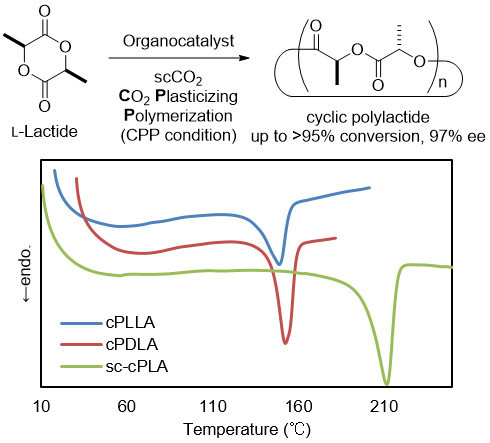
Organocatalytic Stereoselective Cyclic Polylactide Synthesis in Supercritical Carbon Dioxide under Plasticizing Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Characterization
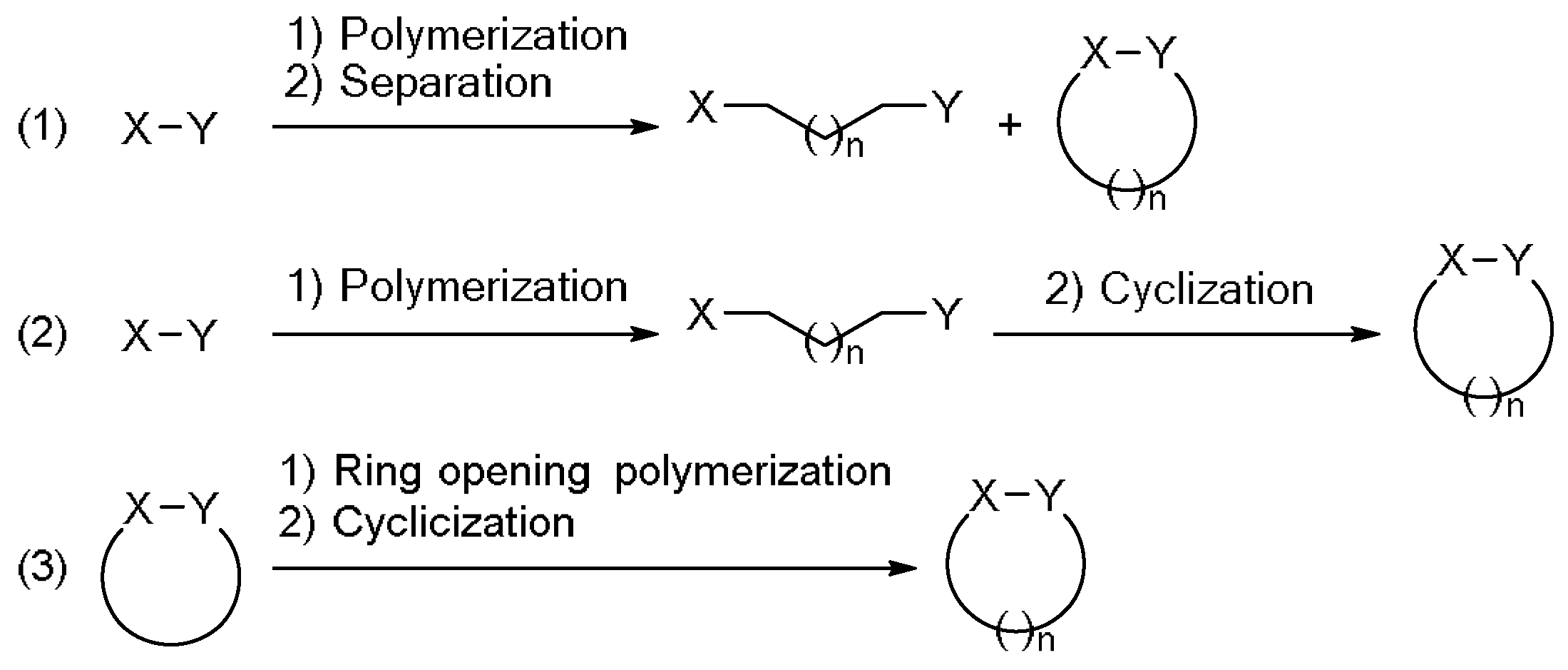
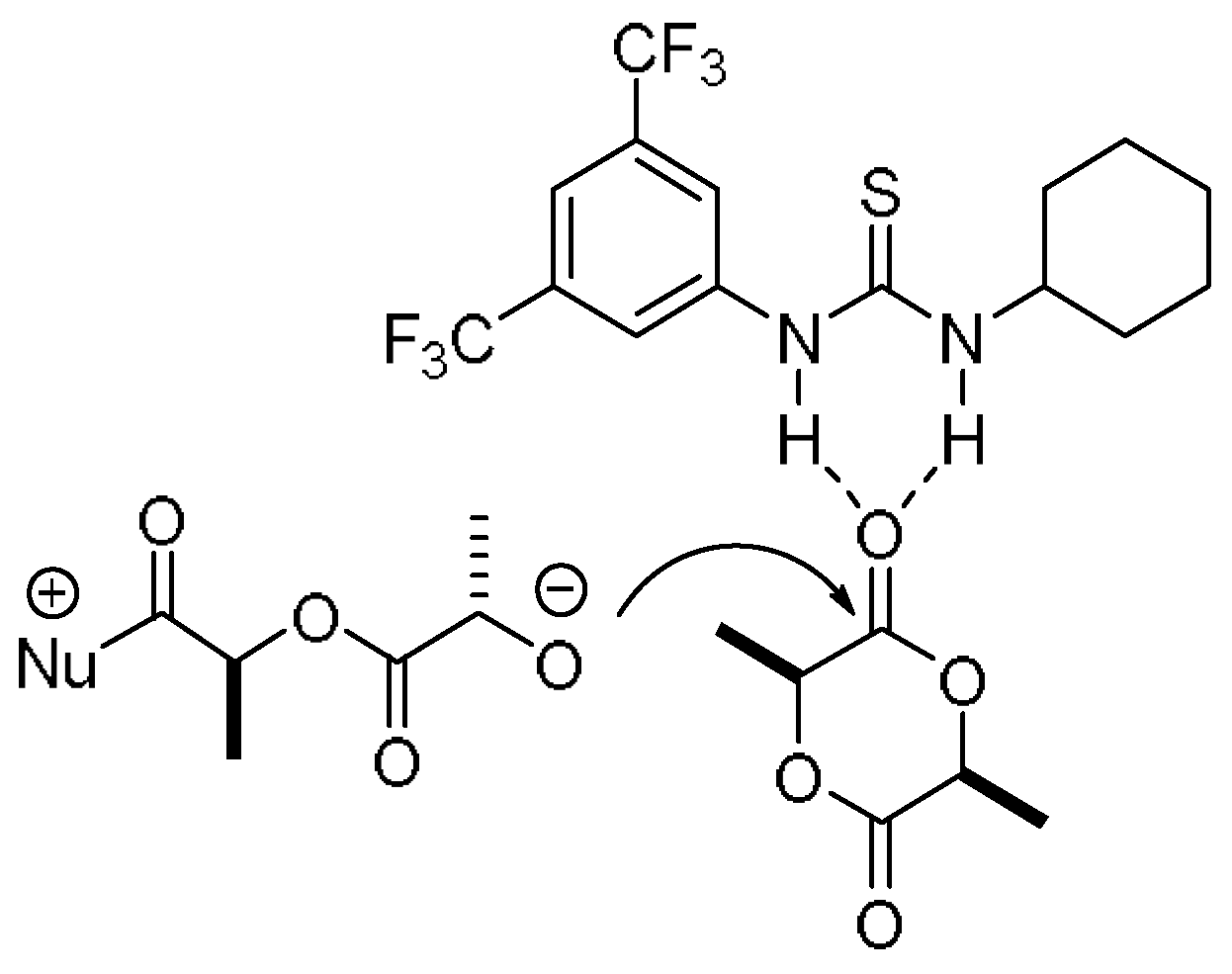
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thakur, V.K.; Thakur, M.K. Handbook of Polymers for Pharmaceutical Technologies: Biodegradable Polymers; Scrivener Publishing LLC: New York, NY, USA, 2015; Volume 3, pp. 1–583. [Google Scholar] [CrossRef]
- Rieger, B.; Kunkel, A.; Coates, G.W.; Reichardt, R.; Dinjus, E.; Zevaco, T.A. Synthetic Biodegradable Polymers; Springer: Berlin/Heidelberg, Germany, 2012; Volume 245, pp. 1–364. [Google Scholar]
- Lendlein, A.; Sisson, A. Handbook of Biodegradable Polymers: Isolation, Synthesis, Characterization and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 1–405. [Google Scholar]
- Nomura, K.; Ohara, H. Synthesis and function of cyclic poly(lactic acid) and oligo(lactic acid). Mini-Rev. Org. Chem. 2017, 14, 35–43. [Google Scholar] [CrossRef]
- Tezuka, Y. Topological Polymer Chemistry: Progress of Cyclic Polymers in Syntheses, Properties and Functions; World Scientific Publishing Co., Pte., Ltd.: Singapore, 2013; pp. 1–352. [Google Scholar]
