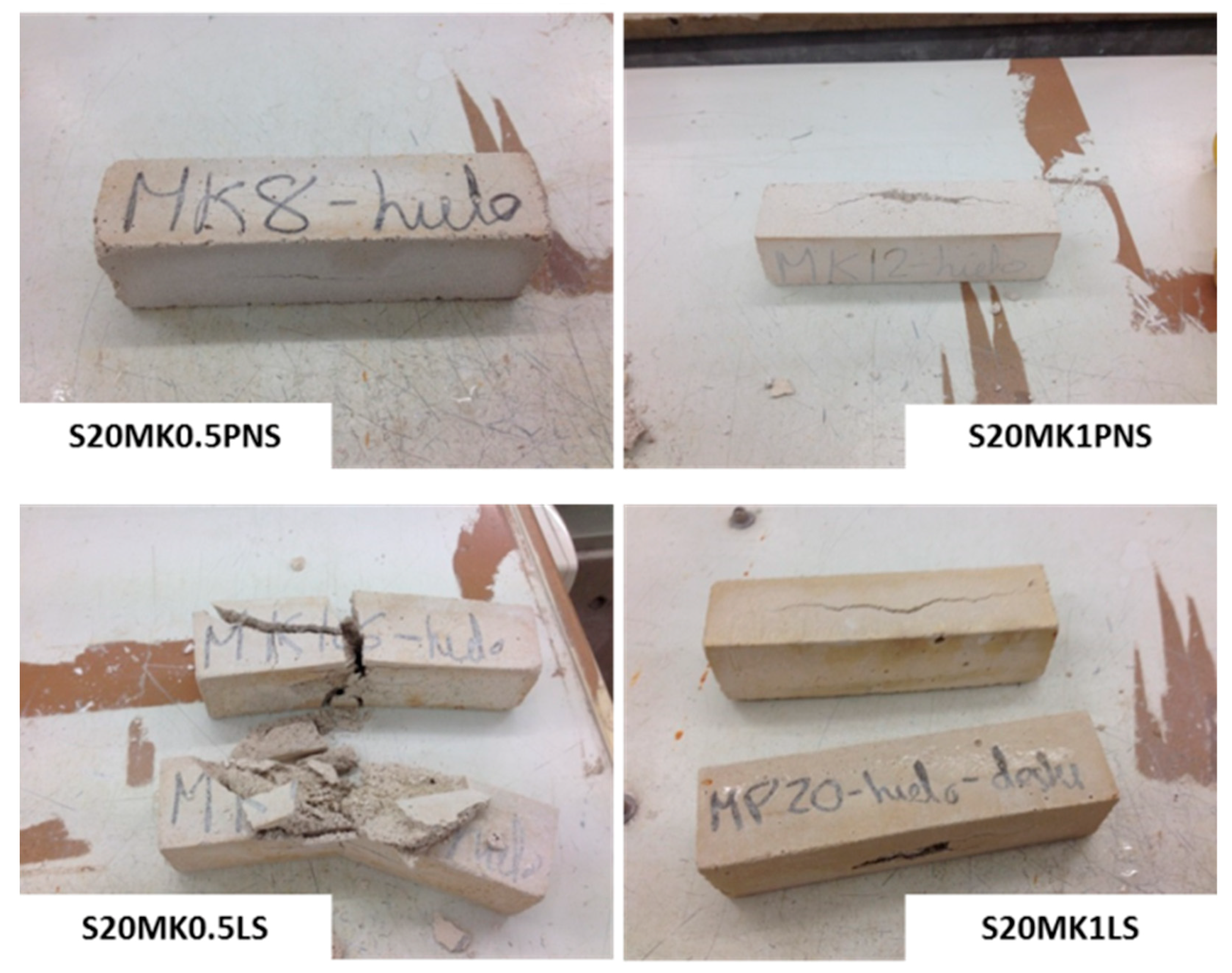
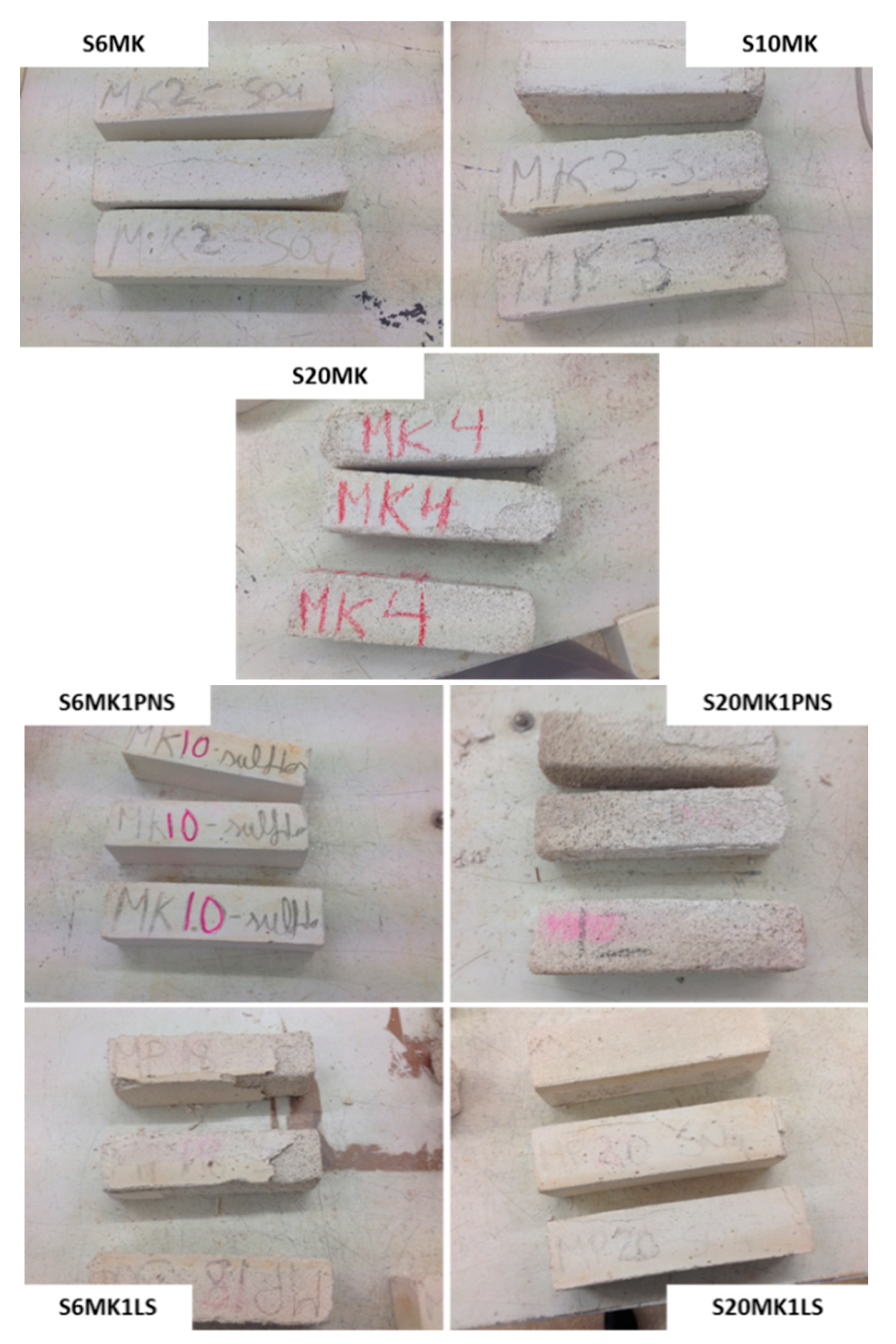
3.3.1. Freezing-Thawing
3.3.2. Magnesium Sulphate Attack
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duran, A.; Robador, M.D.; Perez-Rodriguez, J.L. Degradation of two historic buildings in Northern Spain by formation of oxalate and sulphate-based compounds. Int. J. Archit. Herit. 2012, 6, 342–358. [Google Scholar] [CrossRef]
- Amenta, M.; Karatasios, I.; Maravelaki-Kalaitzaki, P.; Kilikoglou, V. The role of aggregate characteristics on the performance optimization of high hydraulicity restoration mortars. Constr. Build. Mater. 2017, 153, 527–534. [Google Scholar] [CrossRef]
- Borsoi, G.; Lubelli, B.; Van Hees, R.; Veiga, R.; Silva, A.S. Evaluation of the effectiveness and compatibility of nanolime consolidants with improved properties. Constr. Build. Mater. 2017, 142, 385–394. [Google Scholar] [CrossRef]
- Azeiteiro, L.C.; Velosa, A.; Paiva, H.; Mantas, P.Q.; Ferreira, V.M.; Veiga, R. Development of grouts for consolidation of old renders. Constr. Build. Mater. 2014, 50, 352–360. [Google Scholar] [CrossRef]
- Bras, A.; Henriques, F.M.A. Natural hydraulic lime based grouts—The selection of grout injection parameters for masonry consolidation. Constr. Build. Mater. 2012, 26, 135–144. [Google Scholar] [CrossRef]
- Baltazar, L.G.; Henriques, F.M.A.; Jorne, F.; Cidade, M.T. Combined effect of superplasticizer, silica fume and temperature in the performance of natural hydraulic lime grouts. Constr. Build. Mater. 2014, 50, 584–597. [Google Scholar] [CrossRef]
- Baltazar, L.G.; Henriques, F.M.A.; Cidade, M.T. Experimental study and modeling of rheological and mechanical properties of NHL grouts. J. Mater. Civ. Eng. 2015, 27, 04015055. [Google Scholar] [CrossRef]
- Baltazar, L.G.; Henriques, F.M.A.; Jorne, F.; Cidade, M.T. The use of rheology in the study of the composition effects on the fresh behaviour of hydraulic lime grouts for injection of masonry walls. Rheol. Acta 2013, 52, 127–138. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Comp. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Vejmelkova, E.; Keppert, M.; Kersner, Z.; Rovnanikova, P.; Cerny, R. Mechanical, fracture-mechanical, hydric, thermal, and durability properties of lime-metakaolin plasters for renovation of historical buildings. Constr. Build. Mater. 2012, 31, 22–28. [Google Scholar] [CrossRef]
- Frías Rojas, M.; Cabrera, J. The effect of temperature on the hydration rate and stability of the hydration phases of metakaolin-lime-water systems. Cem. Concr. Res. 2002, 32, 133–138. [Google Scholar] [CrossRef]
