A Roadmap towards Successful Nanocapsule Synthesis via Vesicle Templated RAFT-Based Emulsion Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
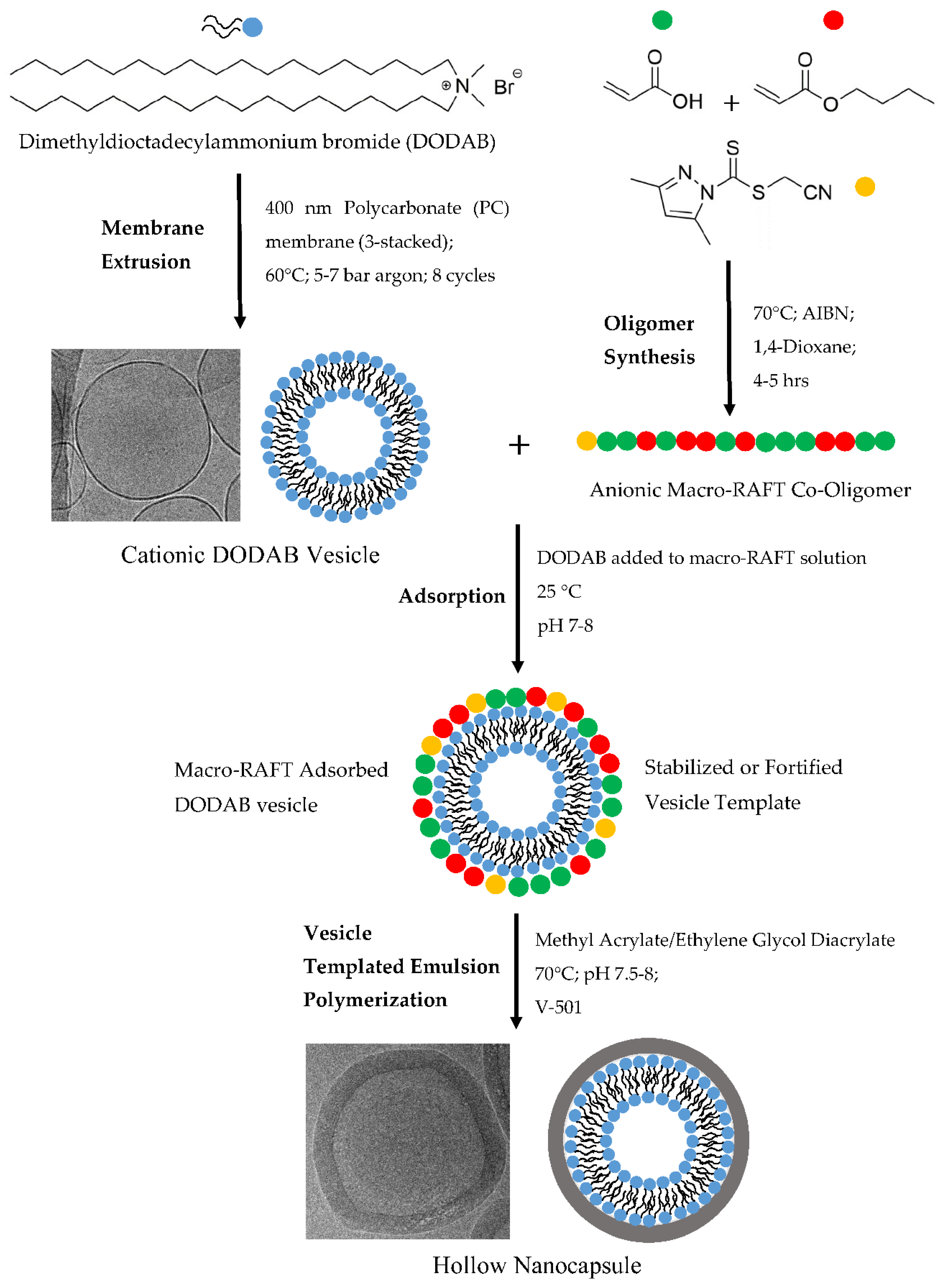
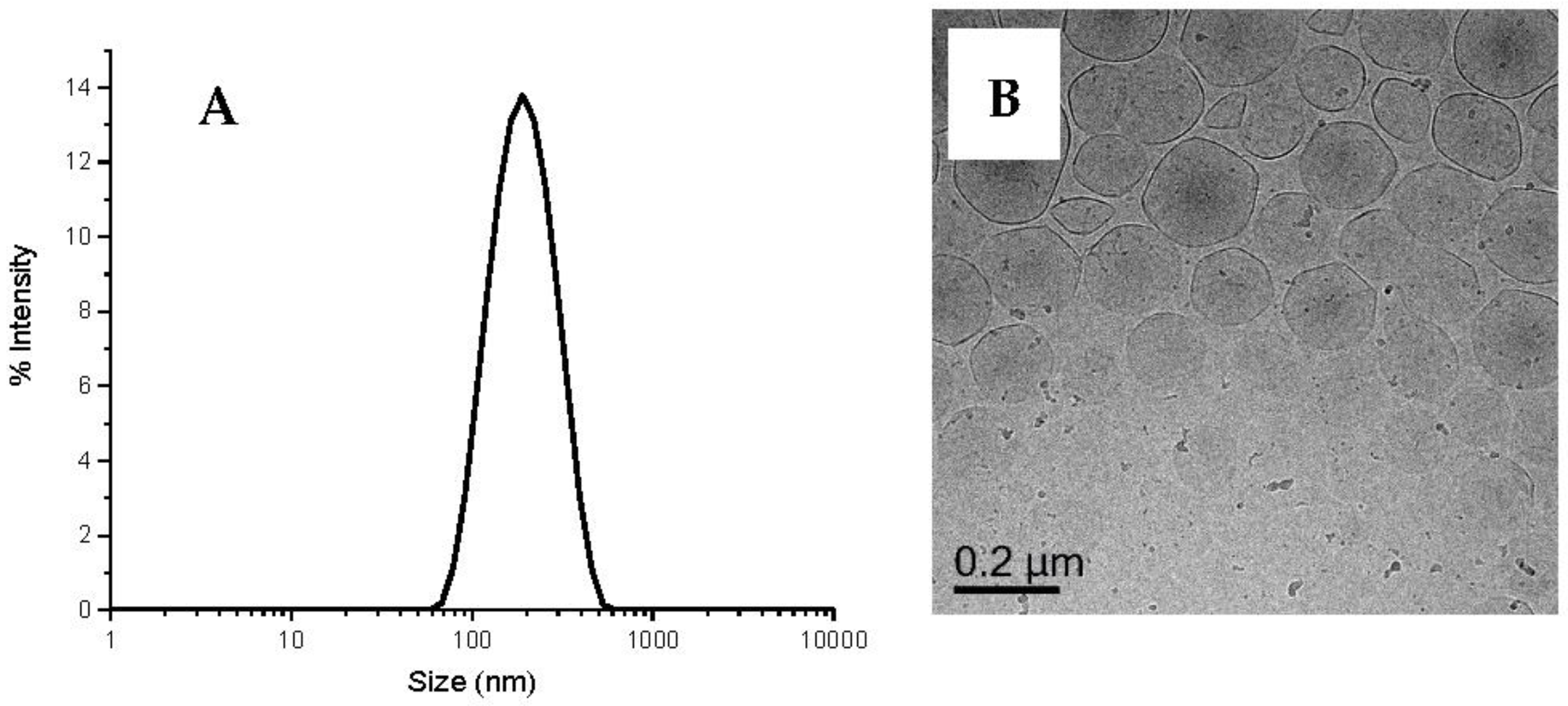
2.2. Preparation of Unilamellar DODAB Vesicles
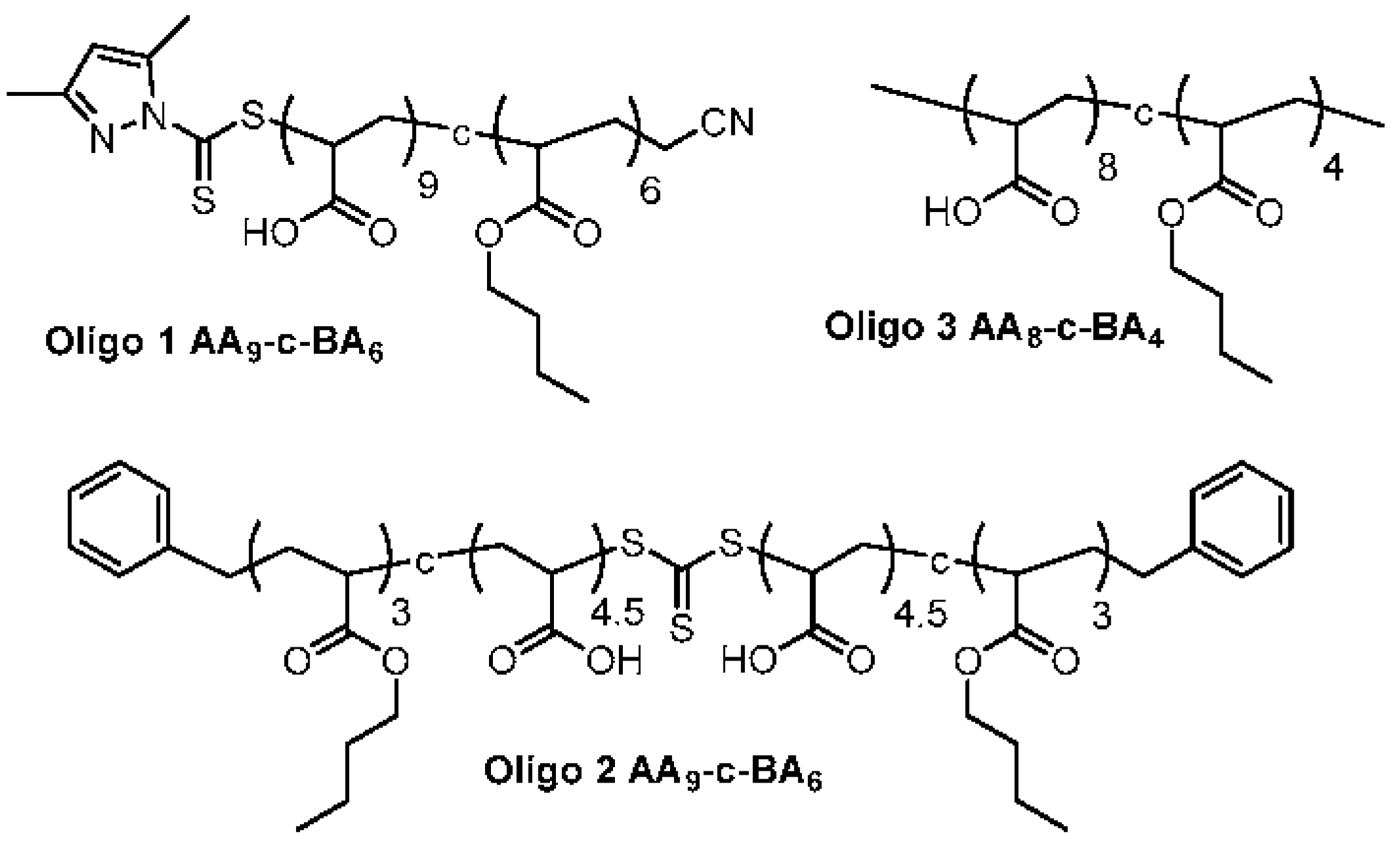
2.3. Preparation of Macro-RAFT Co-Oligomer
2.4. Preparation of RAFT-Less Co-Oligomer
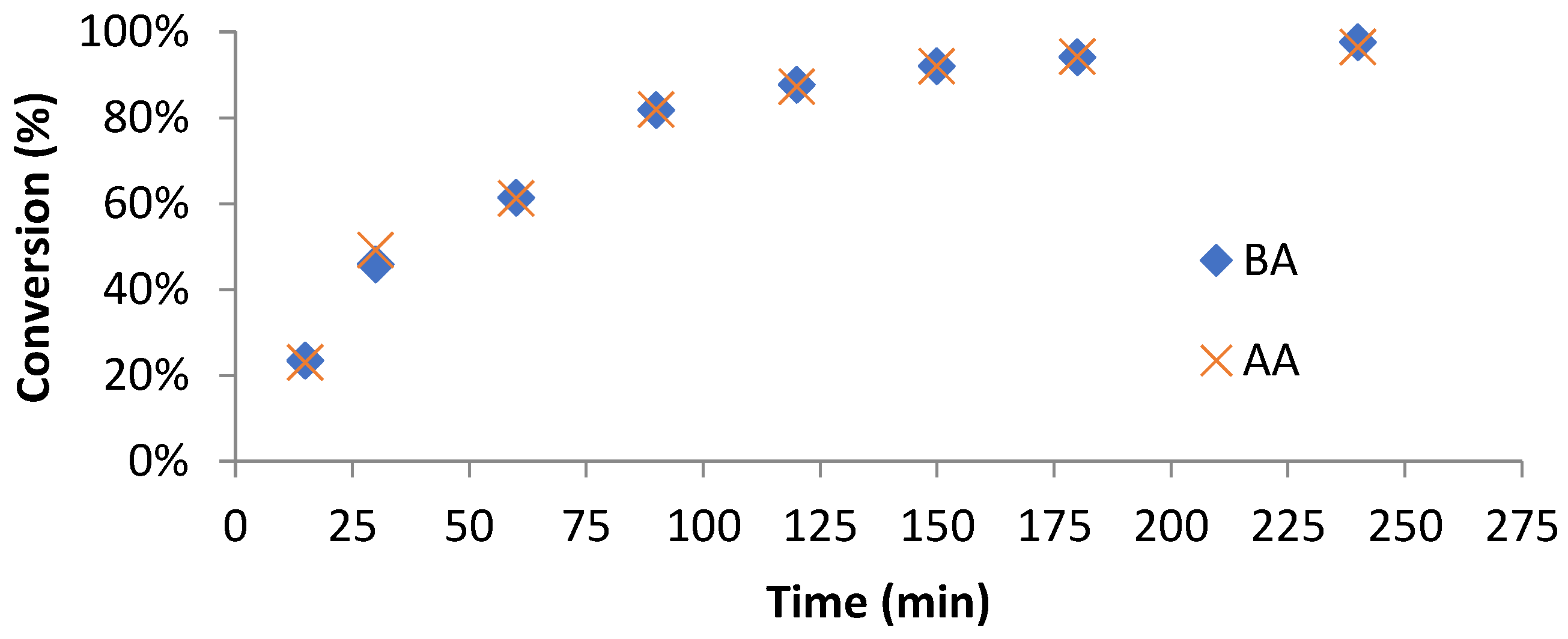
2.5. Solution Polymerization of Macro-RAFT Co-Oligomer
2.6. Adsorption of Macro-RAFT Co-Oligomer to the Vesicle Surface