- Yamamoto, T.; Tezuka, Y. Cyclic and multicyclic topological polymers. In Complex Macromolecular Architectures: Synthesis, Characterization, and Self-Assembly; Hadjichristidis, N., Hirao, A., Tezuka, Y., DuPrez, F., Eds.; John Wiley & Sons (Asia) Pte. Ltd.: Singapore, 2011; pp. 3–19. [Google Scholar]
- Endo, K. Synthesis and properties of cyclic polymers. In New Frontiers in Polymer Synthesis; Kobayashi, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 217, pp. 121–183. [Google Scholar]
- Roovers, J. Organic cyclic polymers. In Cyclic Polymers, 2nd ed.; Semlyen, J.A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 347–384. [Google Scholar]
- Takada, S.; Nagato, Y.; Yamamura, M. Effect of cyclic polylactates on tumor cells and tumor bearing mice. Biochem. Mol. Biol. Int. 1997, 43, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.V.; Biying, A.O.; Ling, W.Y.; Stubbs, L.P.; Zhu, Y. Synthesis and new application of green and recyclable cyclic poly(l-lactide)-clay hybrid. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4167–4174. [Google Scholar] [CrossRef]
- Kato, H.; Naganushi, Y. Preparation of l-Lactic Acid Condensation Products for Cancer Treatment. JPH06336427A, 6 December 1994. [Google Scholar]
- Osaka, I.; Watanabe, M.; Takama, M.; Murakami, M.; Arakawa, R. Characterization of linear and cyclic polylactic acids and their solvolysis products by electrospray ionization mass spectrometry. J. Mass Spectrom. 2006, 41, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Osaka, I.; Yoshimoto, A.; Watanabe, M.; Takama, M.; Murakami, M.; Kawasaki, H.; Arakawa, R. Quantitative determination of cyclic polylactic acid oligomers in serum by direct injection liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2008, 870, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Sugai, N.; Yamamoto, T.; Tezuka, Y. Synthesis of orientationally isomeric cyclic stereoblock polylactides with head-to-head and head-to-tail linkages of the enantiomeric segments. ACS Macro Lett. 2012, 1, 902–906. [Google Scholar] [CrossRef]
- Josse, T.; De Winter, J.; Dubois, P.; Coulembier, O.; Gerbaux, P.; Memboeuf, A. A tandem mass spectrometry-based method to assess the architectural purity of synthetic polymers: A case of a cyclic polylactide obtained by click chemistry. Polym. Chem. 2015, 6, 64–69. [Google Scholar] [CrossRef]