- Jorne, F.; Henriques, F.M.A.; Baltazar, L.G. Influence of superplasticizer, temperature, resting time and injection pressure on hydraulic lime grout injectability. Correlation analysis between fresh grout parameters and grout injectability. J. Build. Eng. 2015, 4, 140–151. [Google Scholar] [CrossRef]
- Navarro-Blasco, I.; Perez-Nicolas, M.; Fenrandez, J.M.; Duran, A.; Sirera, R.; Alvarez, J.I. Assessment of the interaction of polycarboxylate superplasticizers in hydrated lime pastes modified with nanosilica or metakaolin as pozzolanic reactives. Constr. Build. Mater. 2014, 73, 1–12. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Duran, A.; Navarro-Blasco, I.; Fernandez, J.M.; Sirera, R.; Alvarez, J.I. Study on the effectiveness of PNS and LS superplasticizers in aire lime-based mortars. Cem. Concr. Res. 2016, 82, 11–22. [Google Scholar] [CrossRef]
- Zapata, L.E.; Portela, G.; Suarez, O.M.; Carrasquillo, O. Rheological performance and compressive strength of superplasticized cementitious mixtures with micro/nano-SiO2 additions. Constr. Build. Mater. 2013, 41, 708–716. [Google Scholar] [CrossRef]
- Ng, S.; Justness, H. Influence of plasticizers on the rheology and early heat of hydration of blended cements with high content of fly ash. Cem. Concr. Comp. 2016, 65, 41–54. [Google Scholar] [CrossRef]
- Hallal, A.; Kadri, E.H.; Ezziane, K.; Kadri, A.; Khelafi, H. Combined effect of mineral admixtures with superplasticizers on the fluidity of the blended cement paste. Constr. Build. Mater. 2010, 24, 1418–1423. [Google Scholar] [CrossRef]
- Landrou, G.; Brumaud, C.; Plötze, M.L.; Winnefeld, F.; Habert, G. A fresh look at dense clay paste: Deflocculation and thixotropy mechanisms. Colloid Surf. A 2018, 539, 252–260. [Google Scholar] [CrossRef]
- Brumaud, C.; Baumann, R.; Schmitz, M.; Radler, M.; Roussel, N. Cellulose ethers and yield stress of cement pastes. Cem. Concr. Res. 2014, 55, 14–21. [Google Scholar] [CrossRef]
- Bucher, R.; Diederich, P.; Mouret, M.; Escadeillas, G.; Cyr, M. Self-compacting concrete using flash-metakaolin: Design method. Mater. Struct. 2015, 48, 1717–1737. [Google Scholar] [CrossRef]
- Ouyang, X.; Qiu, X.; Chen, P. Physicochemical characterization of calcium lignosulfonate-a potentially useful water reducer. Colloid Surf. A 2006, 282–283, 489–497. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X. Correlations of the dispersing capability of NSF and PCE types of superplasticizer and their impacts on cement hydration with the adsorption in fresh cement pastes. Cem. Concr. Res. 2015, 69, 1–9. [Google Scholar] [CrossRef]
