Preparation and Evaluation of Coal Fly Ash/Chitosan Composites as Magnetic Supports for Highly Efficient Cellulase Immobilization and Cellulose Bioconversion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
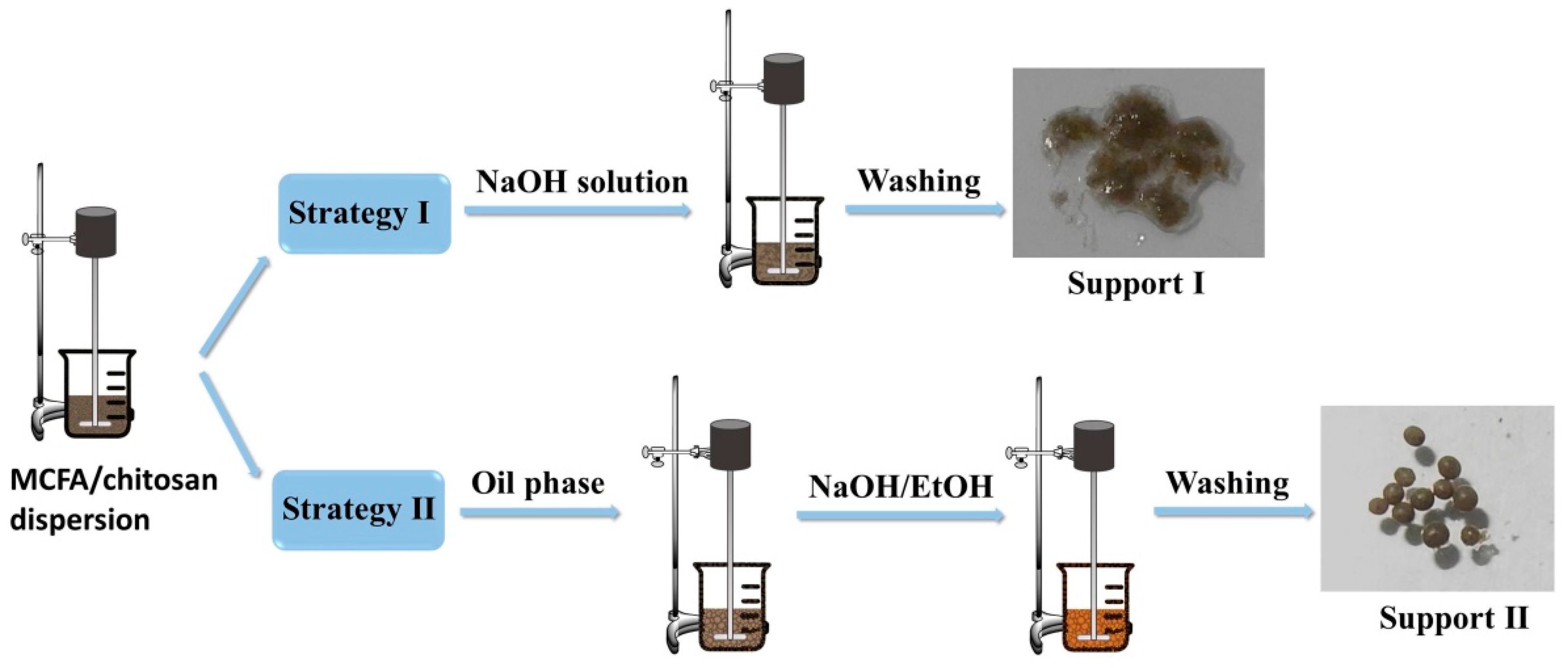
2.2. Preparation of MCFA-Chitosan Supports
2.3. Immobilization of Cellulase
2.4. Characterization
2.5. Activity Assay
2.6. Determination of Thermal Stability
2.7. Reusability Assay
3. Results and Discussion
3.1. Strategy for Preparation of Supports
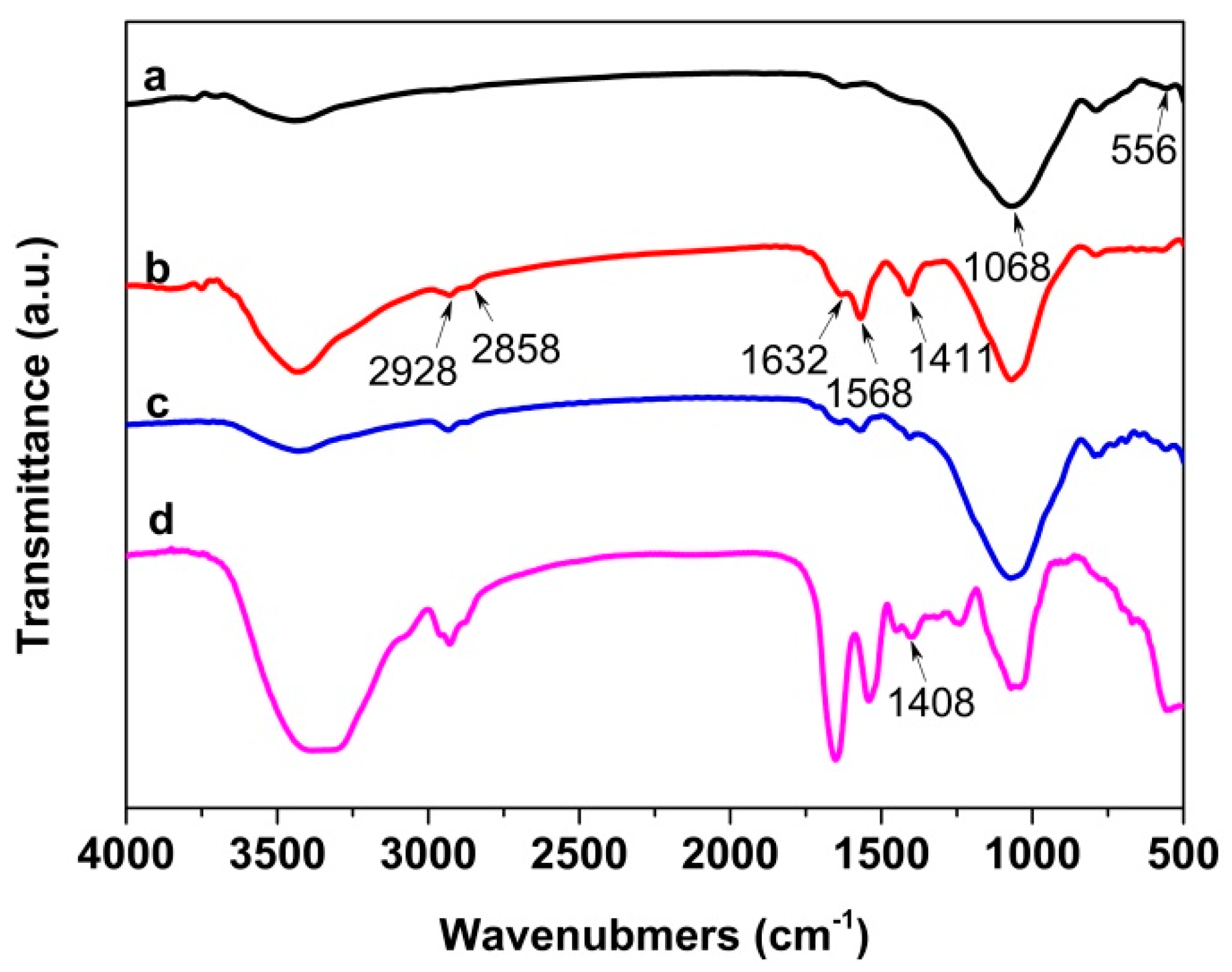
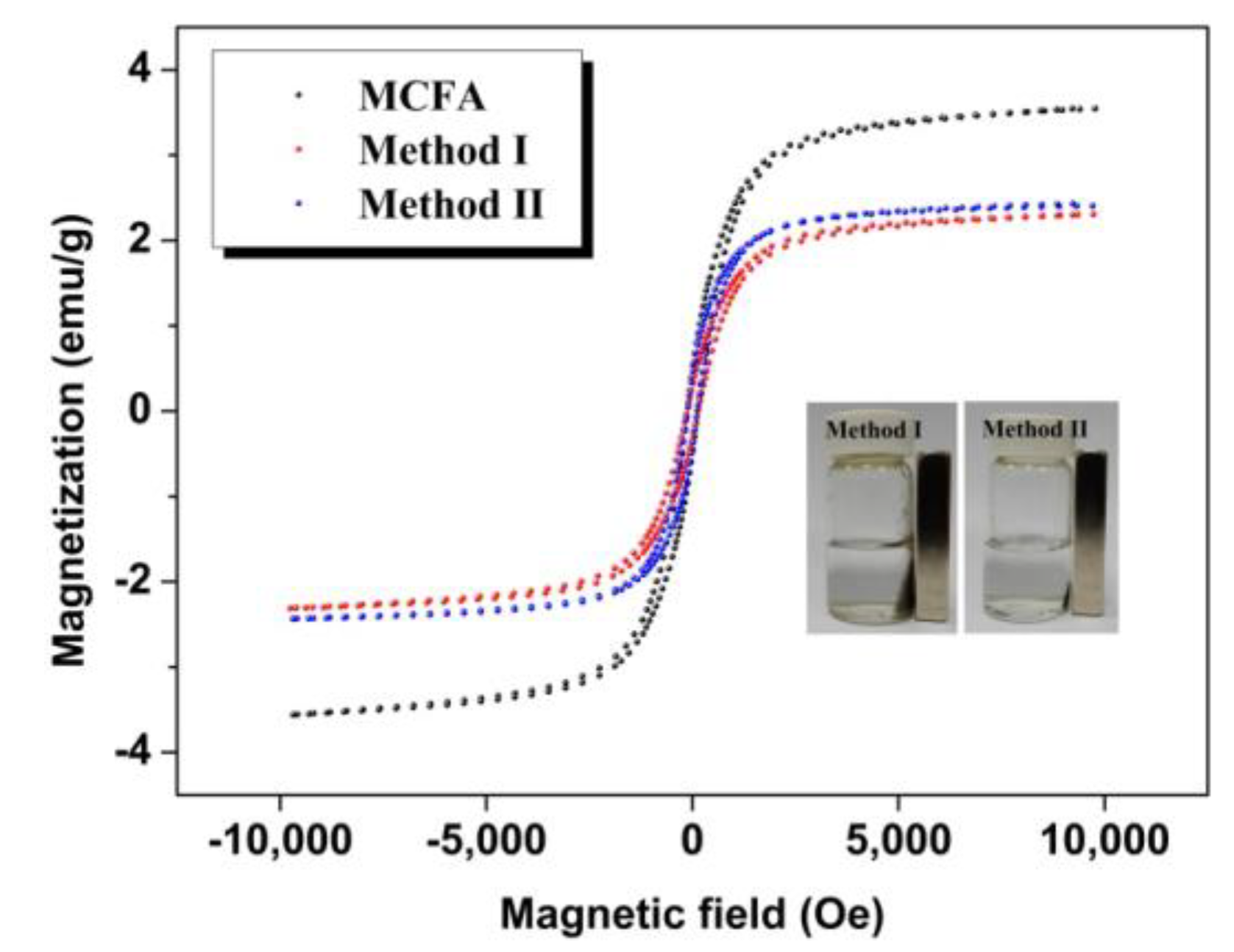
3.2. Characterization of Immobilized Cellulase
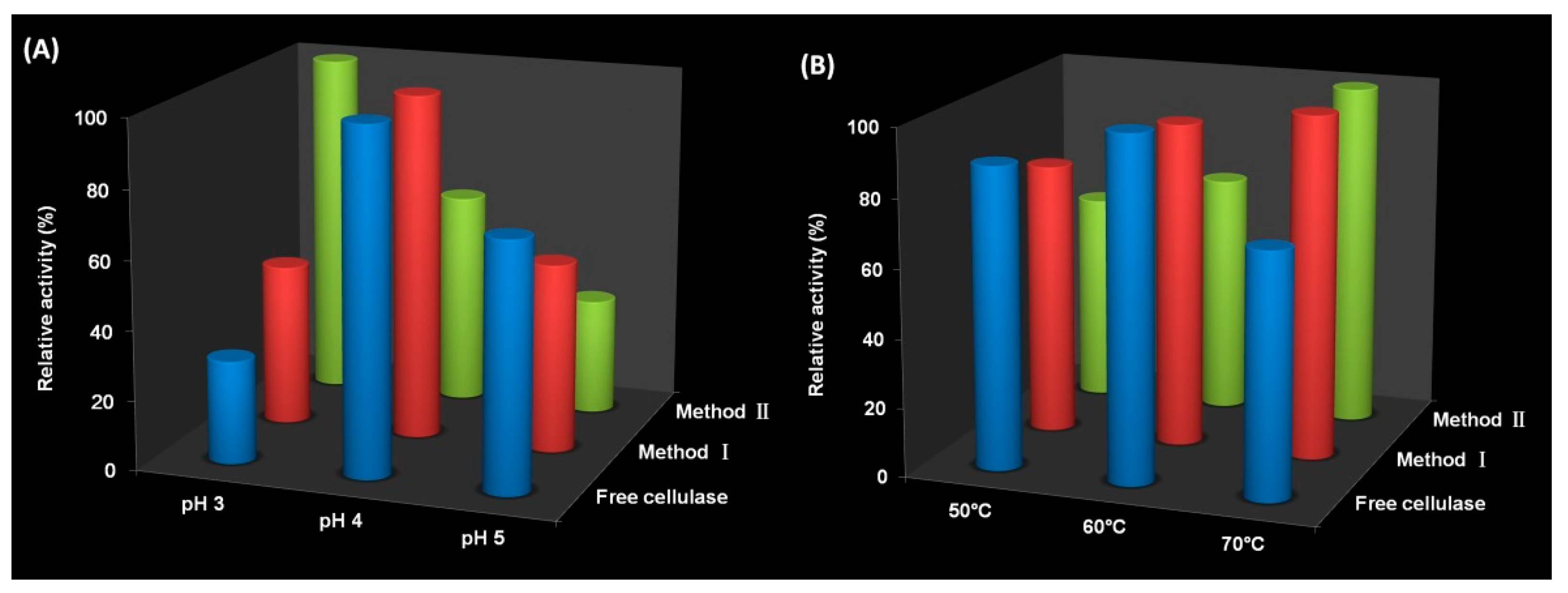
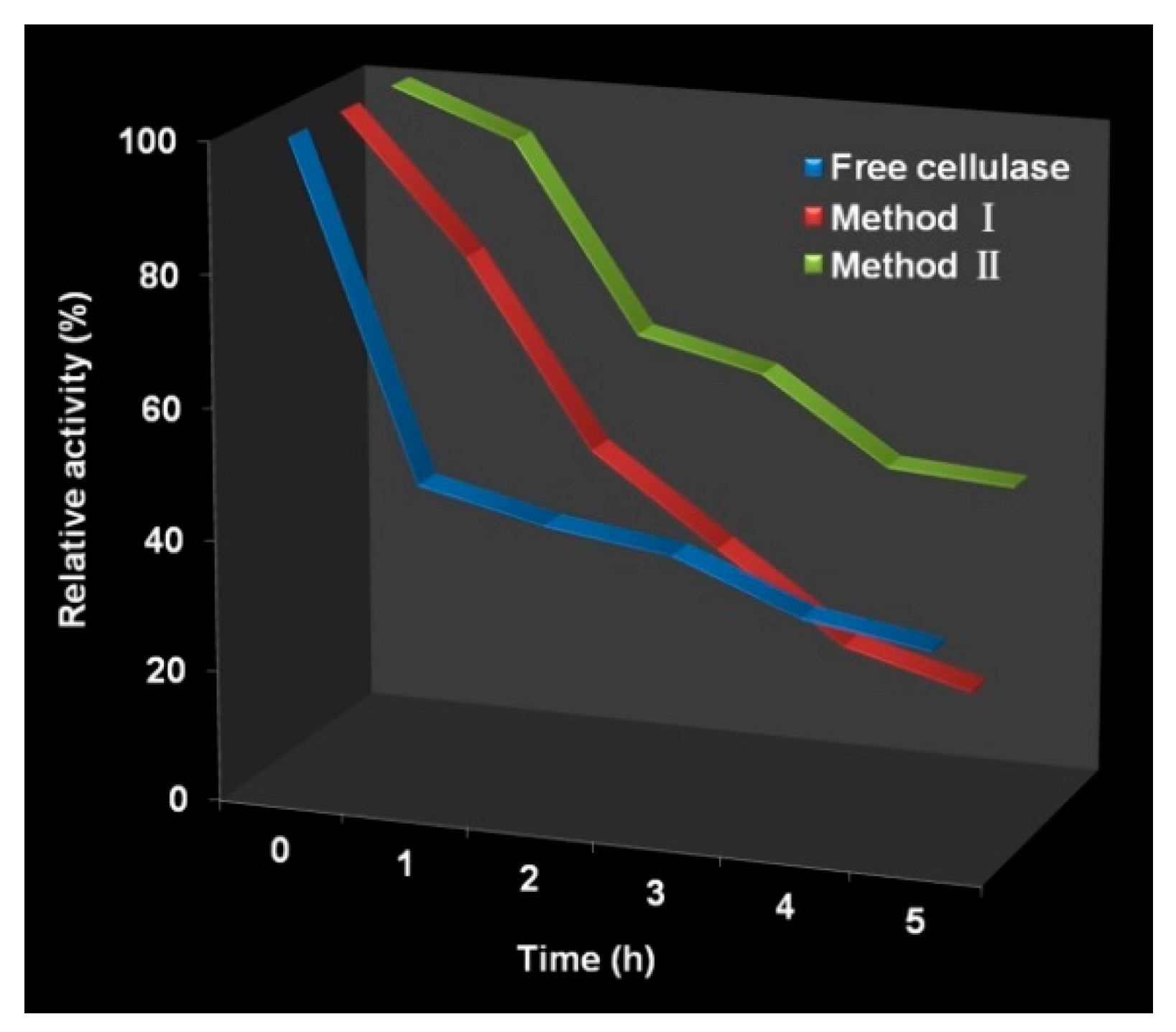
3.3. Activity of Immobilized Cellulase
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Garcia-Galan, C.; Barbosa, O.; Ortiz, C.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Biotechnological prospects of the lipase from Mucor javanicus. J. Mol. Catal. B Enzym. 2013, 93, 34–43. [Google Scholar] [CrossRef]
- Gurung, N.; Ray, S.; Bose, S.; Rai, V. A broader view: Microbial enzymes and their relevance in industries, medicine, and beyond. Biomed. Res. Int. 2013, 2013, 329121. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Esposito, E. Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: A review. Appl. Catal. B 2000, 28, 83–99. [Google Scholar] [CrossRef]