2.7. DODAB Vesicle Polymerization (Transcriptive Synthesis)
2.8. Vitrification and Cryo-Transmission Electron Microscope (Cryo-TEM)
2.9. Dynamic Light Scattering (DLS)
2.10. Matrix Assisted Laser Desorption/Ionization Time of Flight (MALDI-ToF) Mass Spectrometer (MS)
3. Results and Discussion
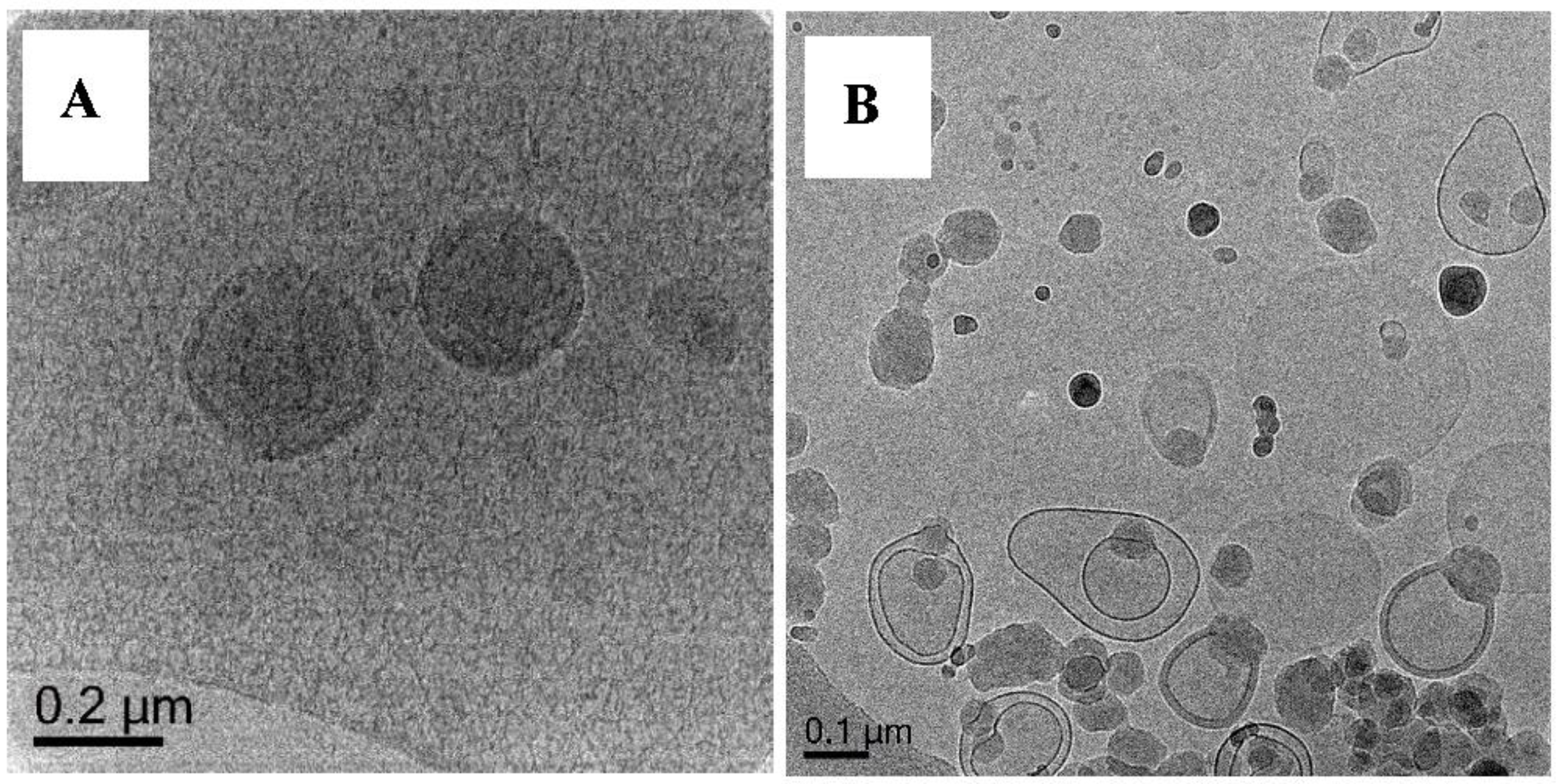
3.1. Preparation and Characterization of DODAB Vesicle as Template
3.2. Synthesis of Macro-RAFT Co-Oligomer for Vesicle Polymerization
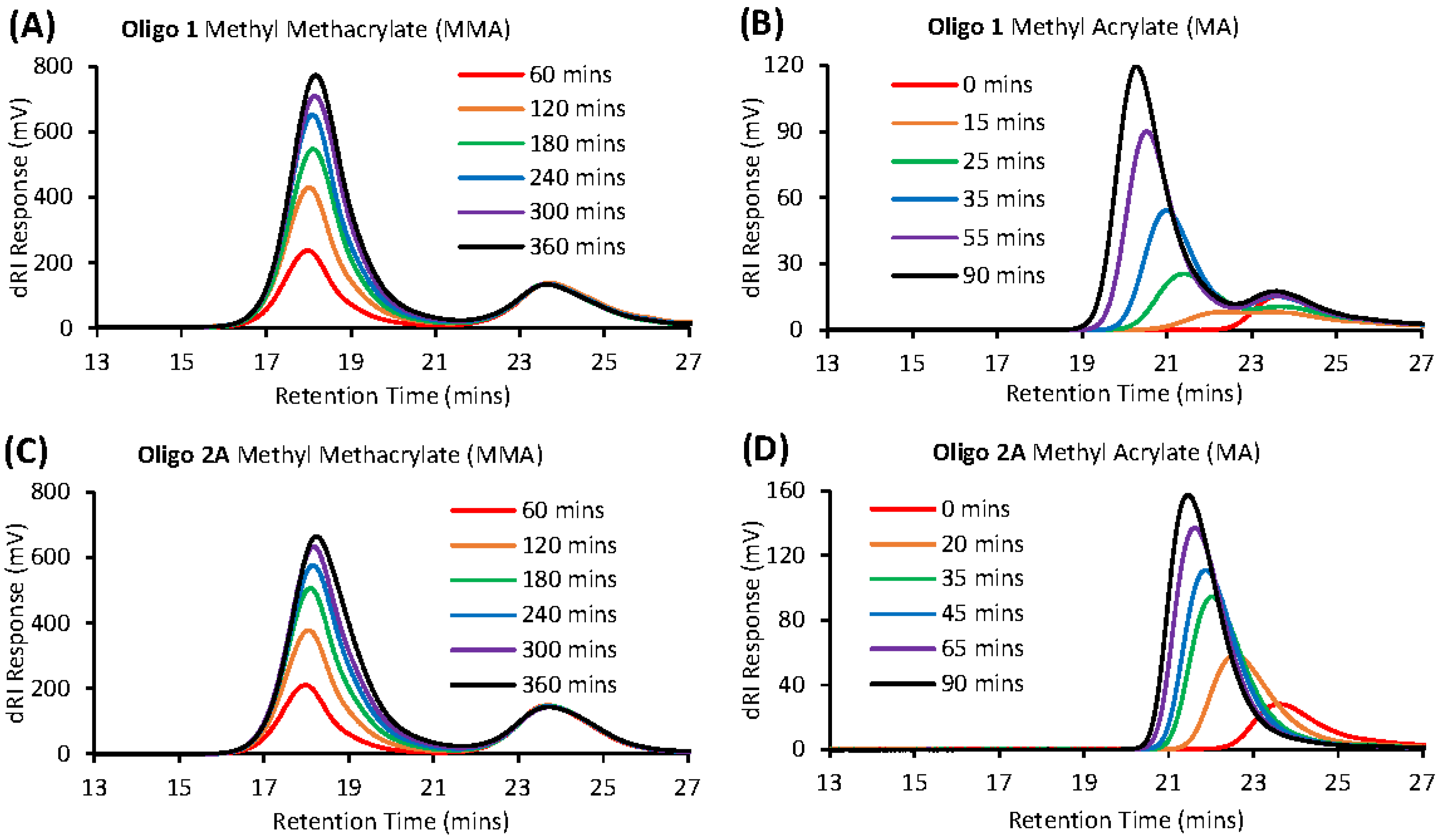
3.3. Chain Extension of Macro-RAFT Co-Oligomer in Solution