- Danner, T.; Justnes, H.; Geiker, M.; Lauten, R.A. Phase changes during the early hydration of Portland cement with Ca-lignosulfonates. Cem. Concr. Res. 2015, 69, 50–60. [Google Scholar] [CrossRef]
- Shi, C.; He, T.-S.; Zhang, G.; Wang, X.; Hu, Y. Effects of superplasticizers on carbonation resistance of concrete. Constr. Build. Mater. 2016, 108, 48–55. [Google Scholar] [CrossRef]
- Topçu, I.B.; Atesin, O. Effect of high dosage lignosulphonate and naphthalene sulphonate based plasticizer usage on micro concrete properties. Constr. Build. Mater. 2016, 120, 189–197. [Google Scholar] [CrossRef]
- Arel, H.S.; Aydin, E. Effects of Ca-, Mg-, K-, and Na-lignosulfonates on the behaviour of fresh concrete. Constr. Build. Mater. 2017, 157, 1084–1091. [Google Scholar] [CrossRef]
- Fernández, J.M.; Duran, A.; Navarro-Blasco, I.; Lanas, J.; Sirera, R.; Alvarez, J.I. Influence of nanosilica and a polycarboxylate ether superplasticizer on the performance of lime mortars. Cem. Concr. Res. 2013, 43, 12–24. [Google Scholar] [CrossRef]
- European Committee for Standardization EN 1015-3. Methods of Test for Mortar for Masonry, Part 3: Determination of Consistence of Fresh Mortar (by Flow Table); CEN: Brussels, Belgium, 2000. [Google Scholar]
- Messina, F.; Ferone, C.; Colangelo, F.; Cioffi, R. Low temperature alkaline activation of weathered fly ash: Influence of mineral admixtures on early age performance. Constr. Build. Mater. 2015, 86, 169–177. [Google Scholar] [CrossRef]
- European Committee for Standardization EN 1015-6. Methods of Test for Mortar for Masonry. Part 6: Determination of Bulk Density of Fresh Mortar; CEN: Brussels, Belgium, 1999. [Google Scholar]
- European Committee for Standardization EN 1015-7. Methods of Test for Mortar for Masonry. Part 7: Determination of Air Content of Fresh Mortar; CEN: Brussels, Belgium, 1999. [Google Scholar]
- European Committee for Standardization EN 1015-9. Methods of Test for Mortar for Masonry, Part 9: Determination of Workable Life and Correction Time of Fresh Mortar; CEN: Brussels, Belgium, 2000. [Google Scholar]
- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements. J. Colloid Interface Sci. 2010, 347, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.M.; Puertas, F. Adsorption of PCE and PNS superplasticisers on cubic and orthorhombic C3A. Effect of sulfate. Constr. Build. Mater. 2015, 78, 324–332. [Google Scholar] [CrossRef]
