Preparation and Characterization of Highly Ordered Mercapto-Modified Bridged Silsesquioxane for Removing Ammonia-Nitrogen from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
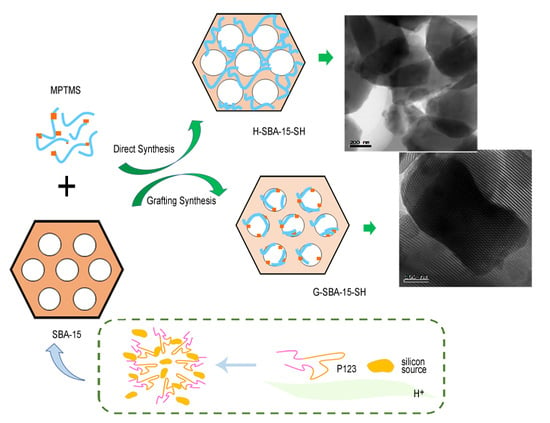
2.2. Direct Synthesis in Hot Water
2.3. Grafting Synthesis
2.4. Structure Characterization
2.5. Effect of the Initial Concentration of Ammonia Nitrogen on the Adsorption of Ammonia Nitrogen
2.6. Statistical Analysis
3. Results and Discussion
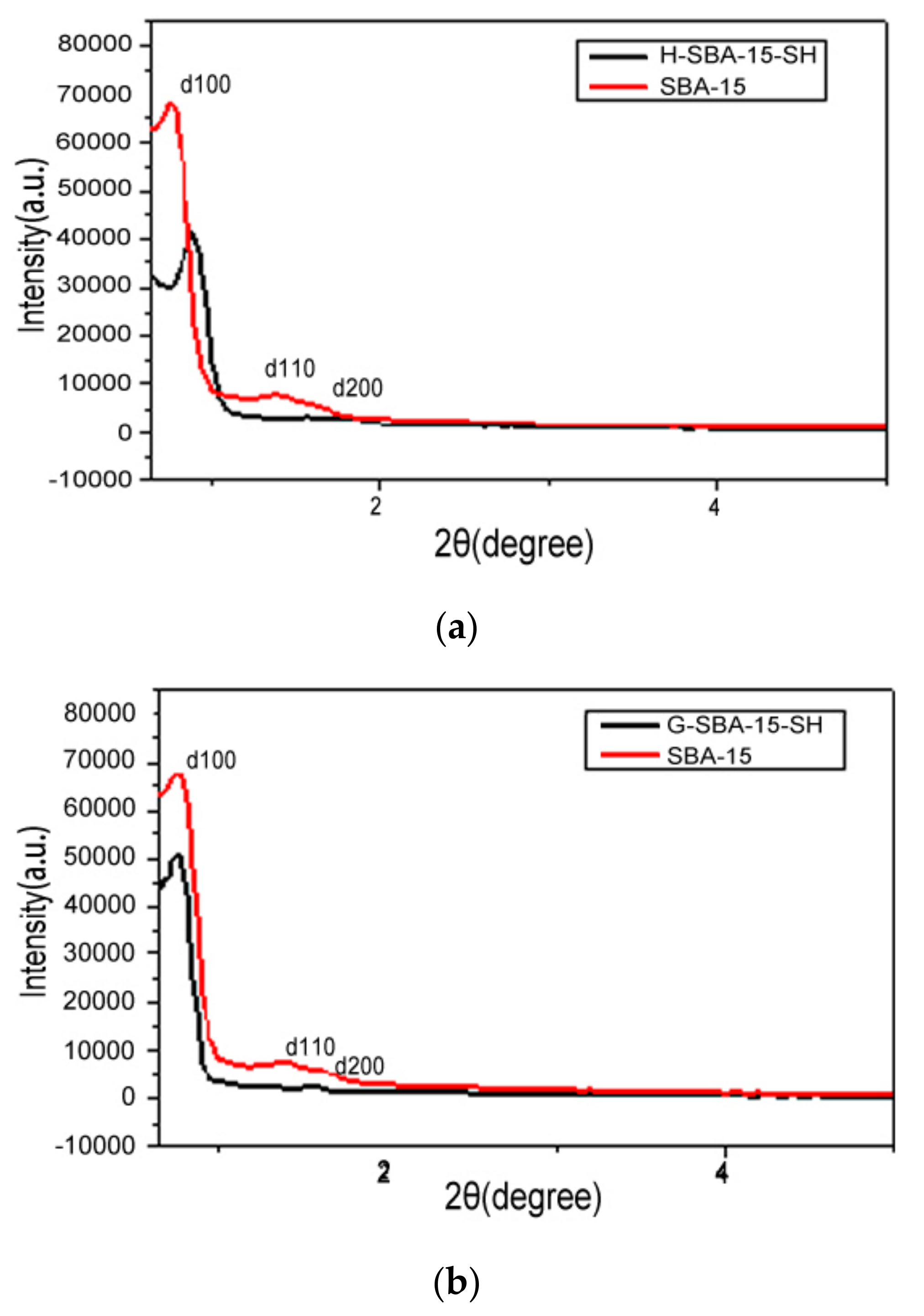
3.1. X-Ray Diffraction
3.2. Fourier Transform Infrared Spectroscopy
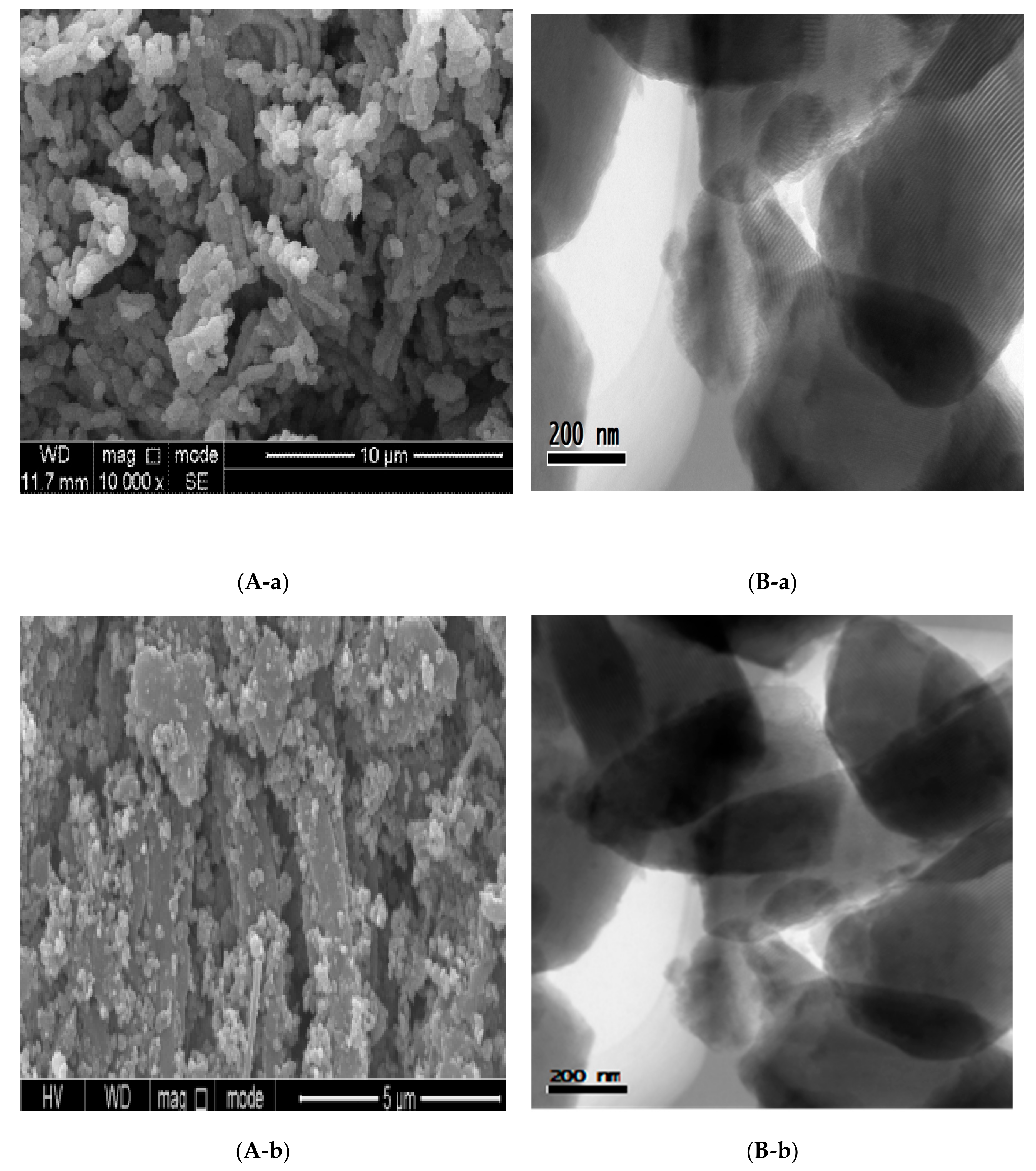
3.3. Scanning Electron Microscopy
3.4. Transmission Electron Microscopy
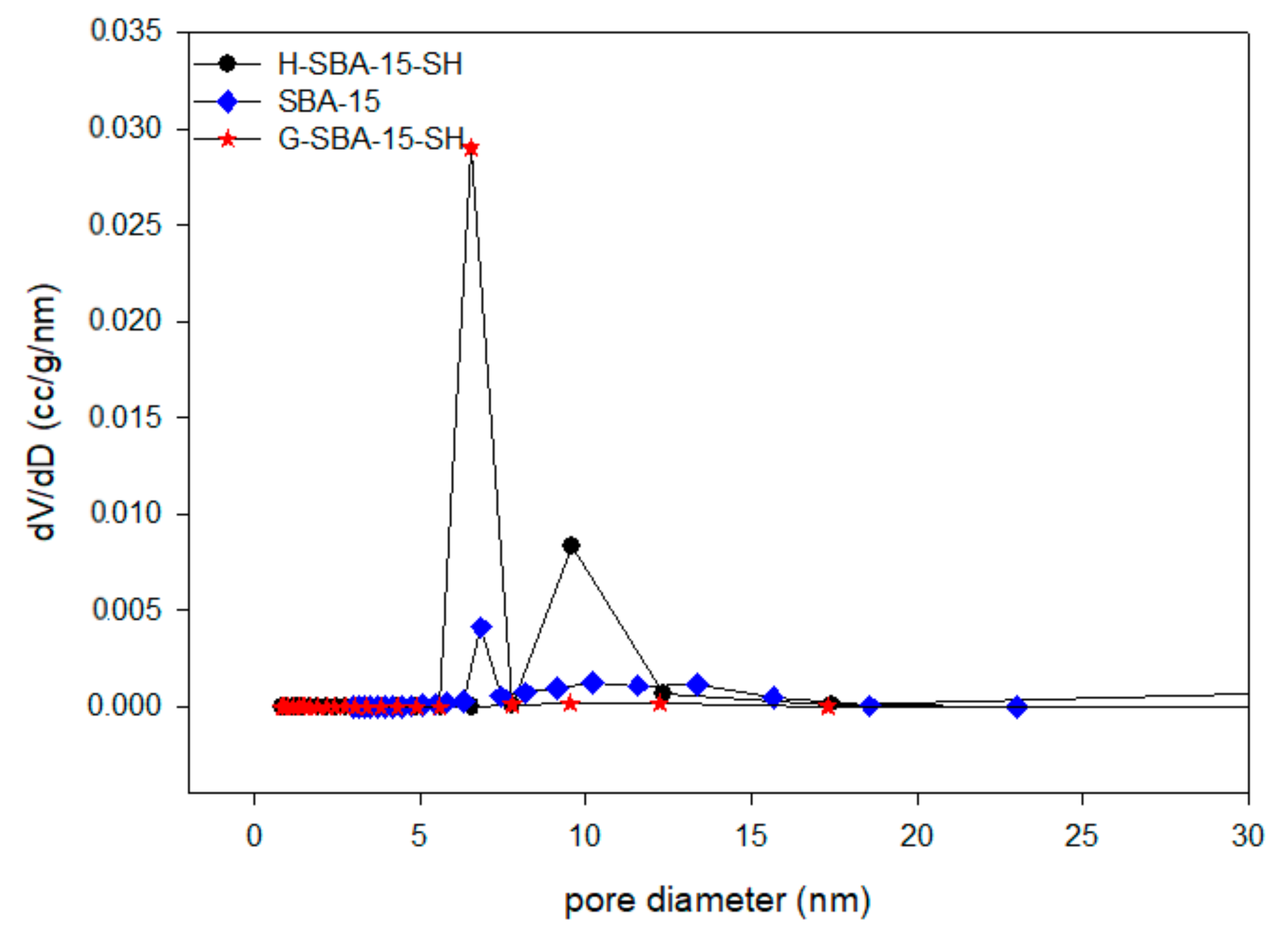
3.5. Analysis of Pore Size Distribution
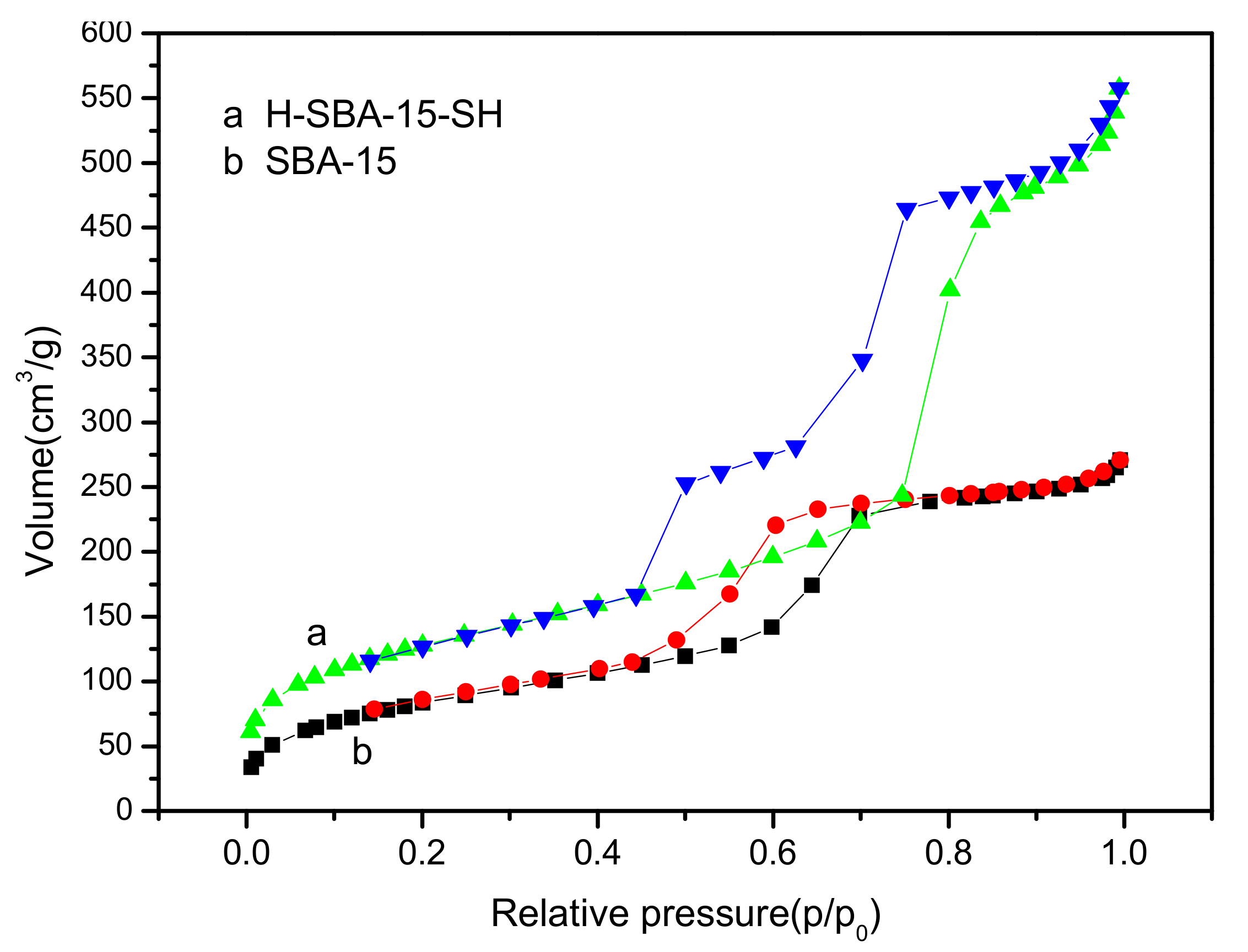
3.6. Analysis of Nitrogen Adsorption–Desorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lin, D.R.; Hu, L.J.; You, H.; Tolbert, S.H.; Loy, D.A. Comparison of new periodic, mesoporous, hexylene-bridged polysilsesquioxanes with Pm3n symmetry versus sol-gel polymerized, hexylene-bridged gels. J. Non-Cryst. Solids 2014, 406, 139–143. [Google Scholar] [CrossRef]
- Lin, D.R.; Hu, L.J.; Tolbert, S.H.; Li, Z.; Loy, D.A. Controlling nanostructure in periodic mesoporous hexylene-bridged polysilsesquioxanes. J. Non-Cryst. Solids 2015, 419, 6–11. [Google Scholar] [CrossRef]
- Lin, D.R.; Hu, L.J.; Li, Z.; Loy, D.A. Influence of alkylene-bridging group length on mesostructure and porosity in cubic (Pm3n) periodic mesoporous bridged polysilsesquioxanes. J. Porous Mater. 2014, 21, 39–44. [Google Scholar] [CrossRef]