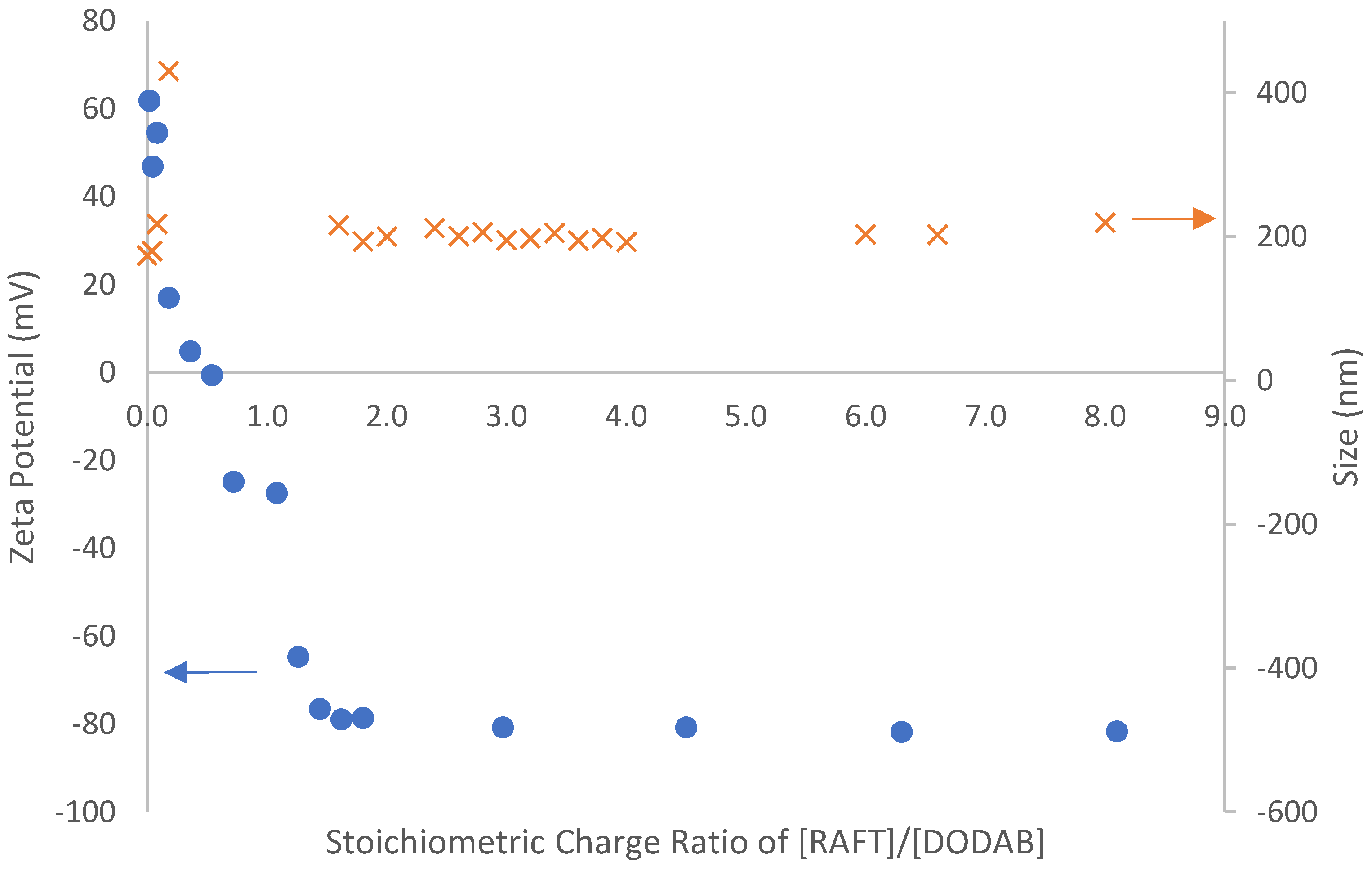
3.4. Adsorption of Macro-RAFT Co-Oligomer on the DODAB Vesicle Surface
3.5. Vesicle Templated Polymerization (Trancriptive Synthesis)
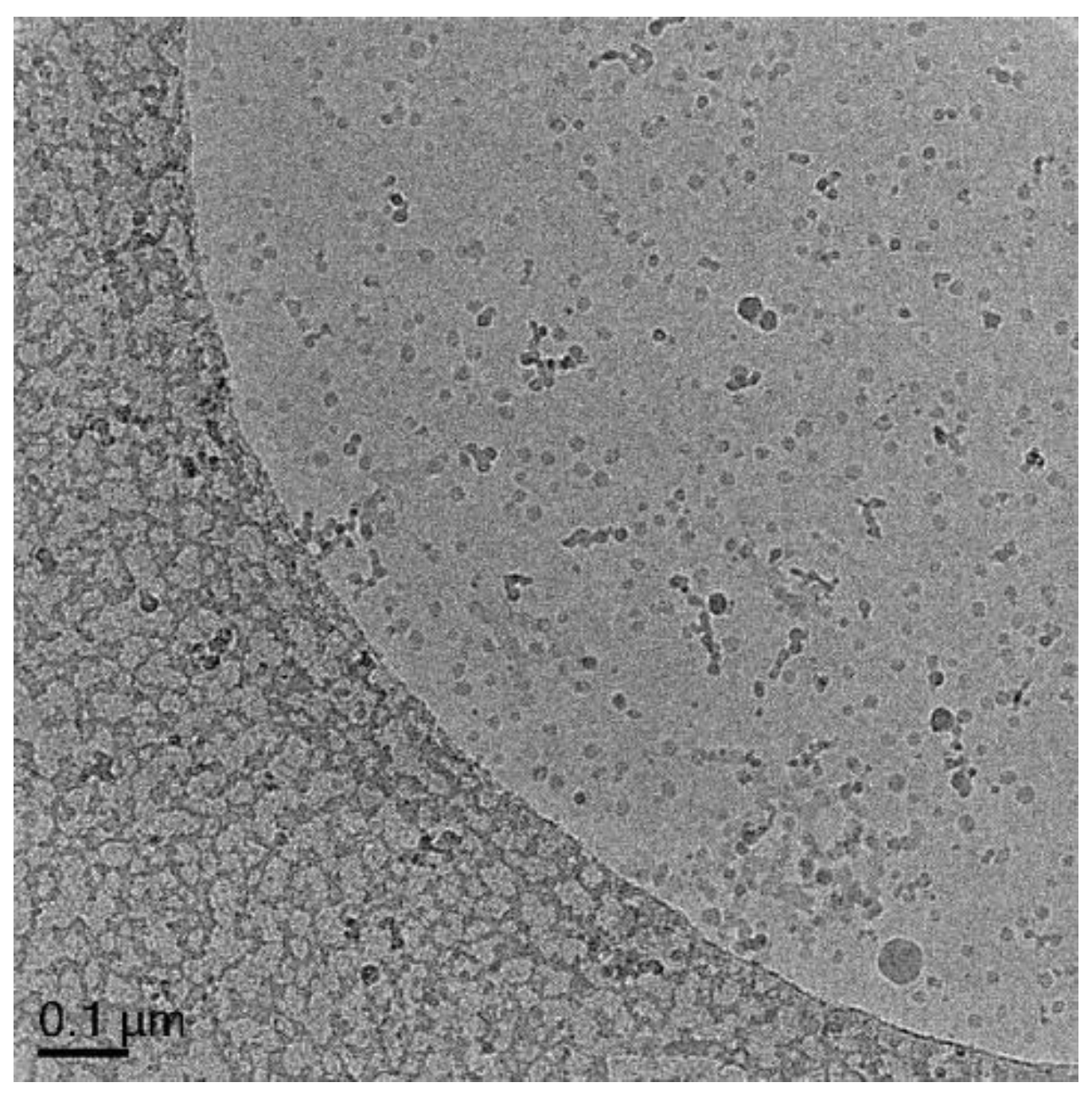
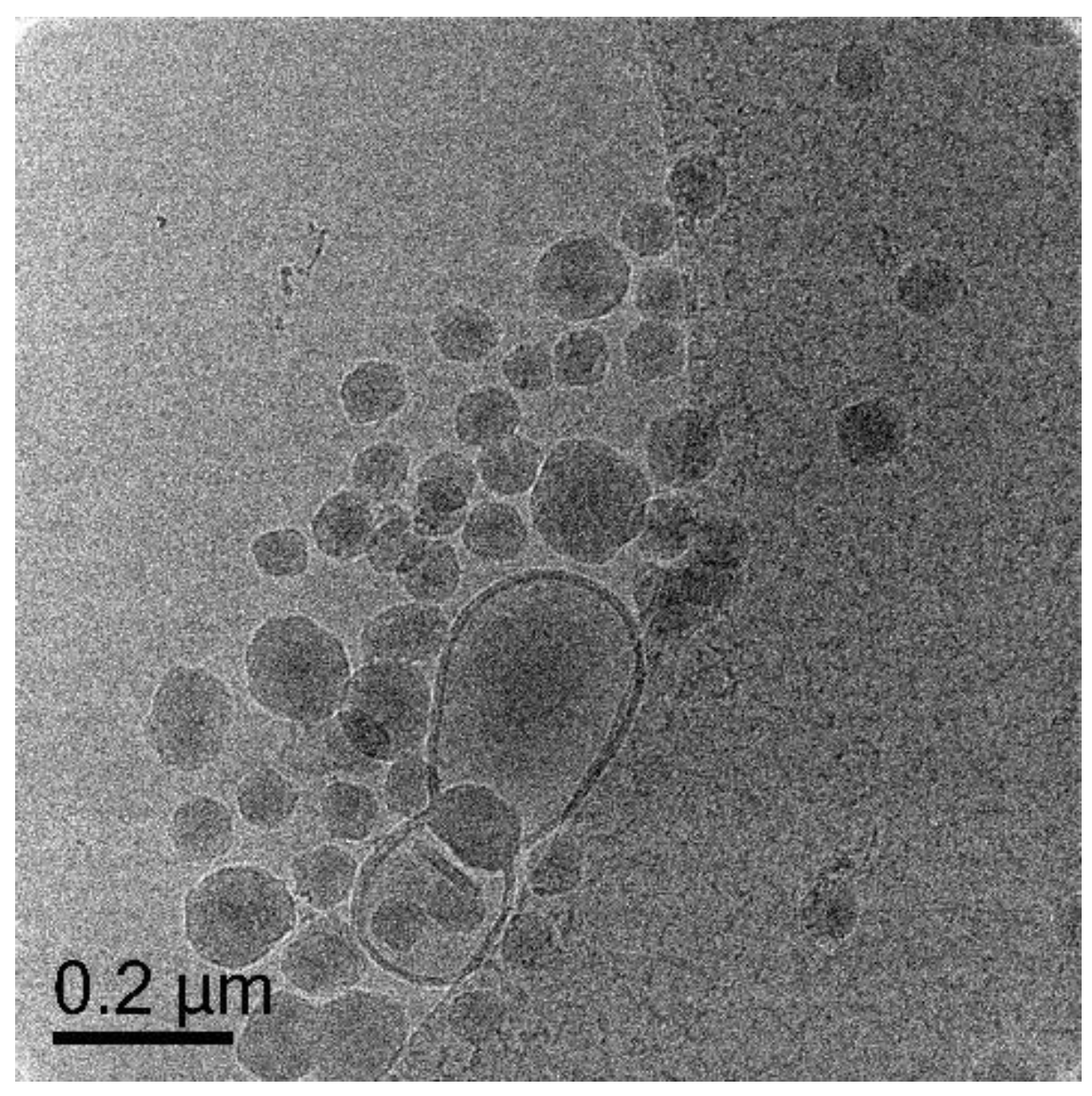
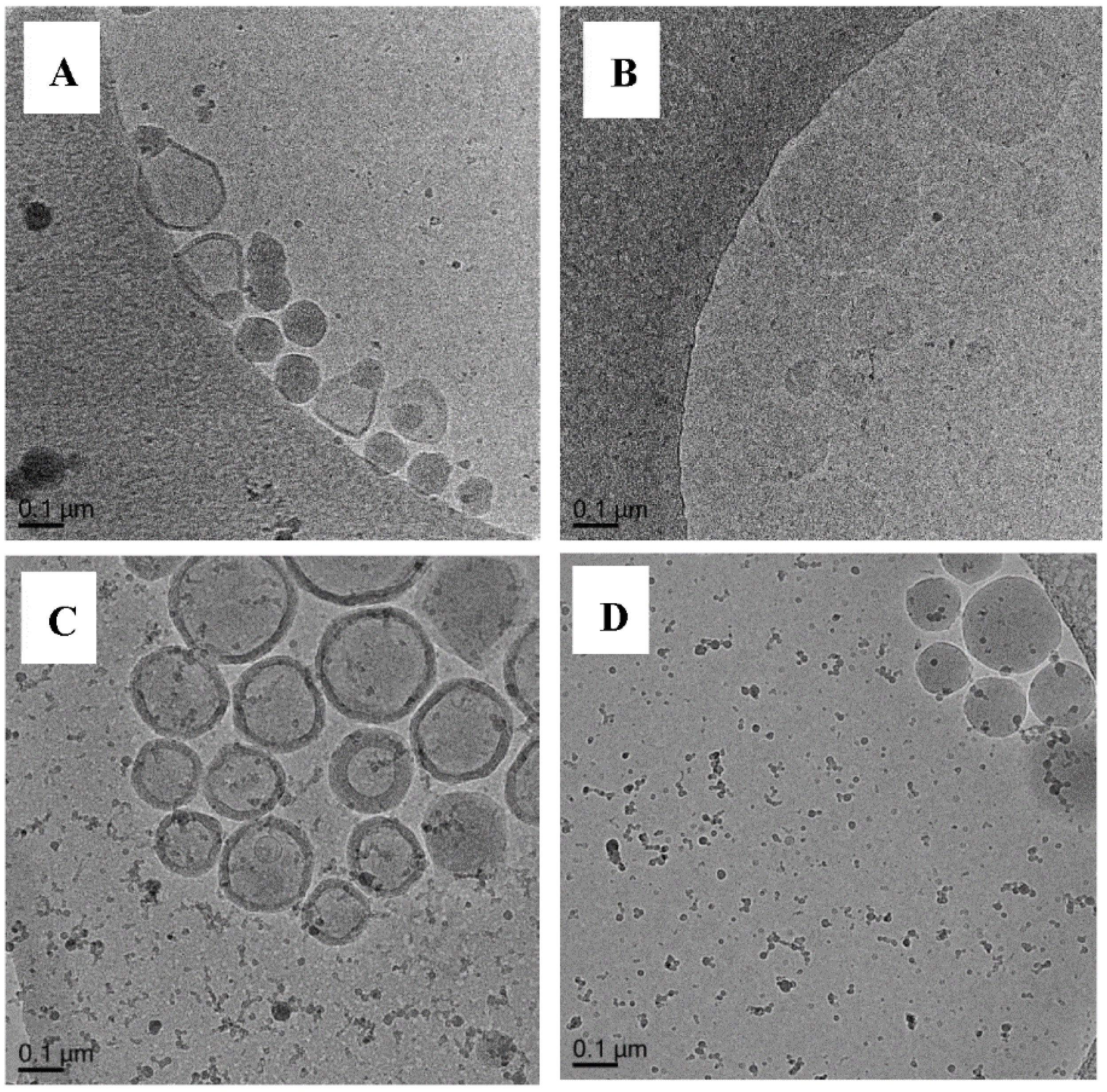
3.5.1. Polymerization of Oligo 1 with DODAB Vesicle (Unextruded) (Exp 1)
3.5.2. Polymerization of MA/EGDA with Oligo 1 without DODAB Vesicle (Exp 2)
3.5.3. Polymerization of MA/EGDA with DODAB Vesicle without Oligo 1 (Exp 3)