- Duran, A.; Navarro-Blasco, I.; Fernandez, J.M.; Alvarez, J.I. Long-term mechanical resistance and durability of air lime mortars. Constr. Build. Mater. 2014, 58, 147–158. [Google Scholar] [CrossRef]
- Lanas, J.; Sirera, R.; Alvarez, J.I. Study of the mechanical behavior of masonry repair lime-based mortars cured and exposed under different conditions. Cem. Concr. Res. 2006, 36, 961–970. [Google Scholar] [CrossRef]
- European Committee for Standardization EN 1015-11. Methods of Test for Mortar for Masonry. Part 11—Determination of Flexural and Compressive Strength of Hardened Mortars; CEN: Brussels, Belgium, 2007. [Google Scholar]
- Sonebi, M. Rheological properties of grouts with viscosity modifying agents as diutan gum and welan gum incorporating pulverised fly ash. Cem. Concr. Res. 2006, 36, 1609–1618. [Google Scholar] [CrossRef]
- Janowska-Renkas, E. The effect of superplasticizers’ chemical structure on their efficiency in cement pastes. Constr. Build. Mater. 2013, 38, 1204–1210. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Sisomphon, K.; Ng, T.S.; Sun, D.J. Effect of superplasticizers on workability retention and initial setting time of cement pastes. Constr. Build. Mater. 2010, 24, 1700–1707. [Google Scholar] [CrossRef]
- Blank, B.; Rossington, D.R.; Weinland, L.A. Adsorption of Admixtures on Portland Cement. J. Am. Ceram. Soc. 1963, 46, 395–399. [Google Scholar] [CrossRef]
- Mittal, A.; Kurup, L.; Mittal, J. Freundlich and Langmuir adsorption isotherms and kinetics for the removal of Tartrazine from aqueous solutions using hen feathers. J. Hazard Mater. 2007, 146, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Recalde-Lummer, N.; Plank, J. Combination of lignosulfonate and AMPS-co-NNDMA water retention agent—An example for dual synergistic interaction between admixtures in cement. Cem. Concr. Res. 2012, 42, 728–735. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zheng, J.; Guo, H.; Yang, C.; Li, Z.; Lu, M. Dispersion and rheological properties of concentrated kaolin suspensions with polycarboxylate copolymers bearing comb-like side chains. J. Eur. Ceram. Soc. 2014, 34, 137–146. [Google Scholar] [CrossRef]
- Pickelmann, J.; Plank, J. A mechanistic study explaining the synergistic viscosity increase obtained from polyethylene oxide (PEO) and β-naphthalene sulfonate (BNS) in shotcrete. Cem. Concr. Res. 2012, 42, 1409–1416. [Google Scholar] [CrossRef]