- Lin, D.R.; Hu, L.J.; You, H.; Williams, R.J.J. Synthesis and characterization of a nanostructured photoluminescent silsesquioxane containing urea and dodecyl groups that can be patterned on carbon films. Eur. Polym. J. 2011, 47, 1526–1533. [Google Scholar] [CrossRef]
- Lin, D.R.; Zhao, Q.; Hu, L.J.; Xing, B.S. Synthesis and characterization of cubic mesoporous bridged polysilsesquioxane for removing organic pollutants from water. Chemosphere 2014, 103, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.C.; Shea, K.J. Organo-silica hybrid functional nanomaterials: How do organic bridging groups and silsesquioxane moieties work hand-in-hand? Chem. Soc. Rev. 2011, 40, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.J.; Moreau, J.; Loy, D.A.; Corriu, R.J.P.; Boury, B. Bridged Polysilsesquioxanes. Molecular-engineering nanostructured hybrid organic-inorganic materials. In Functional Hybrid Materials; Gómez-Romero, P., Sanchez, C., Eds.; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Loy, D.A. Sol-gel processing of hybrid organic-inorganic materials based on polysilsesquioxanes. In Hybrid Materials: Synthesis, Characterization, and Applications; Kickelbick, G., Ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Waiman, C.V.; Chesta, C.A.; Gómez, M.L. Hybrid films based on a bridged silsesquioxane doped with goethite and montmorillonite nanoparticles as sorbents of wastewater contaminants. J. Nanomater. 2016, 40, 6286247. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Wang, T.; Liu, L. Influence of fibre surface oxidation–reduction followed by silsesquioxane coating treatment on interfacial mechanical properties of carbon fibre/polyarylacetylene composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 936–944. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Zhang, W.B. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Dong, X.H.; Yu, X.; Guo, K.; Su, H.; Zhang, W.B. Giant gemini surfactants based on polystyrene–hydrophilic polyhedral oligomeric silsesquioxane shape amphiphiles: Sequential “click” chemistry and solution self-assembly. Chem. Sci. 2013, 4, 1345–1352. [Google Scholar] [CrossRef]
- Lin, D.R.; Hu, L.J.; Xing, B.S.; You, H.; Loy, D.A. Mechanisms of Competitive Adsorption Organic Pollutants on Hexylene-Bridged Polysilsesquioxane. Materials 2015, 8, 5806–5817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.R.; Kong, M.; Li, L.Y.; Li, X.D.; Zhang, X.W. Enhanced Anti-Ultraviolet and Thermal Stability of a Pesticide via Modification of a Volatile Organic Compound (VOC)-Free Vinyl-Silsesquioxane in Desert Areas. Polymers 2016, 8, 282. [Google Scholar] [CrossRef]
- Lin, D.R.; Hu, L.J.; Zhang, Q.; You, H. Design of a POSS-modified zeolite structure and the study of the enhancement of ammonia-nitrogen removal from drinking water. In Molecular Environmental Soil Science at the Interfaces in the Earth’s Critical Zone; Springer: Berlin/Heidelberg, Germany, 2010; pp. 118–120. [Google Scholar]
- Wu, B.P.; Qi, Y.T.; Yuan, X.D.; Shen, J.; Bi, J.; Li, C. Synthesis of tributyl citrate over B-SBA-15 mesoprous molecular sieve catalyst. Ind. Catal. 2004, 12, 32–35. (In Chinese) [Google Scholar]
- Liu, T.H. Preparation of Silcequioxane Hybrid Material from Rice Hush and Properity Study; Harbin Institute of Technology: Harbin, China, 2007. (In Chinese) [Google Scholar]
- Zhai, Q.Z.; Wang, W.; Jiang, T.S.; Wang, Y.; Chen, M.G. Modification for SBA-15 Molecular sieve by Lanthanum(III). J. Inorg. Mater. 2004, 19, 1212–1216, In Chinese. [Google Scholar]