3.5.4. Polymerization of MA/EGDA with Oligo 3 without DODAB Vesicle (Exp 4)
3.5.5. Polymerization of MA/EGDA with Oligo 3 and DODAB Vesicle (Exp 5)
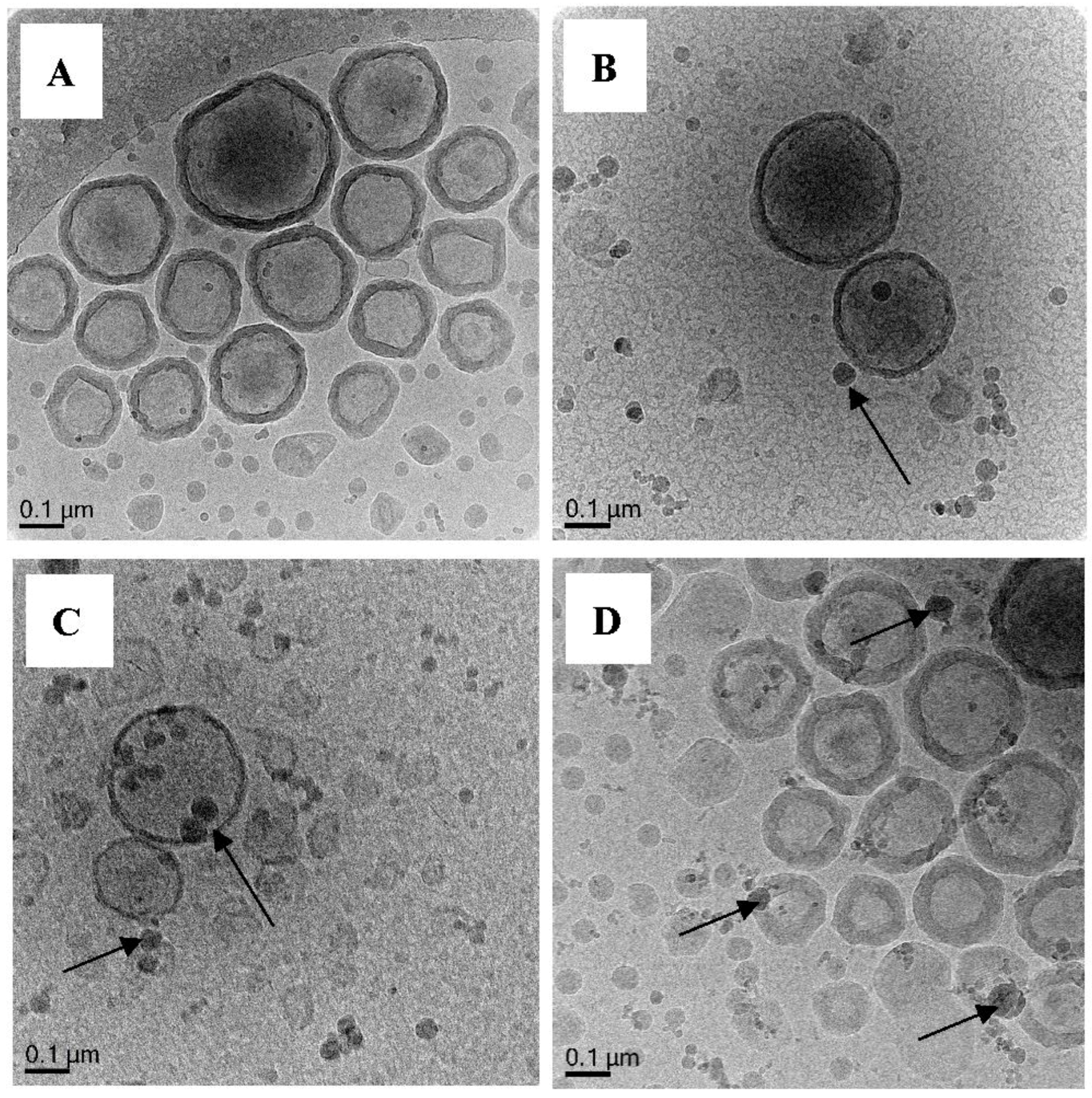
3.5.6. Polymerization of Oligo 1 with DODAB Vesicle (Exp 6–15)
- DODAB vesicles as templates must be homogenous in size and exist as unilamellar particles.
- RAFT moiety in the form of macro-RAFT co-oligomer is required for further chain extension of the co-oligomer on the vesicles surface.
- Colloidal stability after adsorption is critical to retain the vesicular structures.