- Uchikawa, H.; Hanehara, S.; Shirasaka, T.; Sawaki, D. Effect of admixture on hydration of cement, adsorptive behaviour of admixture and fluidity and setting of fresh cement paste. Cem. Concr. Res. 1992, 22, 1115–1129. [Google Scholar] [CrossRef]
- Vikan, H.; Justnes, H.; Figi, R. Adsorption of plasticizers—Influence of plasticizer and cement type. Ann. Trans. Nord. Rheol. Soc. 2005, 13, 127–134. [Google Scholar]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Ortega-Huertas, M.; Hansen, E. Nanostructure and irreversible coloidal behavior of Ca(OH)2: Implications in cultural heritage conservation. Langmuir 2005, 21, 10948–10957. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, A.; Santos Silva, A.; Faria, P.; Grilo, J.; Branco, T.; Veiga, R.; Velosa, A. Physical and chemical assessment of lime-metakaolin mortars: Influence of binder:aggregate ratio. Cem. Concr. Comp. 2014, 45, 264–271. [Google Scholar] [CrossRef]
- El-Gamal, S.M.A.; Amin, M.S.; Ramadan, M. Hydration characteristics and compressive strength of hardened cement pastes containing nano-metakaolin. HRB J. 2017, 13, 114–121. [Google Scholar] [CrossRef]
- Abbas, R.; Abo-El-Enein, S.A.; Ezzat, E.-S. Properties and durability of metakaolin blended cements: Mortar and concrete. Mater. Construct. 2010, 60, 33–49. [Google Scholar] [CrossRef]
- Aguilar-Penagos, A.; Gómez-Soberón, J.M.; Rojas-Valencia, M.N. Physicochemical, Mineralogical and Microscopic Evaluation of Sustainable Bricks Manufactured with Construction Wastes. Appl. Sci. 2017, 7, 1012. [Google Scholar] [CrossRef]
- Lanas, J.; Sirera, R.; Alvarez, J.I. Compositional changes in lime-based mortars exposed to different environments. Thermochim. Acta 2005, 429, 219–226. [Google Scholar] [CrossRef]
- Gameiro, A.; Santos Silva, A.; Veiga, R.; Velosa, A. Hydration products of lime-metakaolin pastes at ambient temperature with ageing. Thermochim. Acta 2012, 535, 36–41. [Google Scholar] [CrossRef]
- Santos Silva, A.; Gameiro, A.; Grilo, J.; Veiga, R.; Velosa, A. Long-term behavior of lime-metakaolin pastes at ambient temperature and humid curing condition. Appl. Clay Sci. 2014, 88–89, 49–55. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Shah, S.P.; Mishra, G.; Ahalawat, S.; Sharma, U. Studies on early stage hydration of tricalcium silicate incorporating silica nanoparticles: Part I. Constr. Build. Mater. 2015, 74, 278–286. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Frías Rojas, M. Study of hydrated phases present in a MK-lime system cured at 60 ºC and 60 months of reaction. Cem. Concr. Res. 2006, 36, 827–831. [Google Scholar] [CrossRef]