- Coutinho, D.; Aeevedo, A.O.; Dieekmann, G.R.; Balkus, K.J., Jr. Molecular imprinting of mesoporous SBA-15 with chiral ruthenium complexes. Microporous Mesoporous Mater. 2002, 54, 297–302. [Google Scholar] [CrossRef]
- Reddy, S.S.; Raju, B.D.; Kumar, V.S.; Padmasri, A.H.; Narayanan, S.; Rao, K.S.R. Sulfonic acid funetionalized mesoporous SBA-15 for selective synthesis of 4-phenyl-1,3-dioxane. Catal. Conunun. 2007, 8, 261–266. [Google Scholar] [CrossRef]
- Asefa, T.; MacLachlan, M.J.; Grondey, H.; Coombs, N.; Ozin, J.A. Metamorphic channels in periodic mesoporous methylenesilica. Angew. Chem. Int. 2000, 112, 1878–1881. [Google Scholar] [CrossRef]
- Sanchez, C. Design of functional materials: From nanostructured hybrid materials to hierarchical structures. Abstr. Pap. Am. Chem. Soc. 2004, 228, U490. [Google Scholar]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New family of mesoporous molecular-sieves prepared with liquid-crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular-sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- He, Q.; Shi, J.; Zhao, J.; Chen, Y.; Chen, F. Bottom-up tailoring of nonionic surfactant-templated mesoporous silica nanomaterials by a novel composite liquid crystal templating mechanism. J. Mater. Chem. 2009, 19, 6498–6503. [Google Scholar] [CrossRef]
- Wu, B.; Tong, Z.; Yuan, X. Synthesis, characterization and catalytic application of mesoporous molecular sieves SBA-15 functionalized with phosphoric acid. J. Porous Mater. 2012, 19, 641–647. [Google Scholar] [CrossRef]
- Ziolek, M.; Nowak, I. Synthesis and characterization of niobium-containing MCM-41. Zeolites 1997, 18, 356–360. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Structural and surface properties of siliceous and titanium-modified HMS molecular sieves. Microporous Mater. 1997, 9, 173–182. [Google Scholar] [CrossRef]