- Stanford, M.J.; Pflughaupt, R.L.; Dove, A.P. Synthesis of stereoregular cyclic poly(lactide)s via “thiol-ene” click chemistry. Macromolecules 2010, 43, 6538–6541. [Google Scholar] [CrossRef]
- Culkin, D.A.; Jeong, W.H.; Csihony, S.; Gomez, E.D.; Balsara, N.R.; Hedrick, J.L.; Waymouth, R.M. Zwitterionic polymerization of lactide to cyclic poly(lactide) by using N-heterocyclic carbene organocatalysts. Angew. Chem. Int. Ed. 2007, 46, 2627–2630. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Shin, E.J.; Culkin, D.A.; Hedrick, J.L.; Waymouth, R.M. Zwitterionic polymerization: A kinetic strategy for the controlled synthesis of cyclic polylactide. J. Am. Chem. Soc. 2009, 131, 4884–4891. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Jones, A.E.; Waymouth, R.M. Stereocomplexation in cyclic and linear polylactide blends. Macromolecules 2012, 45, 595–598. [Google Scholar] [CrossRef]
- Prasad, A.V.; Stubbs, L.P.; Zhun, M.; Zhu, Y.H. Zwitterionic ring opening polymerization of lactide by metal free catalysts: Production of cyclic polymers. J. Appl. Polym. Sci. 2012, 123, 1568–1575. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Lomadze, N.; Schwarz, G. Cyclic polylactides by imidazole-catalyzed polymerization of l-lactide. Macromolecules 2008, 41, 7812–7816. [Google Scholar] [CrossRef]