- Minussi, R.C.; Pastore, G.M.; Durán, N. Potential applications of laccase in the food industry. Trends Food Sci. Technol. 2002, 13, 205–216. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Jia, F.; Narasimhan, B.; Mallapragada, S. Materials-based strategies for multi-enzyme immobilization and co-localization: A review. Biotechnol. Bioeng. 2014, 111, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.J.; Guisan, M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Clark, D.S. Can immobilization be exploited to modify enzyme activity? Trends Biotechnol. 1994, 12, 439–443. [Google Scholar] [CrossRef]
- Mateo, B.C.C.C.; Fuentes, M.; Vian, A.J.; Garca, L.; Carrascosa, A.V.; Guisn, J.M.; Fernandez-Lafuente, R. The immobilization of a thermophilic β-galactosidase on Sepabeads supports decreases product inhibition: Complete hydrolysis of lactose in dairy products. Enzym. Microb. Technol. 2003, 33, 199–205. [Google Scholar]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzym. Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Tanaka, H.; Fujimoto, S.; Fujii, A.; Hino, R.; Kawazoe, T. Microwave assisted two-step process for rapid synthesis of Na-A zeolite from coal fly ash. Ind. Eng. Chem. Res. 2008, 47, 226–230. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.J.; Rangan, B.V. On the development of fly ash-based geopolymer concrete. Mater. J. 2004, 101, 467–472. [Google Scholar]
- Mittra, B.N.; Karmakar, S.; Swain, D.K.; Ghosh, B.C. Fly ash—A potential source of soil amendment and a component of integrated plant nutrient supply system. Fuel 2005, 84, 1447–1451. [Google Scholar] [CrossRef]
- Wee, J.H. A review on carbon dioxide capture and storage technology using coal fly ash. Appl. Energy 2013, 106, 143–151. [Google Scholar] [CrossRef]
- Wang, X.S. Mineralogical and chemical composition of magnetic fly ash fraction. Environ. Earth Sci. 2014, 71, 1673–1681. [Google Scholar] [CrossRef]