- Leary, J.J.; Messick, E.B. Constrained calibration curves: A novel application of Lagrange multipliers in analytical chemistry. Anal. Chem. 1985, 57, 956–957. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distribution of pore volume and area distributions in porous substances 1 computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Ciesla, U.; Schuth, F. Ordered mesoporous materials. Microporous Mesoporous Mater. 1999, 27, 131–149. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Wei, D.; Chueh, W.T.; Haller, G.L.; Neimark, A.V. Evaluation of pore structure parameters of MCM-41 catalyst supports and catalysts by means of nitrogen and argon adsorption. J. Phys. Chem. B 1997, 101, 3671–3679. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Odomhnaill, S.C.; Neimark, A.V.; Schüth, F.; Unger, K.K. Capillary hysteresis in nanopores: Theoretical and experimental studies of nitrogen adsorption on MCM-41. Langmuir 1995, 11, 4765–4772. [Google Scholar] [CrossRef]
- Maddox, M.W.; Olivier, J.P.; Gubbins, K.E. Characterization of MCM-41 using molecular simulation: Heterogeneity effects. Langmuir 1997, 13, 1737–1745. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Relations between structural parameters and adsorption characterization of templated nanoporous materials with cubic symmetry. Langmuir 2000, 16, 2419–2423. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas solid systems with special reference to the determination of surface-area and porosity (recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Ghosh, B.K.; Hazra, S.; Naik, B.; Ghosh, N.N. Preparation of Cu nanoparticle loaded SBA-15 and their excellent catalytic activity in reduction of variety of dyes. Powder Technol. 2015, 269, 371–378. [Google Scholar] [CrossRef]