- Mendivil-Escalante, J.M.; Gómez-Soberón, J.M.; Almaral-Sánchez, J.L.; Cabrera-Covarrubias, F.G. Metamorphosis in the Porosity of Recycled Concretes Through the Use of a Recycled Polyethylene Terephthalate (PET) Additive. Correlations between the Porous Network and Concrete Properties. Materials 2017, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Dinakar, P.; Sahoo, P.K.; Sriram, G. Effect of metakaolin content on the properties of high strength concrete. Int. J. Concr. Struct. Mater. 2013, 7, 215–223. [Google Scholar] [CrossRef]
- Drouet, E.; Poyet, S.; Torrenti, J.-M. Temperature influence on water transport in hardened cement pastes. Cem. Concr. Res. 2015, 76, 37–50. [Google Scholar] [CrossRef]
- Izaguirre, A.; Lanas, J.; Alvarez, J.I. Ageing of lime mortars with admixtures: Durability and strength assessment. Cem. Concr. Res. 2010, 40, 1081–1095. [Google Scholar] [CrossRef]
- Nunes, C.; Slizkova, Z. Freezing and thawing resistance of aerial lime mortar with metakaolin and a traditional water-repellent admixture. Constr. Build. Mater. 2016, 114, 896–905. [Google Scholar] [CrossRef]
- Skaropoulou, A.; Tsivilis, S.; Kakali, G.; Sharp, J.H.; Swamy, R.N. Long-term behaviour of Portland limestone cement mortars exposed to magnesium sulfate attack. Cem. Concr. Comp. 2009, 31, 628–636. [Google Scholar] [CrossRef]
- Aziez, M.N.; Bezzar, A. Magnesium Sulphate Attacks on Mortars—Influence of Temperature, Type of Sand and Type of Cement. J. Eng. Sci. Technol. Rev. 2017, 10, 41–50. [Google Scholar] [CrossRef]
- Lee, S.T.; Moon, H.Y.; Swamy, R.N. Sulfate attack and role of silica fume in resisting strength loss. Cem. Concr. Comp. 2005, 27, 65–76. [Google Scholar] [CrossRef]
- Ganjian, E.; Pouya, H.S. Effect of magnesium and sulfate ions on durability of silica fume blended mixes exposed to the seawater tidal zone. Cem. Concr. Res. 2005, 35, 1332–1343. [Google Scholar] [CrossRef]
- Diab, H.M.; Abd Elmoaty, M.; Abd Elmoaty, M.A.E. Long term study of mechanical properties, durability and environmental impact of limestone cement concrete. Alexandria Eng. J. 2016, 55, 1465–1482. [Google Scholar] [CrossRef]







| Samples | MK (g) | LS (g) | PNS (g) |
|---|---|---|---|
| S0MK (control group) | 0 | 0 | 0 |
| S0MK0.5LS | 0 | 2.5 | 0 |
| S0MK0.5PNS | 0 | 0 | 2.5 |
| S0MK1LS | 0 | 5 | 0 |
| S0MK1PNS | 0 | 0 | 5 |
| S6MK | 30 | 0 | 0 |
| S6MK0.5LS | 30 | 2.5 | 0 |
| S6MK0.5PNS | 30 | 0 | 2.5 |
| S6MK1LS | 30 | 5 | 0 |
| S6MK1PNS | 30 | 0 | 5 |
| S10MK | 50 | 0 | 0 |
| S10MK0.5LS | 50 | 2.5 | 0 |