- Bourliva, A.; Papadopoulou, L.; Aidona, E.; Simeonidis, K.; Vourlias, G.; Devlin, E.; Sanakis, Y. Enrichment and oral bioaccessibility of selected trace elements in fly ash-derived magnetic components. Environ. Sci. Pollut. Res. 2017, 24, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Qi, Z.; Zhu, H. Preparation of core–shell magnetic polydopamine/alginate biocomposite for Candida rugosa lipase immobilization. Colloids Surf. B Biointerfaces 2015, 128, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Borowska, M.; Chelminiak-Dudkiewicz, D.; Siódmiak, T.; Sikora, A.; Wegrzynowska-Drzymalska, K.; Skopinska-Wisniewska, J.; Marszałł, M.P. Chitosan–collagen coated magnetic nanoparticles for lipase immobilization—New type of “enzyme friendly” polymer shell crosslinking with squaric acid. Catalysts 2017, 7, 26. [Google Scholar] [CrossRef]
- Krajewska, B. Chitin and its derivative as supports for immobilization of enzymes. Eng. Life Sci. 1991, 11, 269–277. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a strategy for improving enzyme properties-application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin-and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Krajewska, B.; Zaborska, W.; Leszko, M. Inhibition of chitosan-immobilized urease by boric acid as determined by integration methods. J. Mol. Catal. B Enzym. 1997, 3, 231–238. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Lee, S.M. Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv. Colloid Interface Sci. 2013, 201, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.L.; Wang, J.L. Immobilized lipase on magnetic chitosan microspheres for transesterification of soybean oil. Biomass Bioenergy 2012, 36, 373–380. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Lahtinen, M.; Sillanpää, M. Thermodynamic and isotherm studies of methyl orange adsorption. Chem. Eng. J. 2015, 259, 1–10. [Google Scholar] [CrossRef]
- Chen, A.; Zeng, G.; Chen, G.; Hu, X.; Yan, M.; Guan, S.; Shang, C.; Lu, L.; Zou, Z.; Xie, G. Novel thiourea-modified magnetic ion-imprinted chitosan/TiO2 composite for simultaneous removal of cadmium and 2,4-dichlorophenol. Chem. Eng. J. 2012, 191, 85–94. [Google Scholar] [CrossRef]
- Yang, C.; Mo, H.; Zang, L.; Chen, J.; Wang, Z.; Qiu, J. Surface functionalized natural inorganic nanorod for highly efficient cellulase immobilization. RSC Adv. 2016, 6, 76855–76860. [Google Scholar] [CrossRef]
- Li, X.Y.; Yang, Q.; Ouyang, J.; Yang, H.M.; Chang, S. Chitosan modified halloysite nanotubes as emerging porous microspheres for drug carrier. Appl. Clay Sci. 2016, 126, 306–312. [Google Scholar] [CrossRef]
- Futalan, C.M.; Kan, C.C.; Dalida, M.L.; Hsien, K.J.; Pascua, C.; Wan, M.W. Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite. Carbohydr. Polym. 2011, 83, 528–536. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.L.; Freire, D.M.G.; Manoel, E.A.; Fernandez-Lafuente, R.; de Oliveira, D. Nanomaterials for biocatalyst immobilization–state of the art and future trends. RSC Adv. 2016, 6, 104675–104692. [Google Scholar] [CrossRef]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzym. Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Torres, R.