- Macina, D.; Piwowarska, Z.; Góra-Marek, K.; Tarach, K.; Rutkowska, M.; Girman, V.; Błachowski, A.; Chmielarz, L. SBA-15 loaded with iron by various methods as catalyst for DeNOx process. Mater. Res. Bull. 2016, 78, 72–82. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Dai, C.; Shi, H.; Li, J. Adsorption of Pb2+ on amino-functionalized core–shell magnetic mesoporous SBA-15 silica composite. Chem. Eng. J. 2015, 262, 897–903. [Google Scholar] [CrossRef]
- Hashemikia, S.; Hemmatinejad, N.; Ahmadi, E.; Montazer, M. Optimization of tetracycline hydrochloride adsorption on amino modified SBA-15 using response surface methodology. J. Colloid Interface Sci. 2015, 443, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Gondal, M.A.; Suliman, M.A.; Dastageer, M.A.; Chuah, G.K.; Basheer, C.; Yang, D.; Suwaiyan, A. Visible light photocatalytic degradation of herbicide (Atrazine) using surface plasmon resonance induced in mesoporous Ag-WO3/SBA-15 composite. J. Mol. Catal. A Chem. 2016, 425, 208–216. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Akmal, Z.S. Direct decomposition of methane over Pd promoted Ni/SBA-15 catalysts. Appl. Surf. Sci. 2015, 353, 127–136. [Google Scholar] [CrossRef]
- Palai, Y.N.; Anjali, K.; Sakthivel, A.; Ahmed, M.; Sharma, D.; Badamali, S.K. Cerium Ions Grafted on Functionalized Meso porous SBA-15 Molecular Sieves: Pre paration and Its Catalytic Activity on p-Cresol Oxidation. Catal. Lett. 2018, 148, 465–473. [Google Scholar] [CrossRef]
- Xie, W.; Fan, M. Biodiesel production by transesterification using tetraalkylammonium hydroxides immobilized onto SBA-15 as a solid catalyst. Chem. Eng. J. 2014, 239, 60–67. [Google Scholar] [CrossRef]