- Brown, H.A.; De Crisci, A.G.; Hedrick, J.L.; Waymouth, R.M. Amidine-mediated zwitterionic polymerization of lactide. ACS Macro Lett. 2012, 1, 1113–1115. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Waymouth, R.M. Zwitterionic ring opening polymerization with isothioureas. ACS Macro Lett. 2014, 3, 1024–1028. [Google Scholar] [CrossRef]
- Yao, L.; Wang, L.; Pan, X.; Tang, N.; Wu, J. Synthesis, characterization and catalytic activity of salen-(sodium)2 and (salen)2-lanthanum-sodium complexes. Inorg. Chim. Acta 2011, 373, 219–225. [Google Scholar] [CrossRef]
- Weil, J.; Mathers, R.T.; Getzler, Y.D.Y.L. Lactide cyclopolymerization by an alumatrane-inspired catalyst. Macromolecules 2012, 45, 1118–1121. [Google Scholar] [CrossRef]
- Piedra-Arroni, E.; Ladavière, C.; Amgoune, A.; Bourissou, D. Ring-opening polymerization with Zn(C6F5)2-based lewis pairs: Original and efficient approach to cyclic polyesters. J. Am. Chem. Soc. 2013, 135, 13306–13309. [Google Scholar] [CrossRef] [PubMed]