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Heterofunctional supports in enzyme immobilization: From traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 2013, 14, 2433–2462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betancor, L.; Fuentes, M.; Dellamora-Ortiz, G.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Mateo, C.; Guisán, J.M.; Fernández-Lafuente, R. Dextran aldehyde coating of glucose oxidase immobilized on magnetic nanoparticles prevents its inactivation by gas bubbles. J. Mol. Catal. B Enzym. 2005, 32, 97–101. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Wilson, L.; Ferrarotti, S.A.; Guisan, J.M.; Fernandez-Lafuente, R.; Mateo, C. Improvement of the stability of alcohol dehydrogenase by covalent immobilization on glyoxyl-agarose. J. Biotechnol. 2006, 125, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Mateo, G.D.O.C.; Fernández-Lafuente, R.; Guisán, J.M. Different mechanisms of protein immobilization on glutaraldehyde activated supports: Effect of support activation and immobilization conditions. Enzym. Microb. Technol. 2006, 39, 877–882. [Google Scholar] [CrossRef]
- López-Gallego, F.; Betancor, L.; Mateo, C.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; Fernández-Lafuente, R. Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J. Biotechnol. 2005, 119, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lorente, G.; Palomo, J.M.; Mateo, C.; Munilla, R.; Ortiz, C.; Cabrera, Z.; Fernández-Lafuente, R. Glutaraldehyde cross-linking of lipases adsorbed on aminated supports in the presence of detergents leads to improved performance. Biomacromolecules 2006, 7, 2610–2615. [Google Scholar] [CrossRef] [PubMed]
