PLGA Microspheres Loaded with β-Cyclodextrin Complexes of Epigallocatechin-3-Gallate for the Anti-Inflammatory Properties in Activated Microglial Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Epigallocatechin-3-Gallate-β-Cyclodextrin (CD-EG) Complexes
2.3. Preparation of Epigallocatechin-3-Gallate-β-Cyclodextrin Complex-Loaded PLGA Microspheres
2.4. Crude Yield and Loading Efficiency
2.5. Fourier Transform Infrared Spectrometry (FTIR)
2.6. Morphology of CD-EG Complex-Loaded PLGA Microspheres
2.7. Release Profile of CD-EG Complex-Loaded PLGA Microspheres
2.8. BV-2 Cell Culture
2.9. Cytotoxicity
2.10. NO Qualification
2.11. Western Blot
2.12. Statistical Analyses
3. Results and Discussion
3.1. Preparation of Epigallocatechin-3-Gallate-β-Cyclodextrin (CD-EG) Complexes
3.2. Preparation of CD-EG Complex-Loaded PLGA Microspheres
3.3. FTIR Characterization
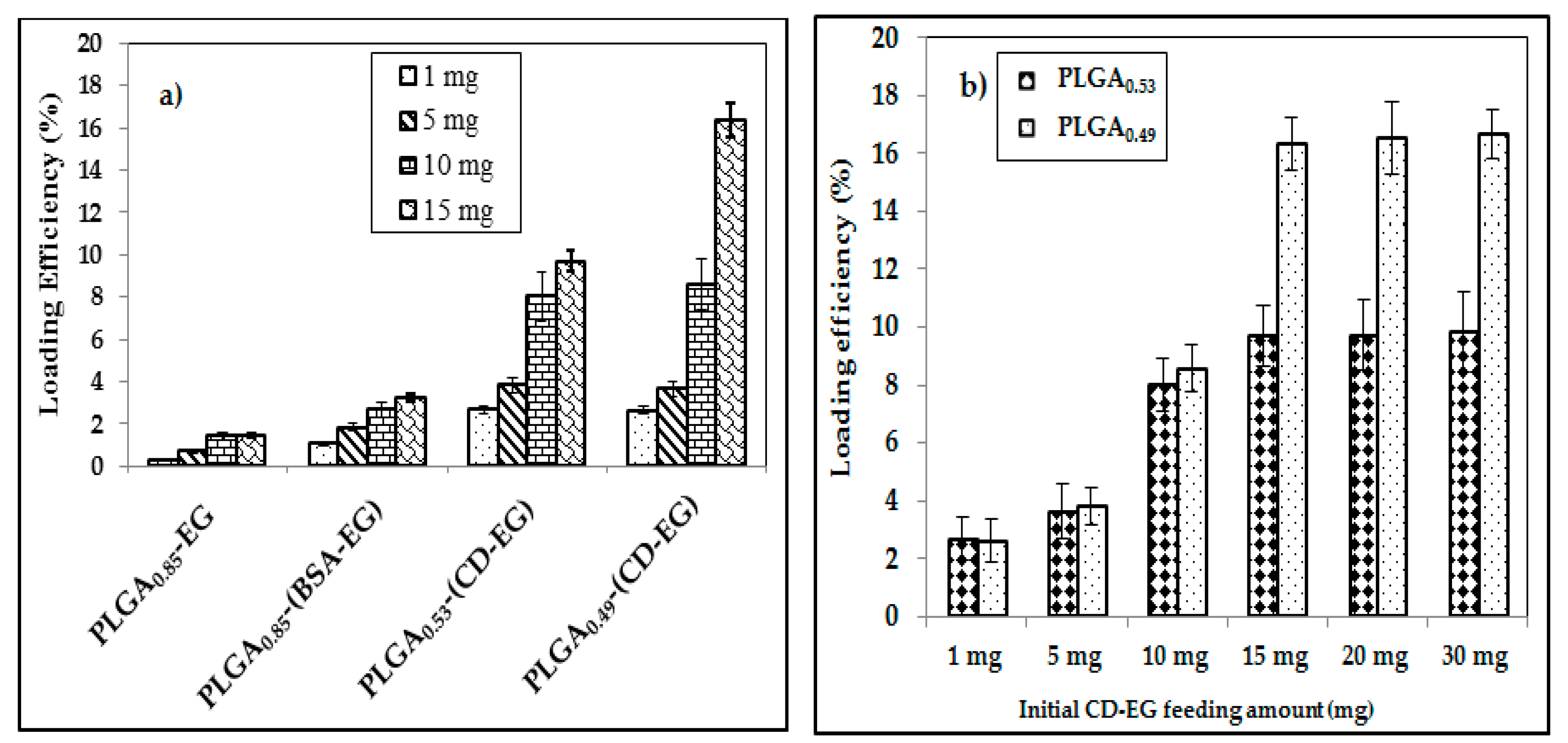
3.4. Loading Efficiency
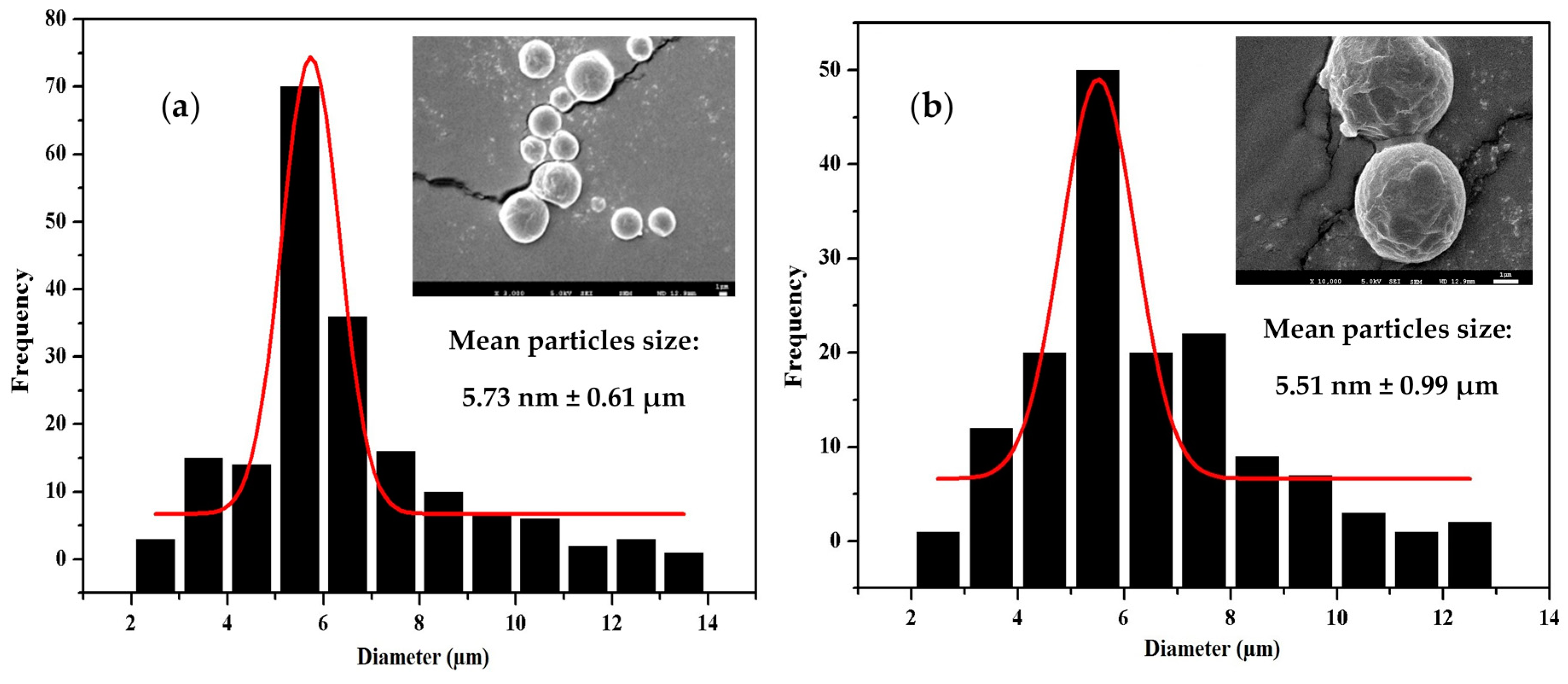
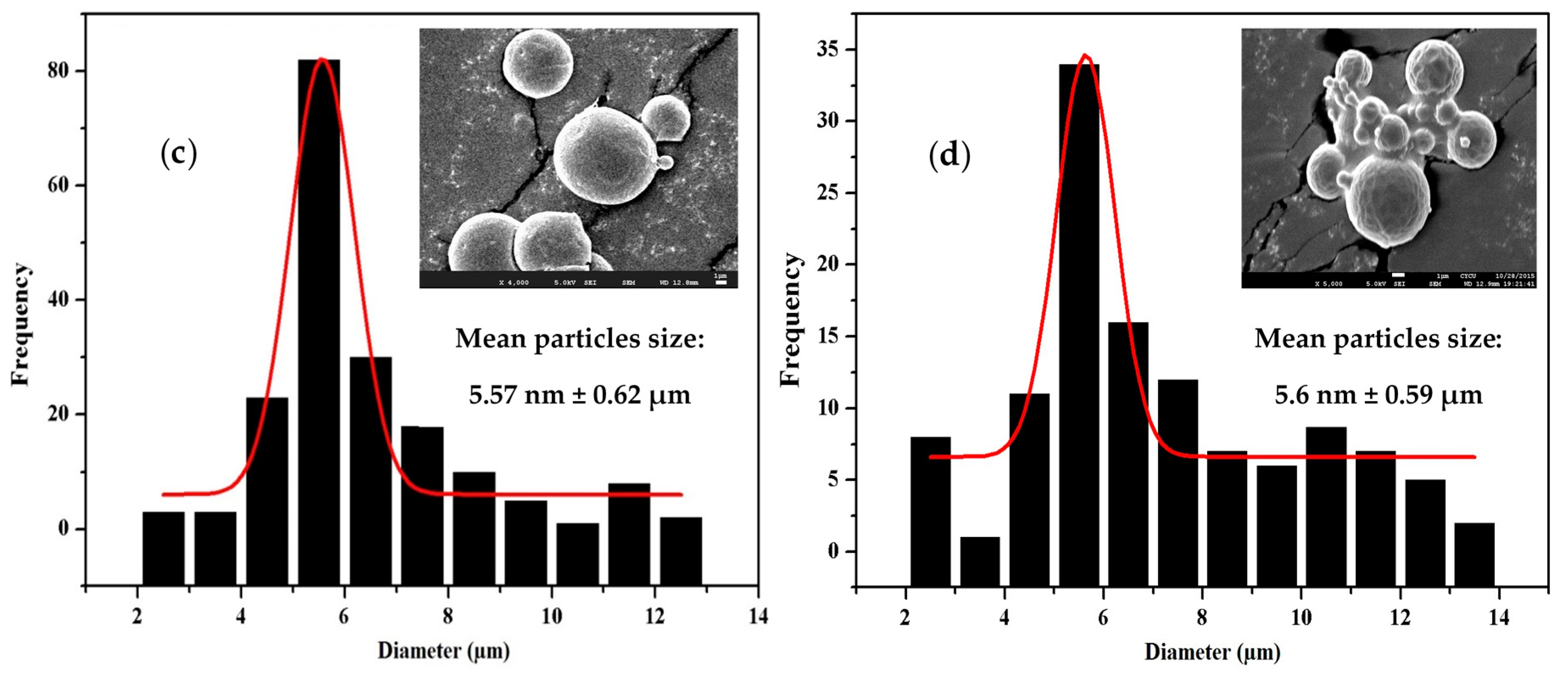
