Intrinsic Fluorescence of PAMAM Dendrimers—Quenching Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
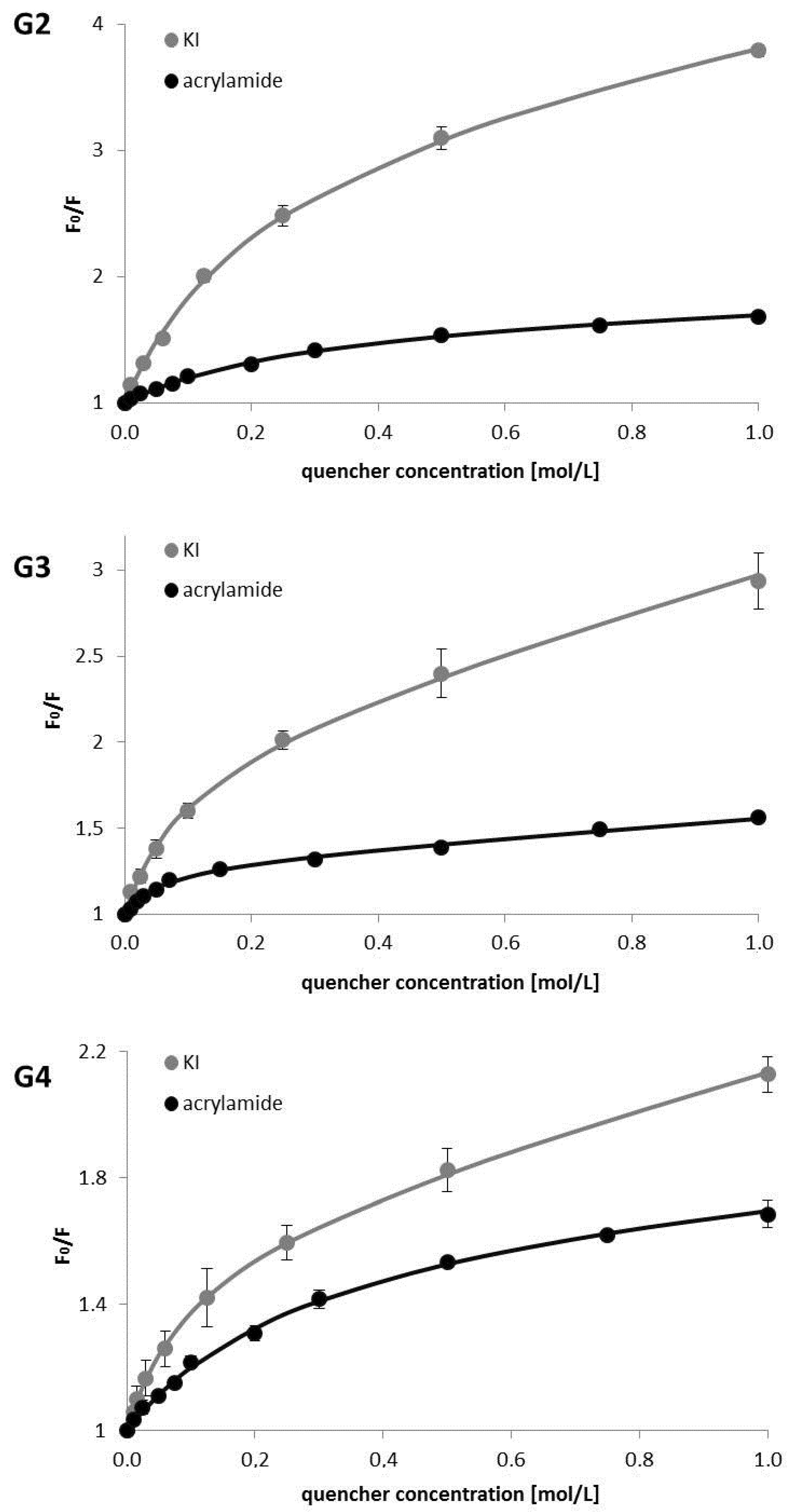
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef] [PubMed]
- Caminati, G.; Turro, N.J.; Tomalia, D.A. Photophysical investigation of starburst dendrimers and their interactions with anionic and cationic surfactants. J. Am. Chem. Soc. 1990, 112, 8515–8522. [Google Scholar] [CrossRef]
- Pistolis, G.; Malliaris, A.; Paleos, C.M.; Tsiourvas, D. Study of poly(amidoamine) starburst dendrimers by fluorescence probing. Langmuir 1997, 13, 5870–5875. [Google Scholar] [CrossRef]
- Wade, D.A.; Torres, P.A.; Tucker, S.A. Spectrochemical investigations in dendritic media: Evaluation of nitromethane as a selective fluorescence quenching agent in aqueous carboxylate-terminated polyamido amine (PAMAM) dendrimers. Anal. Chim. Acta 1999, 397, 17–31. [Google Scholar] [CrossRef]
- Larson, C.; Tucker, S. Intrinsic fluorescence of carboxylate-terminated polyamido amine dendrimers. Appl. Spectr. 2001, 55, 679–683. [Google Scholar] [CrossRef]
- Saravanan, G.; Abe, H. Influence of pH on dendritic structure of strongly fluorescent persulfate-treated poly(amidoamine) dendrimer. J. Photochem. Photobiol. A Chem. 2011, 224, 102–109. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Wu, T.-H.; Jao, Y.-C.; Liu, C.-P.; Lin, H.-Y.; Lo, L.-W.; Yang, C.-S. Unraveling the photoluminescence puzzle of PAMAM dendrimers. Chem. Eur. J. 2011, 17, 7158–7161. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.Z.; Zhang, Y. Nonconventional macromolecular luminogens with aggregation-induced emission characteristics. J. Polym. Sci. A Polym. Chem. 2017, 55, 560–574. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.L.; Qian, Y. Polyamidoamine structure characterization: Amide resonance structure of imidic acid (HO-C=N) and tertiary ammonium. RSC Adv. 2014, 4, 49535–49540. [Google Scholar] [CrossRef]
- Wang, D.; Imae, T. Fluorescence emission from dendrimers and its pH dependence. J. Am. Chem. Soc. 2004, 126, 13204–13205. [Google Scholar] [CrossRef] [PubMed]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Deliv. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Maiti, P.K.; Çağin, T.; Lin, S.-T.; Goddard, W.A. Effect of solvent and pH on the structure of PAMAM dendrimers. Macromolecules 2005, 38, 979–991. [Google Scholar] [CrossRef]
- Raut, S.; Enciso, A.E.; Pavan, G.M.; Lee, C.; Yepremyan, A.; Tomalia, D.A.; Simanek, E.E.; Gryczynski, Z. Intrinsic fluorescence of triazine dendrimers provides a new approach to study dendrimer structure and conformational dynamics. J. Phys. Chem. C 2017, 121, 6946–6954. [Google Scholar] [CrossRef]
- Eftink, M.R.; Hagaman, K.A. Fluorescence quenching of the buried tryptophan residue of cod parvalbumin. Biophys. Chem. 1986, 22, 173–180. [Google Scholar] [CrossRef]