- The choice of monomer is important to achieve molecular control over the polymerization (based on the result from solution polymerization of macro-RAFT co-oligomer).
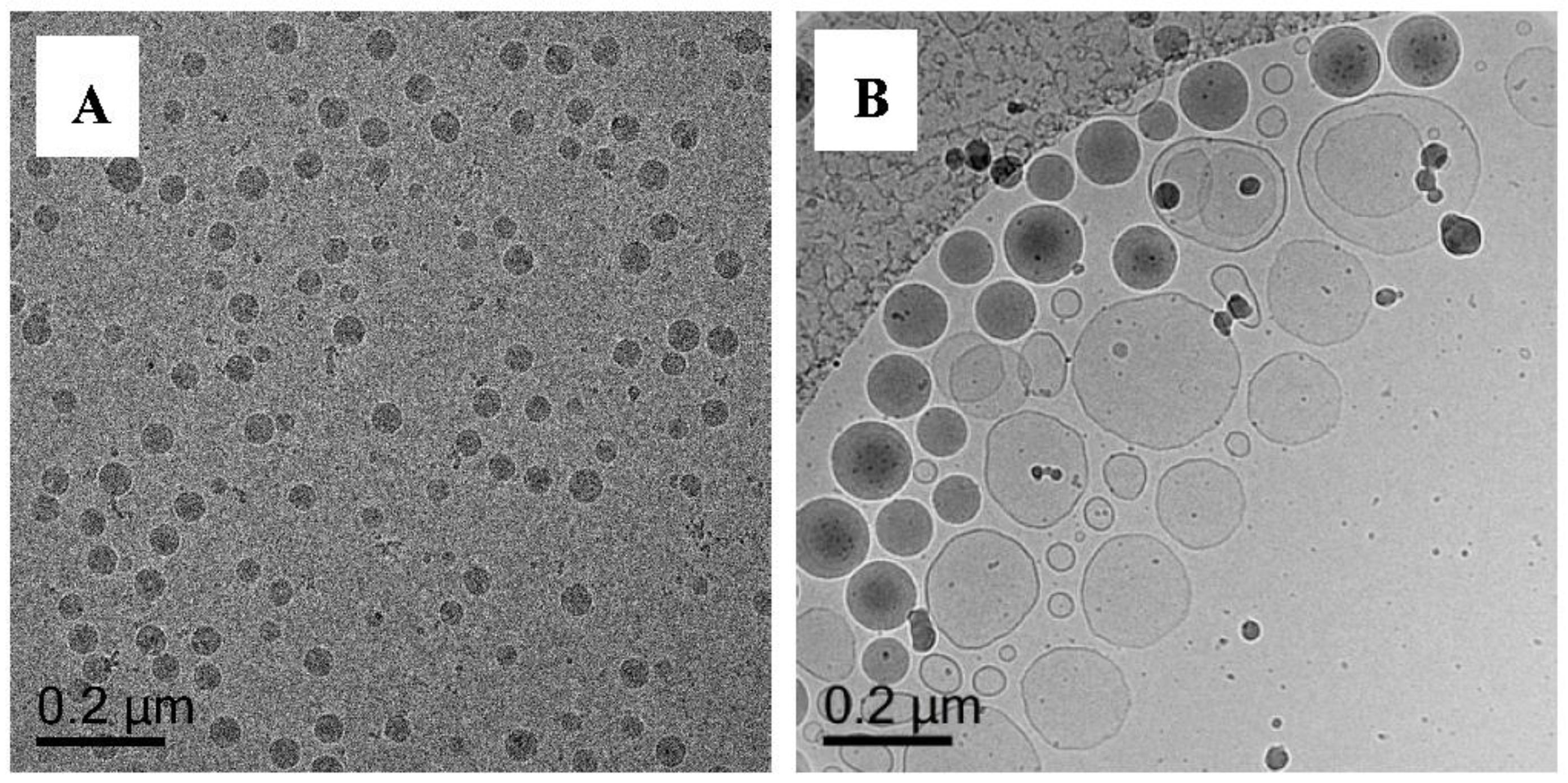
Polymerization of DODAB-Oligo 1 with MA (Exp 6) and MMA (Exp 7)
Polymerization of (DODAB-Oligo 1) with MA or MMA with Crosslinker (Exp 8–12)
- macro-RAFT co-oligomer. The free co-oligomer in the aqueous phase can chain extend in the presence of monomer and initiator. The co-oligomer is added in excess to make sure that there is enough co-oligomer to adsorb to the vesicle surface and also to provide electrostatic charge to stabilize the particles. The chemical composition distribution of the co-oligomer, however, is quite broad from the MALDI-ToF analysis and surface active species could be there.
- Polymerization technique. Even with slow monomer addition a diffusion into the vesicle bilayer might occur, that can lead to polymerization and phase separation into a pro-trusion morphology.
- Cross-linker. Addition of small amount of crosslinker (0.5–1 mmol) is enough to fixate and immobilize the polymer chain on the vesicle surface.