- Cakiryilmaz, N.; Arbag, H.; Oktar, N.; Dogu, G.; Dogu, T. Effect of W incorporation on the product distribution in steam reforming of bio-oil derived acetic acid over Ni based Zr-SBA-15 catalyst. Int. J. Hydrogen Energy 2018, 43, 3629–3642. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Akmal, Z.S. Direct decomposition of methane over SBA-15 supported Ni, Co and Fe based bimetallic catalysts. Appl. Surf. Sci. 2015, 330, 418–430. [Google Scholar] [CrossRef]
- Guan, S.; Inagaki, S.; Ohsuna, T.; Terasaki, O. Cubic hybrid organic-inorganic mesoporous crystal with a decaoctahedral shape. J. Am. Chem. Soc. 2000, 122, 5660–5661. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]


| Molecular Sieve Samples | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| SBA-15 | 310.1 | 0.43 | 6.85 |
| H-SBA-15-SH | 571.3 | 0.88 | 9.26 |
| G-SBA-15-SH | 463.8 | 0.85 | 6.53 |
| Concentration of Ammonia Nitrogen Solution (mg/L) | Ammonia Nitrogen Content in 50 mL Solution (mg) | The Content of Ammonia Nitrogen in the Solution after Adsorption (mg) | The Additive Amount of Molecular Sieve (g) | Ammonia Nitrogen Adsorption Capacity (mg/g) |
|---|---|---|---|---|
| 0.1 | 0.005 | 0.0048 | 0.1 | 1.667 |
| 0.2 | 0.01 | 0.0096 | 0.1 | 3.667 |
| 0.4 | 0.02 | 0.0193 | 0.1 | 7.444 |
| 0.8 | 0.04 | 0.0387 | 0.1 | 13.22 |
| 1.6 | 0.08 | 0.0759 | 0.1 | 21.00 |
| 2 | 0.1 | 0.0971 | 0.1 | 29.00 |
| Concentration of Ammonia Nitrogen Solution (mg/L) | Ammonia Nitrogen Content In 50 mL Solution (mg) | The Content of Ammonia Nitrogen in the Solution after Adsorption (mg) | The Additive Amount of Molecular Sieve (g) | Ammonia Nitrogen Adsorption Capacity (mg/g) |