- Bezbradica, D.I.; Mateo, C.; Guisan, J.M. Novel support for enzyme immobilization prepared by chemical activation with cysteine and glutaraldehyde. J. Mol. Catal. B Enzym. 2014, 102, 218–224. [Google Scholar] [CrossRef]
- Barros, R.M.; Extremina, C.I.; Gonçalves, I.C.; Braga, B.O.; Balcão, V.M.; Malcata, F.X. Hydrolysis of α-lactalbumin by cardosin A immobilized on highly activated supports. Enzym. Microb. Technol. 2003, 33, 908–916. [Google Scholar] [CrossRef]
- Zang, L.; Qiu, J.; Wu, X.; Zhang, W.; Sakai, E.; Wei, Y. Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind. Eng. Chem. Res. 2014, 533, 3448–3454. [Google Scholar] [CrossRef]
- Xue, Z.X.; Yang, G.P.; Wang, G.C.; Niu, J.F.; Cao, X.Y. Preparation of porous chitosan/agarose microsphere and its R-phycoerythrin release properties. J. Appl. Polym. Sci. 2007, 103, 2759–2766. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Pu, Z.; Zhao, Y.; Jia, D.; Chen, H.; Wen, T.; Hu, B.; Alsaedi, A.; Hayat, T.; et al. Synthesis of magnetic Fe3O4/CFA composites for the efficient removal of U(VI) from wastewater. Chem. Eng. J. 2017, 320, 448–457. [Google Scholar] [CrossRef]
- Mohan, S.; Gandhimathi, R. Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. J. Hazard. Mater. 2009, 169, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.X.; Li, Y.F.; Lin, L. Synthesis of a mesoporous functional copolymer bead carrier and its properties for glucoamylase immobilization. Appl. Microbiol. Biotechnol. 