Polymerization of (DODAB-Oligo 1) with MA/BA and BA-(EGDA) (Exp 13–15)
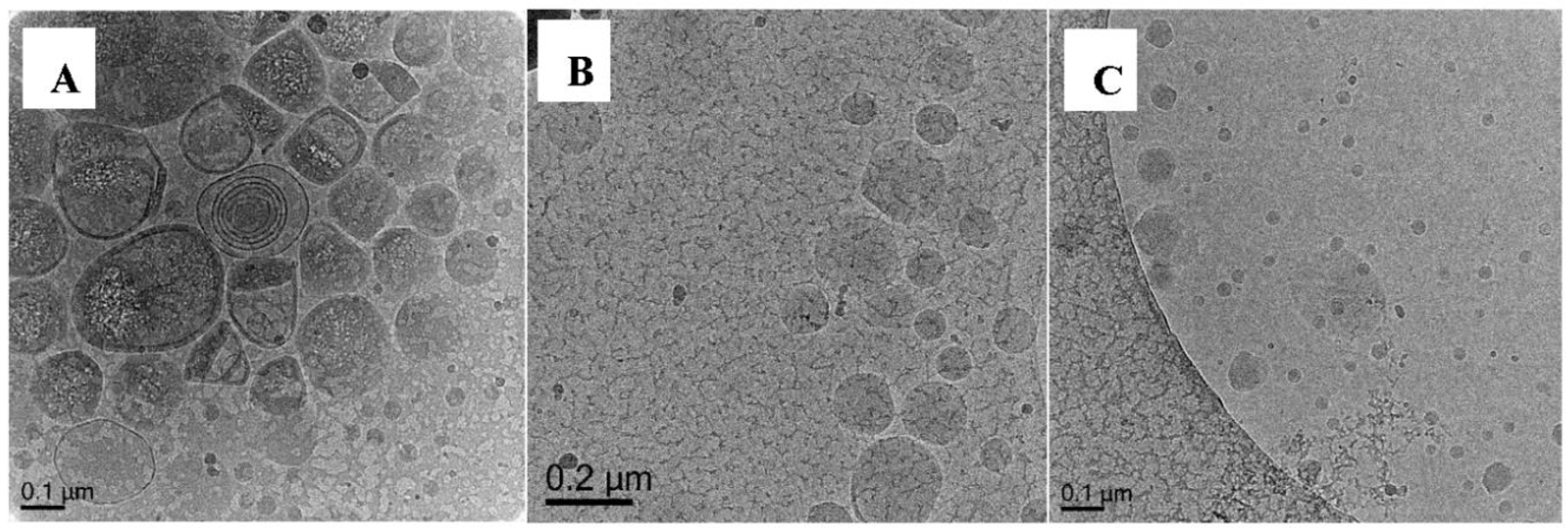
3.5.7. Polymerization of Oligo 2A with DODAB Vesicle (Exp 16-19)
4. Conclusions
- starting with a stable and relatively mono-disperse vesicle dispersion
- adsorption of oppositely charged macro-RAFT co-oligomer and RAFT controlled chain extension with a monomer that is slightly water soluble (MA works and BA does not work)
- having some crosslinking to retain the non-equilibrium morphology
- a good compatibility between RAFT agent and monomer
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Herk, A.M. Chemistry and Technology of Emulsion Polymerization, 2nd ed.; Wiley: Chichester, UK, 2013; ISBN 978-1-119-95372-2. [Google Scholar]
- Jung, M.; Hubert, D.H.W.; Bomans, P.; Frederik, P.M.; van Herk, A.M.; German, A.L. A Topology Map for Novel Vesicle-Polymer Architectures. Adv. Mater. 2000, 12, 210–213. [Google Scholar] [CrossRef]
- Jung, M.; den Ouden, I.; Montoya-Goñi, A.; Hubert, D.H.W.; Frederik, P.M.; van Herk, A.M.; German, A.L. Polymerisation in Polymerizable Vesicle Bilayer Membranes. Langmuir 2000, 16, 4185–4195. [Google Scholar] [CrossRef]
- Nguyen, D.; Zondanos, H.S.; Farrugia, J.M.; Serelis, A.K.; Such, C.H.; Hawkett, B.S. Pigment Encapsulation by Emulsion Polymerization using Macro-RAFT Copolymers. Langmuir 2008, 24, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.; Heuts, J.P.A.; Hawkett, B.S.; van Herk, A.M. Polymer Encapsulated Gibbsite Nanoparticles: Efficient Preparation of Anisotropic Composite Latex Particles by RAFT-based Starved Feed Emulsion Polymerization. Langmuir 2009, 25, 10523–10533. [Google Scholar] [CrossRef] [PubMed]
- Garnier, J.; Warnant, J.; Lacroix-Desmazes, P.; Dufils, P.E.; Vinas, J.; Vanderveken, Y.; van Herk, A.M. An Emulsifier-Free RAFT-Mediated Process for the Efficient Synthesis of Cerium Oxide/Polymer Hybrid Latexes. Macromol. Rapid Commun. 2012, 33, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Mballa Mballa, M.A.; Ali, S.I.; Heuts, J.P.A.; van Herk, A.M. Control of the Anisotropic Morphology of Latex Nanocomposites Containing Single Montmorillonite Clay Particles Prepared by Conventional and Reversible Addition-Fragmentation Chain Transfer Based Emulsion Polymerization. Polym. Int. 2012, 61, 861–865. [Google Scholar] [CrossRef]
- Van Herk, A.M. Polymer Encapsulation of Single Clay Platelets by Emulsion Polymerization Approaches, Thermodynamic and Kinetic Factors. Macromol. React. Eng. 2016, 10, 22–28. [Google Scholar] [CrossRef]
- Loiko, O.P.; Spoelstra, A.B.; van Herk, A.M.; Meuldijk, J.; Heuts, J.P.A. An ATRP-based approach towards water-borne anisotropic polymer-Gibbsite nanocomposites. Polym. Chem. 2016, 7, 3383–3391. [Google Scholar] [CrossRef]
- Loiko, O.P.; Spoelstra, A.B.; van Herk, A.M.; Meuldijk, J.; Heuts, J.P.A. Encapsulation of Gibbsite Platelets with Free Radical and Controlled Radical Emulsion Polymerization Approaches, a Small Review. Macromol. Symp. 2016, 370, 66–74. [Google Scholar] [CrossRef]
- Loiko, O.P.; Spoelstra, A.B.; van Herk, A.M.; Meuldijk, J.; Heuts, J.P.A. ATRP mediated encapsulation of Gibbsite: Fixation of the morphology by using a cross-linker. Polym. Chem. 2017, 8, 2909–2912. [Google Scholar] [CrossRef]
- Ali, S.I.; Heuts, J.P.A.; van Herk, A.M. Controlled Synthesis of Polymeric Nanocapsules by RAFT-Based Vesicle Templating. Langmuir 2010, 26, 7848–7858. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.; Heuts, J.P.A.; van Herk, A.M. Vesicles-templated pH-responsive polymeric nanocapsules. Soft Matter 2011, 7, 5382–5390. [Google Scholar] [CrossRef]
- Moradi, M. Functional Nanomaterials and Nanocapsules. Application of Semi-Quantitative Imaging on Multi-Component Nanosystems. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2017. [Google Scholar]
- Van Herk, A.M.; Forcada, J.; Pastorin, G. Controlled Release Systems: Advances in Nanobottles and Active Nanoparticles; Pan Stanford Publishing: Singapore, 2016; pp. 63–67. ISBN 978-981-4613-21-7. [Google Scholar]
- Rusli, W.; van Herk, A.M. Preparation of Unilamellar DODAX (X = Br− or Cl−) Vesicle Templates: Effect of Salts on Vesicular Morphology. 2018; submitted. [Google Scholar]
- Coppola, L.; Youssry, M.; Nicotera, I.; Gentile, L. Rheological investigation of thermal transitions in vesicular dispersion. J. Colloid Interface Sci. 2009, 338, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Hubert, D.H.W.; Bomans, P.H.H.; Frederik, P.M.; Meuldijk, J.; van Herk, A.M.; Fischer, H.; German, A.L. New Vesicle-Polymer Hybrids: The Parachute Architecture. Langmuir 1997, 13, 6877–6880. [Google Scholar] [CrossRef]
- Jung, M.; Hubert, D.H.W.; van Veldhoven, E.; Frederik, P.; van Herk, A.M.; German, A.L. Vesicle-Polymer Hybrid Architectures: A Full Account of the Parachute Architecture. Langmuir 2000, 16, 3165–3174. [Google Scholar] [CrossRef]