- Piromjitpong, P.; Ratanapanee, P.; Thumrongpatanaraks, W.; Kongsaeree, P.; Phomphrai, K. Synthesis of cyclic polylactide catalysed by bis(salicylaldiminato)tin(II) complexes. Dalton Trans. 2012, 41, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Wongmahasirikun, P.; Prom-On, P.; Sangtrirutnugul, P.; Kongsaeree, P.; Phomphrai, K. Synthesis of cyclic polyesters: Effects of alkoxy side chains in salicylaldiminato tin(II) complexes. Dalton Trans. 2015, 44, 12357–12364. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorf, H.R.; Weidner, S.M.; Scheliga, F. Cyclic poly(l-lactide)s via ring-expansion polymerizations catalysed by 2,2-dibutyl-2-stanna-1,3-dithiolane. Polym. Chem. 2017, 8, 1589–1596. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.; Avérous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Mase, N.; Nakaya, N. Organocatalytic synthesis of highly pure linear- and cyclic-polylactide in supercritical carbon dioxide. In Proceedings of the Symposium on Molecular Chirality Asia 2016, Osaka, Japan, 22 April 2016. No. PB-33. [Google Scholar]
- Pratt, R.C.; Lohmeijer, B.G.G.; Long, D.A.; Lundberg, P.N.P.; Dove, A.P.; Li, H.; Wade, C.G.; Waymouth, R.M.; Hedrick, J.L. Exploration, optimization, and application of supramolecular thiourea-amine catalysts for the synthesis of lactide (co)polymers. Macromolecules 2006, 39, 7863–7871. [Google Scholar] [CrossRef]