2009, 83, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Bordbar, A.K.; Zare, D.; Davoodi, D.; Noruzi, M.; Barkhi, M.; Tabatabaei, M. Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem. Eng. J. 2011, 171, 669–673. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; Kumar, C.S.S.R.; Theegala, C. Preparation and characterization of cellulase-bound magnetite nanoparticles. J. Mol. Catal. B Enzym. 2011, 68, 139–146. [Google Scholar] [CrossRef]
- Wang, F.; Guo, C.; Yang, L.R.; Liu, C.Z. Magnetic mesoporous silica nanoparticles: Fabrication and their laccase immobilization performance. Bioresour. Technol. 2010, 101, 8931–8935. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, L.; Qiao, X.; Hu, L.; Yang, C.; Liu, Q.; Wei, C.; Qiu, J.; Mo, H.; Song, G.; Yang, J.; et al. Preparation and Evaluation of Coal Fly Ash/Chitosan Composites as Magnetic Supports for Highly Efficient Cellulase Immobilization and Cellulose Bioconversion. Polymers 2018, 10, 523. https://doi.org/10.3390/polym10050523
Zang L, Qiao X, Hu L, Yang C, Liu Q, Wei C, Qiu J, Mo H, Song G, Yang J, et al. Preparation and Evaluation of Coal Fly Ash/Chitosan Composites as Magnetic Supports for Highly Efficient Cellulase Immobilization and Cellulose Bioconversion. Polymers. 2018; 10(5):523. https://doi.org/10.3390/polym10050523
Chicago/Turabian StyleZang, Limin, Xuan Qiao, Lei Hu, Chao Yang, Qifan Liu, Chun Wei, Jianhui Qiu, Haodao Mo, Ge Song, Jun Yang, and et al. 2018. "Preparation and Evaluation of Coal Fly Ash/Chitosan Composites as Magnetic Supports for Highly Efficient Cellulase Immobilization and Cellulose Bioconversion" Polymers 10, no. 5: 523. https://doi.org/10.3390/polym10050523