| S10MK0.5PNS | 50 | 0 | 2.5 |
| S10MK1LS | 50 | 5 | 0 |
| S10MK1PNS | 50 | 0 | 5 |
| S20MK | 100 | 0 | 0 |
| S20MK0.5LS | 100 | 2.5 | 0 |
| S20MK0.5PNS | 100 | 0 | 2.5 |
| S20MK1LS | 100 | 5 | 0 |
| S20MK1PNS | 100 | 0 | 5 |
| Samples | Bulk Density of the Fresh Paste (g·mL−1) | Air Content (%) |
|---|---|---|
| S0MK (control group) | 1.89 | 3.2 |
| S0MK0.5LS | 1.90 | 3.4 |
| S0MK0.5PNS | 1.90 | 2.6 |
| S0MK1LS | 1.89 | 3.4 |
| S0MK1PNS | 1.93 | 1.6 |
| S6MK | 1.89 | 3.0 |
| S6MK0.5LS | 1.89 | 3.2 |
| S6MK0.5PNS | 1.88 | 3.0 |
| S6MK1LS | 1.89 | 3.3 |
| S6MK1PNS | 1.90 | 2.2 |
| S10MK | 1.89 | 3.3 |
| S10MK0.5LS | 1.89 | 3.2 |
| S10MK0.5PNS | 1.89 | 2.7 |
| S10MK1LS | 1.88 | 3.4 |
| S10MK1PNS | 1.89 | 2.5 |
| S20MK | 1.87 | 3.1 |
| S20MK0.5LS | 1.87 | 3.4 |
| S20MK0.5PNS | 1.89 | 3.0 |
| S20MK1LS | 1.87 | 3.2 |
| S20MK1PNS | 1.89 | 3.0 |
| Polynaphthalene Sulfonate (PNS) | ||||||
| Langmuir | Freundlich | |||||
| qm (mg·g−1) | b | R2 | K | 1/n | R2 | |
| S0MK | 51.2 | 0.00011 | 0.7738 | 0.01210 | 0.8658 | 0.9757 |
| S6MK | 46.9 | 0.00011 | 0.8019 | 0.01215 | 0.8582 | 0.9768 |
| S10MK | 43.4 | 0.00012 | 0.8469 | 0.01247 | 0.8490 | 0.9775 |
| S20MK | 44.7 | 0.00010 | 0.7458 | 0.00986 | 0.8708 | 0.9766 |
| Lignosulfonate (LS) | ||||||
| Langmuir | Freundlich | |||||
| qm (mg·g−1) | b | R2 | K | 1/n | R2 | |
| S0MK | 32.1 | 0.00016 | 0.9509 | 0.01983 | 0.7812 | 0.9775 |
| S6MK | 28.7 | 0.00018 | 0.9242 | 0.01837 | 0.7830 | 0.9735 |
| S10MK | 31.5 | 0.00015 | 0.9647 | 0.01582 | 0.7977 | 0.9825 |
| S20MK | 29.1 | 0.00015 | 0.9400 | 0.01346 | 0.8076 | 0.9809 |
| Samples | Weight Loss (%) | ||||
|---|---|---|---|---|---|
| 7 Days | 28 Days | 91 Days | 182 Days | 365 Days | |
| S0MK (Plain lime) | 0.38 | 0.74 | 0.45 | 0.30 | 0.31 |
| S20MK | 1.03 | 0.88 | 0.57 | 0.73 | 0.66 |
| S20MK1PNS | 0.76 | 1.00 | 0.75 | 0.93 | 0.58 |
| S20MK1LS | 0.51 | 0.70 | 0.61 | 0.76 | 0.80 |
| Number of FT Cycles | ||||
|---|---|---|---|---|
| Samples | 5 | 10 | 15 | 20 |
| S20MK1LS | 2 | 4 | 5 | - |
| S10MK1LS | 5 | - | - | - |
| S6MK1LS | 5 | - | - | - |
| S20MK0.5LS | 3 | 5 | - | - |
| S10MK0.5LS | 3 | 5 | - | - |
| S6MK0.5LS | 4 | 5 | - | - |
| S20MK1PNS | 2 | 3 | 4 | 5 |
| S10MK1PNS | 3 | 5 | - | - |
| S6MK1PNS | 4 | 5 | - | - |
| S20MK0.5PNS | 0 | 2 | 4 | 5 |
| S10MK0.5PNS | 3 | 5 | - | - |
| S6MK0.5PNS | 5 | - | - | - |
| S0MK0.5PNS | 5 | - | - | - |
| S20MK | 0 | 1 | 2 | 3 |
| S10MK | 3 | 4 | 5 | - |
| S6MK | 5 | - | - | - |
| S0MK (control group) | 5 | - | - | - |
| Number of Sulphate Attack Cycles | |||||