- Wesch, A.; Dahmen, N.; Ebert, K.H. Measuring the static dielectric constants of pure carbon dioxide and carbon dioxide mixed with ethanol and toluene at elevated pressures. Ber. Bunsengesellsch. Phys. Chem. 1996, 100, 1368–1371. [Google Scholar] [CrossRef]
- Moriyoshi, T.; Kita, T.; Uosaki, Y. Static relative permittivity of carbon dioxide and nitrous oxide up to 30 MPa. Ber. Bunsengesellsch. Phys. Chem. 1993, 97, 589–596. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]


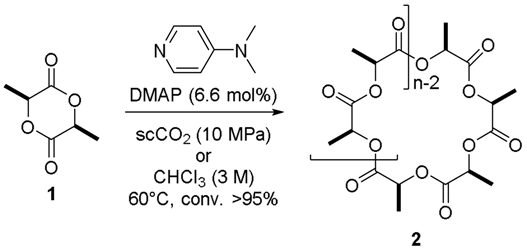
| Entry | Solvent | Time (h) | Mn (a) | PDI (b) | ee (%) (c) |
|---|---|---|---|---|---|
| 1 | CHCl3 | 120 | 12,700 | 3.11 | 39.0 |
| 2 | scCO2 | 5 | 5500 | 1.40 | 90.5 |
| 3 (d) | scCO2 | 2.5 | 11,000 | 1.60 | 93.5 |
| 4 (e) | scCO2 | 5 | 6000 | 1.31 | 91.0 |
| 5 (d,e) | scCO2 | 2.5 | 6800 | 1.20 | 97.0 |
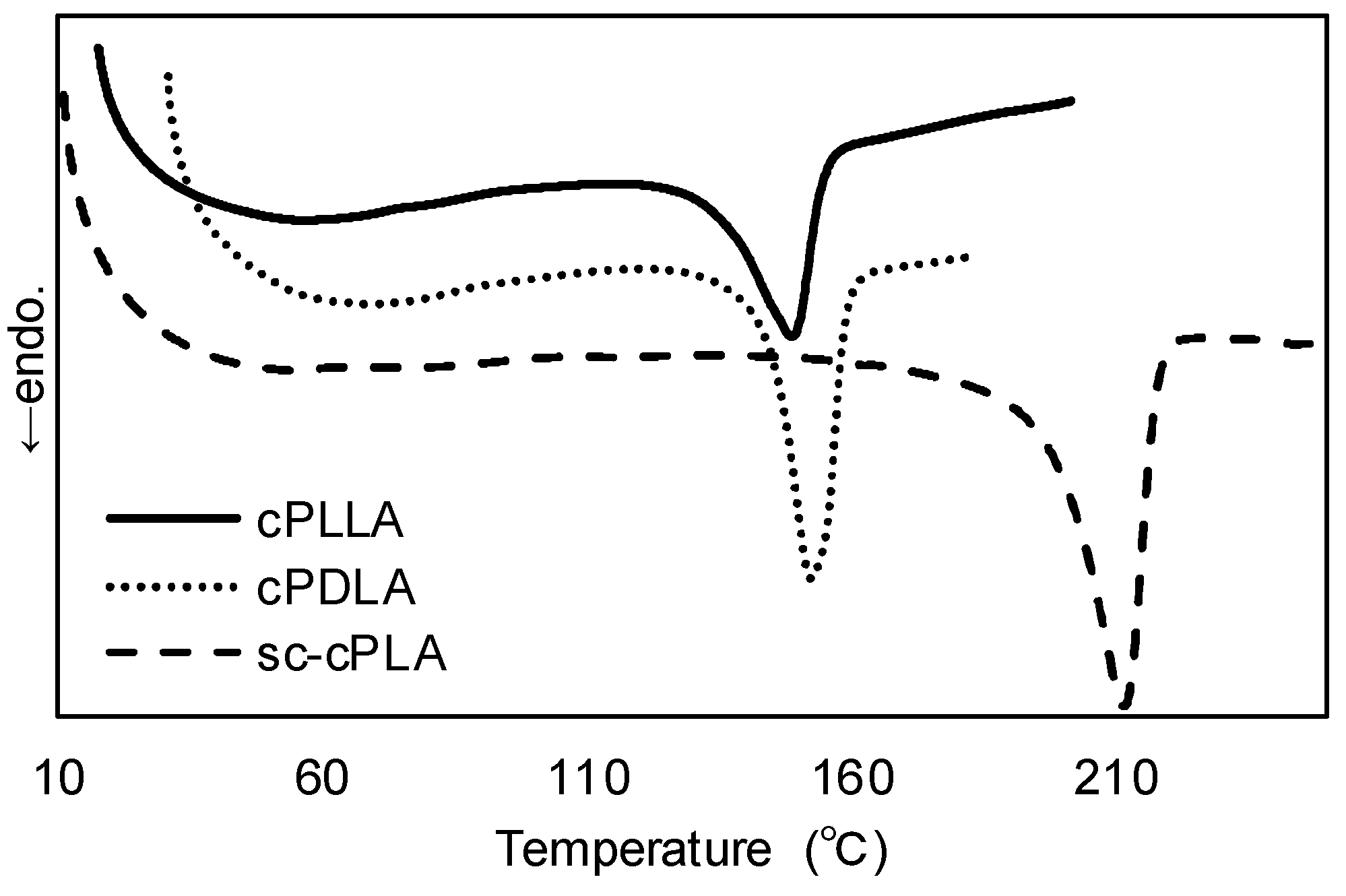
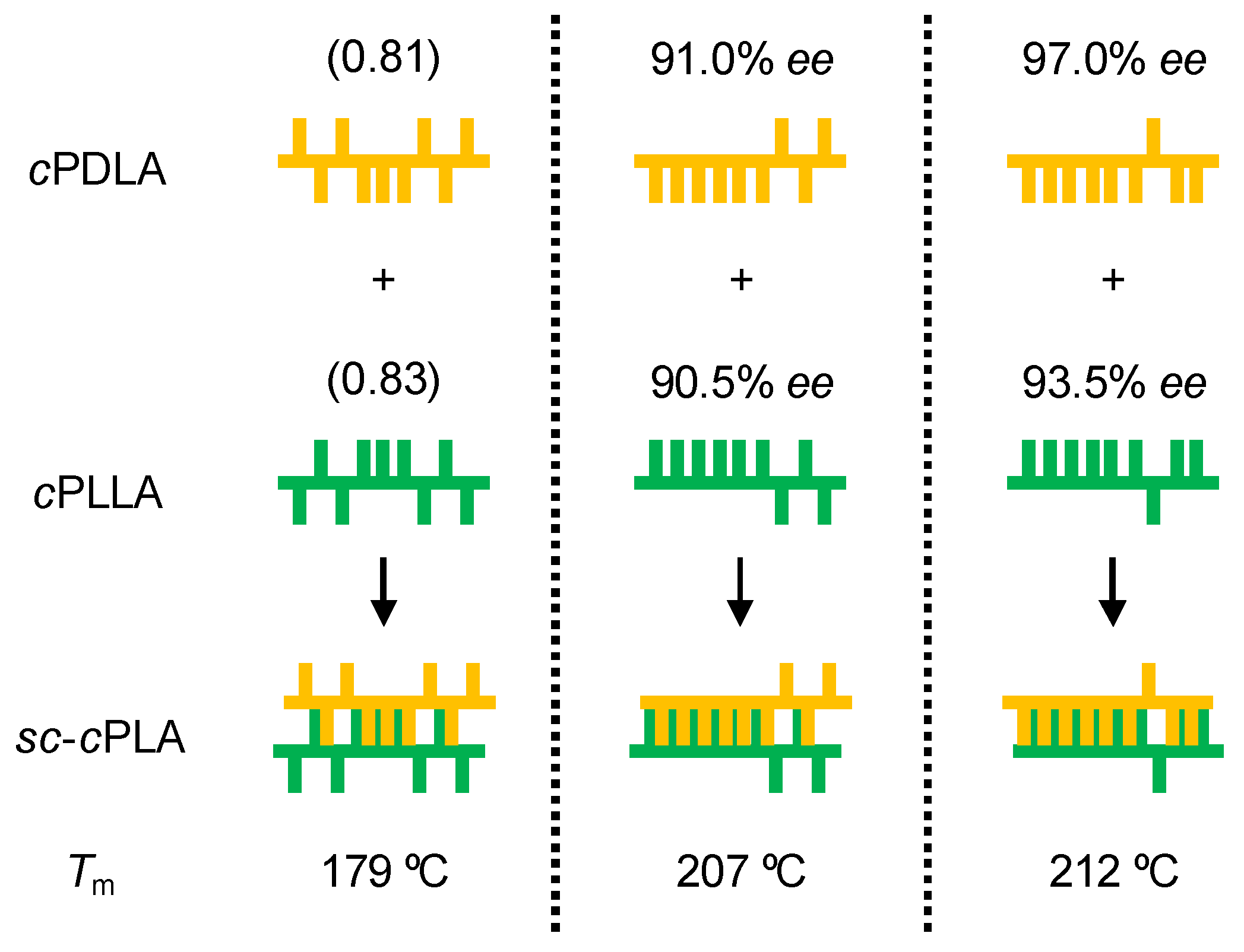
| Entry | cPLA | Mn | PDI | Ee (%) | Tm (°C) | Tm (sc-cPLA) (°C) |
|---|---|---|---|---|---|---|
| 1 | cPDLA | 6800 | 1.20 | 97.0 | 152 | 212 |
| cPLLA | 11,000 | 1.60 | 93.5 | 149 | ||
| 2 | cPDLA | 6000 | 1.31 | 91.0 | 143 | 207 |
| cPLLA | 5500 | 1.40 | 90.5 | 145 | ||
| 3 (a) | cPDLA | 26,000 | 1.40 | (0.81) (b) | 132 | 179 |