- Lopes, A.; Edwards, K.; Feitosa, E. Extruded vesicles of dioctadecyldimethylammonium bromide and chloride investigated by light scattering and cryogenic transmission electron microscopy. J. Colloid Interface Sci. 2008, 322, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, E.; Jansson, J.; Lindman, B. The effect of chain length on the melting temperature and size of dialkyldimethylammonium bromide vesicles. Chem. Phys. Lipids 2006, 142, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, E.; Karlsson, G.; Edwards, K. Unilamellar vesicles obtained by simply mixing dioctadecyldimethylammonium chloride and bromide with water. Chem. Phys. Lipids 2006, 140, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.J.; Hughes, R.J.; Pham, B.T.T.; Hawkett, B.S.; Gilbert, R.G.; Serelis, A.K.; Such, C.H. Effective ab Initio Emulsion Polymerization under RAFT control. Macromolecules 2002, 35, 9243–9245. [Google Scholar] [CrossRef]
- Charleux, B.; Delaittre, G.; Rieger, J.; D’Agosto, F. Polymerization-Induced Self-Assembly: From Soluble Macromolecules to Block Copolymer Nano-Objects in One Step. Macromolecules 2012, 45, 6753–6765. [Google Scholar] [CrossRef]
- Canning, S.L.; Smith, G.N.; Armes, S.P. A Critical Appraisal of RAFT-Mediated Polymerization-Induced Self Assembly. Macromolecules 2016, 49, 1985–2001. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Sun, H.; Yu, M.; Sumerlin, B.S.; Zhang, L. Photo-PISA: Shedding Light on Polymerization-Induced Self-Assembly. ACS Macro Lett. 2015, 4, 1249–1253. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Davis, T.P. Handbook of Radical Polymerization; John Wiley & Sons: Hoboken, NJ, USA, 2003; p. 306. ISBN 978-047-1392-74-3. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics. A Ready-Reference Book of Chemical and Physical Data, 86th ed.; Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 3–334. ISBN 978-0-8493-0486-6. [Google Scholar]
- Oda, Y.; Kanaoka, S.; Sato, T.; Aoshima, S. and Kuroda, K. Block versus Random Amphiphilic Copolymers as Antibacterial Agents. Biomacromolecules 2011, 12, 3581–3591. [Google Scholar] [CrossRef] [PubMed]


| Co-Oligomer | Mn, theoretical (g/mol) | Mn, NMR (g/mol) | Mn, GPC (g/mol) | PDI, GPC |
|---|---|---|---|---|
| Oligo 1 BM1481 (BA6-co-AA9) ^ | 1629 | 1631 | 2259 | 1.26 |
| Oligo 2A BM1361 (BA6-co-AA9) ^ | 1708 | 1820 | 2244 | 1.28 |
| Oligo 2B BM1361 (BA6-co-AA9) * | 1708 | 1372 | 1920 | 1.31 |
| Oligo 2C BM1361 (BA6-co-AA9) # | 1708 | 1421 | 1870 | 1.35 |
| Oligo 2D BM1361 (BA6-co-AA9) ′ | 1708 | 1510 | 1953 | 1.32 |
| Oligo 3 (BA4-co-AA8)^ | 1418 | 1090 | 1241 | 1.74 |
| (RAFT) Oligomer (10 mM, pH 7) | Volume (mL) | [Oligo]/[V-501] | |||
|---|---|---|---|---|---|
| RAFT Oligomer Solution | DI Water | DODAB (10 mM) | |||
| Oligo 1 [BM1481 (BA6-co-AA9) | 50 | 50 | 100 | 4.5 | 2 |
| Oligo 2A [BM1361 (BA6-co-AA9) | 33 | 67 | 100 | 3.0 | 2 |
| Oligo 3 (BA4-co-AA8) | 80 | 20 | 100 | 7.2 | 2 |
| Exp | Oligomer (10 mM) | DODAB Vesicles (10 mM) | Pre-polymerization Stability (Adsorption) | RAFT Control | Monomer | Cross-Linker | Post-polymerization Stability | Cryo-TEM (Particles Morphologies) |
|---|---|---|---|---|---|---|---|---|
| 1. | Oligo 1 | ✓ (unextruded) | ✕ | - | - | - | - | - |
| 2. | Oligo 1 | ✕ | ✓ | ✓ | MA (11.6 mmol) | EGDA (0.59 mmol) | ✓ | Solids (10–20 nm) |
| 3. | ✕ | ✓ | ✓ | - | MA (11.6 mmol) | EGDA (0.59 mmol) | ✕ | - |
| 4. | Oligo 3 | ✕ | ✓ | - | MA (11.6 mmol) | EGDA (0.59 mmol) | ✓ | Solids (30–70 nm) |
| 5. | Oligo 3 | ✓ | ✓ | - | MA (11.6 mmol) | EGDA (0.59 mmol) | ✓ | Solids (100–150 nm), vesicles |
| 6. | Oligo 1 | ✓ | ✓ | ✓ | MA (11.6 mmol) | - | ✓ | Capsules, solids |
| 7. | Oligo 1 | ✓ | ✓ | ✕ | MMA (10 mmol) | - | ✓ | Pro-trusion, vesicles, solids |
| 8. | Oligo 1 | ✓ | ✓ | ✕ | MMA (10 mmol) | EGDMA (0.50 mmol) | ✓ | Pro-trusion, solids |
| 9. | Oligo 1 | ✓ | ✓ | ✓ | MA (11.6 mmol) | EGDA (0.59 mmol) | ✓ | Capsules |
| 10. | Oligo 1 | ✓ | ✓ | ✓ | MA (11.6 mmol) | EGDA (1.17 mmol) | ✓ | Capsules |
| 11. | Oligo 1 | ✓ | ✓ | ✓ | MA (11.6 mmol) | EGDA (2.94 mmol) | ✓ | Capsules |
| 12. | Oligo 1 | ✓ | ✓ | ✓ | MA (23.2 mmol) | EGDA (1.18 mmol) | ✓ | Capsules (thicker shells) |
| 13. | Oligo 1 | ✓ | ✓ | ✓ | MA/BA (11.6/0.78 mmol) | - | ✓ | Pro-trusion, solids |
| 14. | Oligo 1 | ✓ | ✓ | ✓ | BA (7.8 mmol) | - | ✓ | Flattened, film-like |
| 15. | Oligo 1 | ✓ | ✓ | ✓ | BA (7.8 mmol) | EGDA (0.59 mmol) | ✓ | Flattened, film-like |
| Exp | Oligomer (10 mM) | DODAB Vesicles (10 mM) | Pre-Polymerization Stability (Adsorption) | RAFT Control | Monomer | Cross-Linker | Post-Polymerization Stability | Cryo-TEM (Particles Morphologies) |
|---|---|---|---|---|---|---|---|---|
| 16. | Oligo 2A | ✓ | ✓ | ✕ | MMA (10 mmol) | - | ✓ | Pro-trusion, solids |
| 17. | Oligo 2A | ✓ | ✓ | ✓ | MA (11.6 mmol) | - | ✓ | Film-like particles, no clear shells |
| 18. | Oligo 2A | ✓ | ✓ | ✓ | MA (11.6 mmol) | EGDA (0.59 mmol) | ✓ | Capsules |
| 19. | Oligo 2A | ✓ | ✓ | ✓ | BA (7.8 mmol) | EGDA (0.59 mmol) | ✓ | Solids, flattened, film-like particles |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusli, W.; Jackson, A.W.; Van Herk, A. A Roadmap towards Successful Nanocapsule Synthesis via Vesicle Templated RAFT-Based Emulsion Polymerization. Polymers 2018, 10, 774. https://doi.org/10.3390/polym10070774
Rusli W, Jackson AW, Van Herk A. A Roadmap towards Successful Nanocapsule Synthesis via Vesicle Templated RAFT-Based Emulsion Polymerization. Polymers. 2018; 10(7):774. https://doi.org/10.3390/polym10070774
Chicago/Turabian StyleRusli, Wendy, Alexander W. Jackson, and Alexander Van Herk. 2018. "A Roadmap towards Successful Nanocapsule Synthesis via Vesicle Templated RAFT-Based Emulsion Polymerization" Polymers 10, no. 7: 774. https://doi.org/10.3390/polym10070774