|---|---|---|---|---|---|
| Samples | 5 | 10 | 15 | 20 | 25 |
| S20MK1LS | 1 | 3 | 3 | 4 | 5 |
| S10MK1LS | 4 | 4 | 4 | 5 | - |
| S6MK1LS | 4 | 5 | - | - | - |
| S20MK0.5LS | 0 | 1 | 2 | 3 | 3 |
| S10MK0.5LS | 1 | 2 | 4 | 4 | 4 |
| S6MK0.5LS | 1 | 2 | 4 | 4 | 4 |
| S20MK1PNS | 4 | 5 | - | - | - |
| S10MK1PNS | 1 | 4 | 4 | 5 | - |
| S6MK1PNS | 0 | 1 | 2 | 3 | 4 |
| S20MK0.5PNS | 2 | 5 | - | - | - |
| S10MK0.5PNS | 1 | 2 | 4 | 4 | 5 |
| S6MK0.5PNS | 3 | 5 | - | - | - |
| S0MK0.5PNS | 0 | 2 | 4 | 5 | - |
| S20MK | 3 | 5 | - | - | - |
| S10MK | 4 | 4 | 5 | - | - |
| S6MK | 1 | 2 | 2 | 3 | 3 |
| S0MK (control group) | 1 | 5 | - | - | - |
| Samples | Phase (wt %) | |||||
|---|---|---|---|---|---|---|
| Calcite | Portlandite | Brucite | Quartz | Gypsum | Hexahydrite | |
| S20MK | 86.2 | - | - | 0.7 | 5.6 | 7.5 |
| S20MK1PNS | 82.6 | 0.4 | 1.0 | 0.4 | 10.3 | 5.3 |
| S20MK1LS | 81.1 | 4.3 | 3.0 | 0.4 | 9.1 | 2.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duran, A.; González-Sánchez, J.F.; Fernández, J.M.; Sirera, R.; Navarro-Blasco, Í.; Alvarez, J.I. Influence of Two Polymer-Based Superplasticizers (Poly-naphthalene Sulfonate, PNS, and Lignosulfonate, LS) on Compressive and Flexural Strength, Freeze-Thaw, and Sulphate Attack Resistance of Lime-Metakaolin Grouts. Polymers 2018, 10, 824. https://doi.org/10.3390/polym10080824
Duran A, González-Sánchez JF, Fernández JM, Sirera R, Navarro-Blasco Í, Alvarez JI. Influence of Two Polymer-Based Superplasticizers (Poly-naphthalene Sulfonate, PNS, and Lignosulfonate, LS) on Compressive and Flexural Strength, Freeze-Thaw, and Sulphate Attack Resistance of Lime-Metakaolin Grouts. Polymers. 2018; 10(8):824. https://doi.org/10.3390/polym10080824
Chicago/Turabian StyleDuran, Adrián, Jesús F. González-Sánchez, José M. Fernández, Rafael Sirera, Íñigo Navarro-Blasco, and José I. Alvarez. 2018. "Influence of Two Polymer-Based Superplasticizers (Poly-naphthalene Sulfonate, PNS, and Lignosulfonate, LS) on Compressive and Flexural Strength, Freeze-Thaw, and Sulphate Attack Resistance of Lime-Metakaolin Grouts" Polymers 10, no. 8: 824. https://doi.org/10.3390/polym10080824
APA StyleDuran, A., González-Sánchez, J. F., Fernández, J. M., Sirera, R., Navarro-Blasco, Í., & Alvarez, J. I. (2018). Influence of Two Polymer-Based Superplasticizers (Poly-naphthalene Sulfonate, PNS, and Lignosulfonate, LS) on Compressive and Flexural Strength, Freeze-Thaw, and Sulphate Attack Resistance of Lime-Metakaolin Grouts. Polymers, 10(8), 824. https://doi.org/10.3390/polym10080824