|---|---|---|---|---|
| 0.1 | 0.005 | 0.0050 | 0.1 | 2.551 |
| 0.2 | 0.01 | 0.0096 | 0.1 | 6.332 |
| 0.4 | 0.02 | 0.0198 | 0.1 | 11.04 |
| 0.8 | 0.04 | 0.0391 | 0.1 | 17.01 |
| 1.6 | 0.08 | 0.0796 | 0.1 | 24.72 |
| 2 | 0.1 | 0.0997 | 0.1 | 34.33 |
| Concentration of Ammonia Nitrogen Solution (mg/L) | Ammonia Nitrogen Content in 50 mL Solution (mg) | The Content of Ammonia Nitrogen in the Solution after Adsorption (mg) | The Additive Amount of Molecular Sieve (g) | Ammonia Nitrogen Adsorption Capacity (mg/g) |
|---|---|---|---|---|
| 0.1 | 0.005 | 0.0050 | 0.1 | 3.312 |
| 0.2 | 0.01 | 0.0096 | 0.1 | 7.898 |
| 0.4 | 0.02 | 0.0120 | 0.1 | 14.17 |
| 0.8 | 0.04 | 0.0390 | 0.1 | 19. 02 |
| 1.6 | 0.08 | 0.0781 | 0.1 | 30. 32 |
| 2 | 0.1 | 0.0970 | 0.1 | 41.31 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.; Huang, Y.; Yang, Y.; Long, X.; Qin, W.; Chen, H.; Zhang, Q.; Wu, Z.; Li, S.; Wu, D.; et al. Preparation and Characterization of Highly Ordered Mercapto-Modified Bridged Silsesquioxane for Removing Ammonia-Nitrogen from Water. Polymers 2018, 10, 819. https://doi.org/10.3390/polym10080819
Lin D, Huang Y, Yang Y, Long X, Qin W, Chen H, Zhang Q, Wu Z, Li S, Wu D, et al. Preparation and Characterization of Highly Ordered Mercapto-Modified Bridged Silsesquioxane for Removing Ammonia-Nitrogen from Water. Polymers. 2018; 10(8):819. https://doi.org/10.3390/polym10080819
Chicago/Turabian StyleLin, Derong, Yichen Huang, Yuanmeng Yang, Xiaomei Long, Wen Qin, Hong Chen, Qing Zhang, Zhijun Wu, Suqing Li, Dingtao Wu, and et al. 2018. "Preparation and Characterization of Highly Ordered Mercapto-Modified Bridged Silsesquioxane for Removing Ammonia-Nitrogen from Water" Polymers 10, no. 8: 819. https://doi.org/10.3390/polym10080819