| cPLLA | 30,000 | 1.30 | (0.83) (b) | 135 |
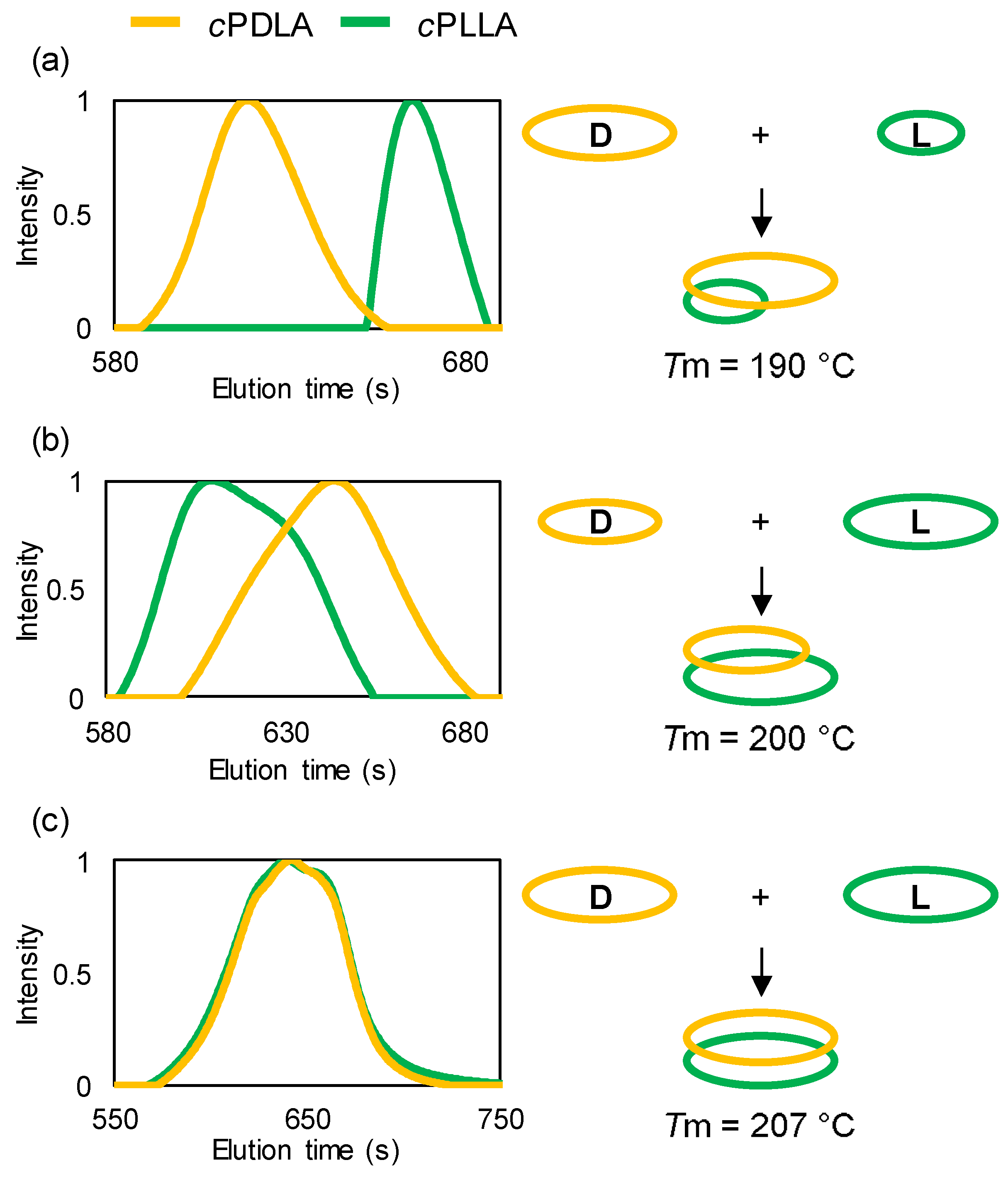
| Entry | cPLA | Mn | PDI | Tm (sc-cPLA) |
|---|---|---|---|---|
| 1 | cPDLA | 7900 | 1.18 | 190 |
| cPLLA | 2600 | 1.20 | ||
| 2 | cPDLA | 5100 | 1.23 | 200 |
| cPLLA | 7400 | 1.23 | ||
| 3 | cPDLA | 6000 | 1.31 | 207 |
| cPLLA | 5500 | 1.40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mase, N.; Moniruzzaman; Yamamoto, S.; Nakaya, Y.; Sato, K.; Narumi, T. Organocatalytic Stereoselective Cyclic Polylactide Synthesis in Supercritical Carbon Dioxide under Plasticizing Conditions. Polymers 2018, 10, 713. https://doi.org/10.3390/polym10070713
Mase N, Moniruzzaman, Yamamoto S, Nakaya Y, Sato K, Narumi T. Organocatalytic Stereoselective Cyclic Polylactide Synthesis in Supercritical Carbon Dioxide under Plasticizing Conditions. Polymers. 2018; 10(7):713. https://doi.org/10.3390/polym10070713
Chicago/Turabian StyleMase, Nobuyuki, Moniruzzaman, Shoji Yamamoto, Yoshitaka Nakaya, Kohei Sato, and Tetsuo Narumi. 2018. "Organocatalytic Stereoselective Cyclic Polylactide Synthesis in Supercritical Carbon Dioxide under Plasticizing Conditions" Polymers 10, no. 7: 713. https://doi.org/10.3390/polym10070713