- Das, N.K.; Pawar, L.; Kumar, N.; Mukherjee, S. Quenching interaction of BSA with DTAB is dynamic in nature: A spectroscopic insight. Chem. Phys. Lett. 2015, 635, 50–55. [Google Scholar] [CrossRef]
- Zeno, W.F.; Hilt, S.; Risbud, S.H.; Voss, J.C.; Longo, M.L. Spectroscopic characterization of structural changes in membrane scaffold proteins entrapped within mesoporous silica gel monoliths. ACS Appl. Mater. Interfaces 2015, 7, 8640–8649. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.A.; Olivares-Ortega, C.; Soto-Arriaza, M.A.; Carmona-Ribeiro, A.M. Interaction of gramicidin with DPPC/DODAB bilayer fragments. Biochim. Biophys. Acta Biomembr. 2012, 1818, 3064–3071. [Google Scholar] [CrossRef] [PubMed]
- Bagul, R.S.; Rajesh, Y.B.R.D.; Yayamurugan, G.; Bera, A.; Sood, A.K.; Yayaraman, N. Photophysical behavior of poly(propyl ether imine) dendrimer in the presence of nitroaromatic compounds. J. Photochem. Photobiol. A Chem. 2013, 253, 1–6. [Google Scholar] [CrossRef]
- Koley, S.; Ghosh, S. The study of electron transfer reactions in a dendrimeric assembly: Proper utilization of dendrimer fluorescence. Phys. Chem. Chem. Phys. 2016, 18, 24830–24834. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jia, D.; Liu, C.; Wang, D. Synthesis and fluorescent properties of hyperbranched poly(amido amine) with new fluorescence-emitting moieties. Adv. Mater. Res. 2011, 348–351. [Google Scholar] [CrossRef]
- Ji, A.; Qian, Y. A study using quantum chemical theory methods on the intrinsic fluorescence emission mechanism of PAMAM. RSC Adv. 2014, 4, 58788–58794. [Google Scholar] [CrossRef]


| Generation | λmax (nm) | Stokes Shift (nm) | Fmax (a.u.) |
|---|---|---|---|
| G2 | 417 | 84 | 415 |
| G3 | 415 | 85 | 383 |
| G4 | 414 | 80 | 353 |
| Quencher | Generation | K1 [M−1] | f1 | K2 [M−1] | f2 | R2 |
|---|---|---|---|---|---|---|
| Acrylamide | G2 | 0.23 ± 0.10 | 0.68 ± 0.07 | 7.41 ± 2.35 | 0.32 ± 0.06 | 0.992 |
| G3 | 0.21 ± 0.04 | 0.76 ± 0.02 | 22.06 ± 4.50 | 0.24 ± 0.02 | 0.994 | |
| G4 | 0.07 ± 0.02 | 0.56 ± 0.02 | 5.91 ± 0.50 | 0.44 ± 0.02 | 0.998 | |
| KI | G2 | 0.25 ± 0.21 | 0.28 ± 0.04 | 16.13 ± 2.01 | 0.72 ± 0.04 | 0.997 |
| G3 | 0.50 ± 0.14 | 0.47 ± 0.04 | 20.75 ± 2.94 | 0.53 ± 0.04 | 0.997 | |
| G4 | 0.33 ± 0.04 | 0.59 ± 0.02 | 16.03 ± 1.24 | 0.41 ± 0.02 | 0.999 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopka, M.; Janaszewska, A.; Klajnert-Maculewicz, B. Intrinsic Fluorescence of PAMAM Dendrimers—Quenching Studies. Polymers 2018, 10, 540. https://doi.org/10.3390/polym10050540
Konopka M, Janaszewska A, Klajnert-Maculewicz B. Intrinsic Fluorescence of PAMAM Dendrimers—Quenching Studies. Polymers. 2018; 10(5):540. https://doi.org/10.3390/polym10050540
Chicago/Turabian StyleKonopka, Malgorzata, Anna Janaszewska, and Barbara Klajnert-Maculewicz. 2018. "Intrinsic Fluorescence of PAMAM Dendrimers—Quenching Studies" Polymers 10, no. 5: 540. https://doi.org/10.3390/polym10050540