3.5. Morphology, Particle Size, and Size Distribution
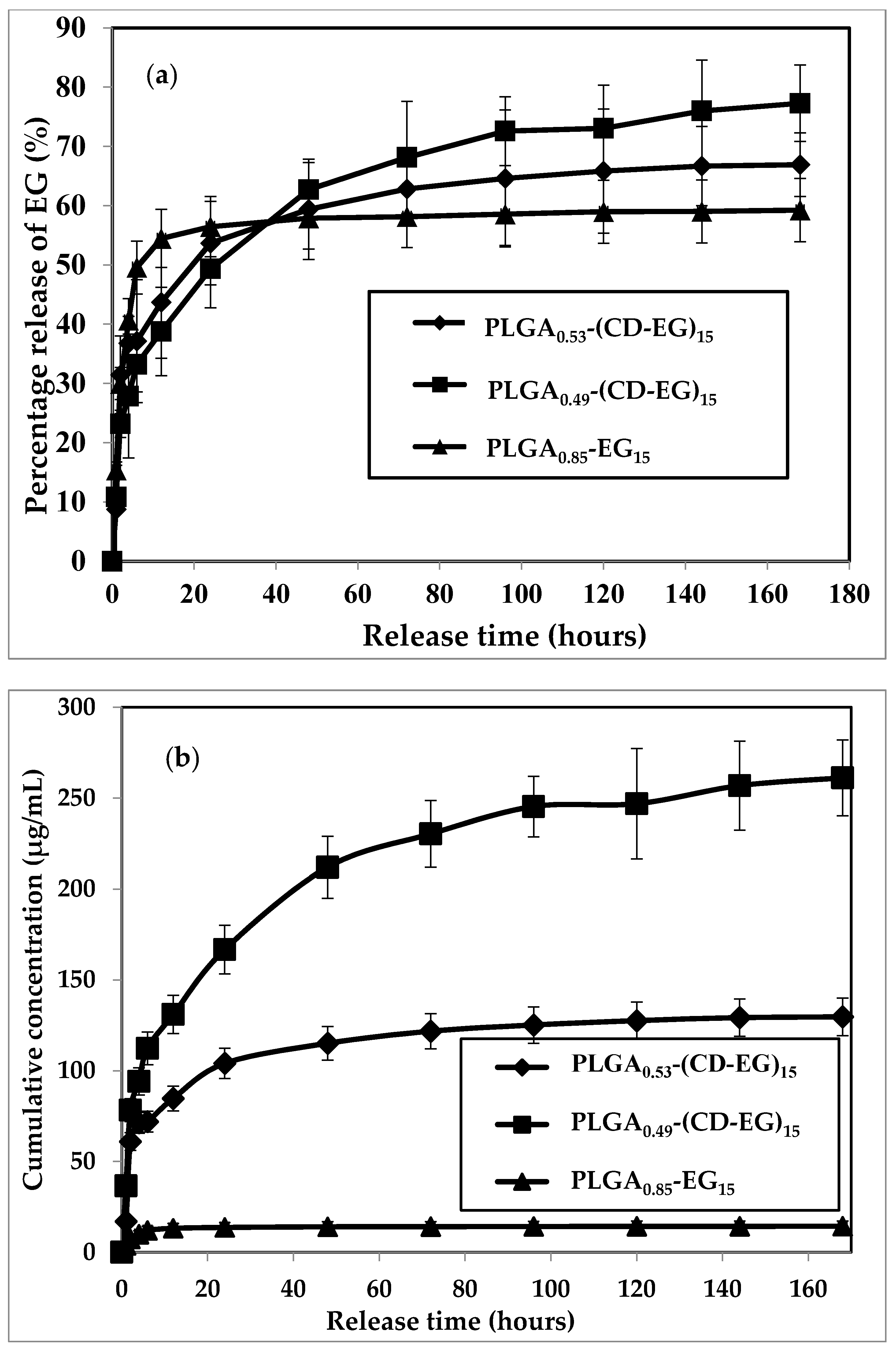
3.6. Release Profile
3.7. Cell Viability
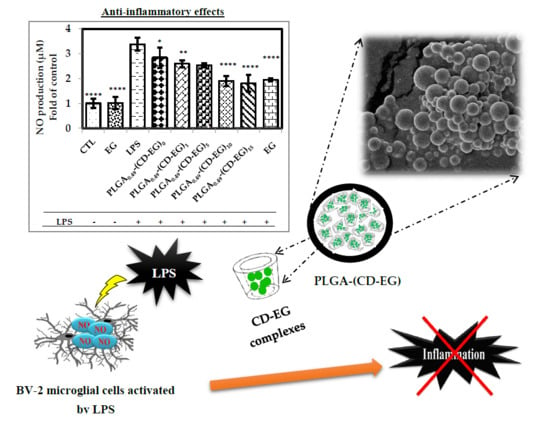
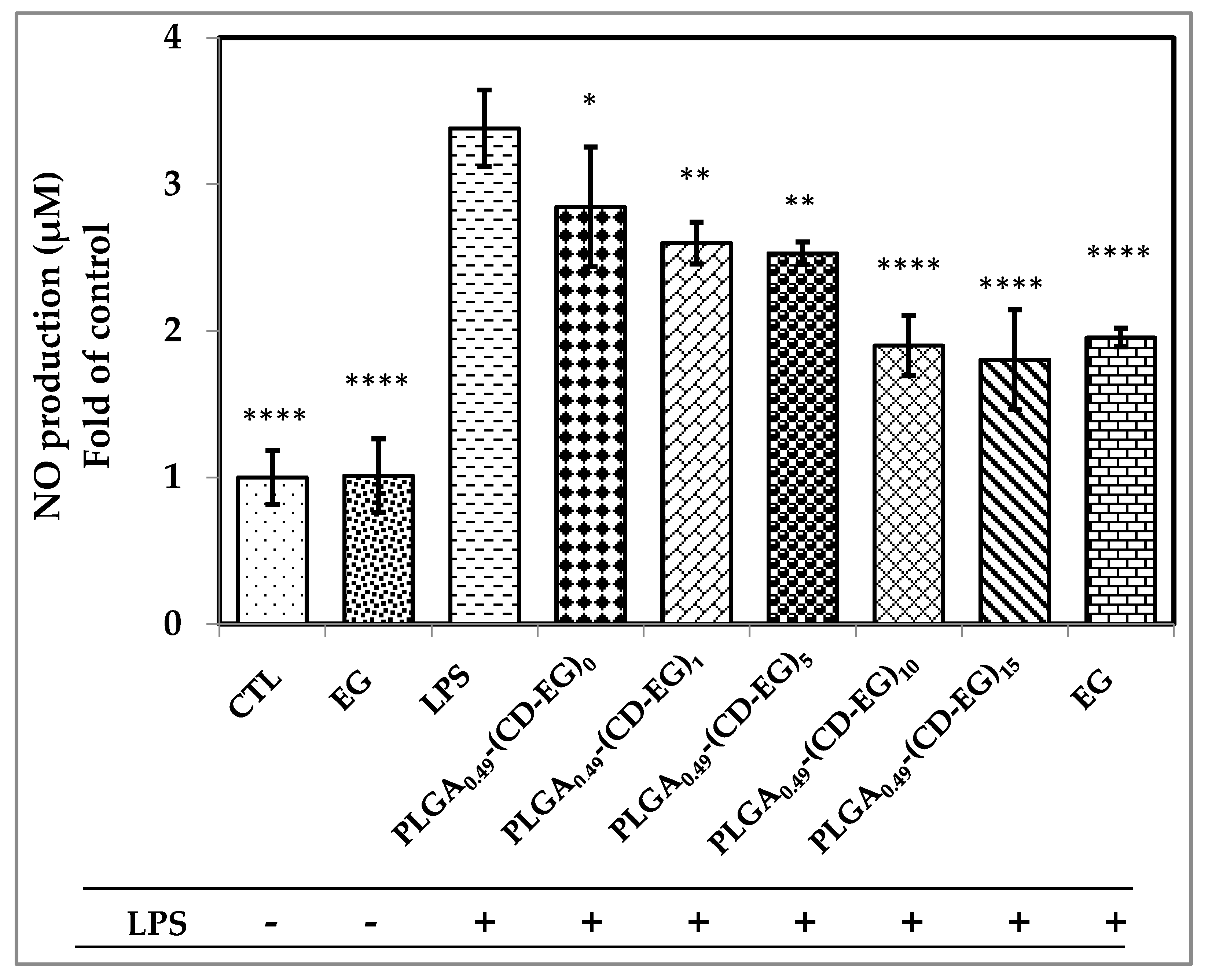
3.8. Investigation of NO Production
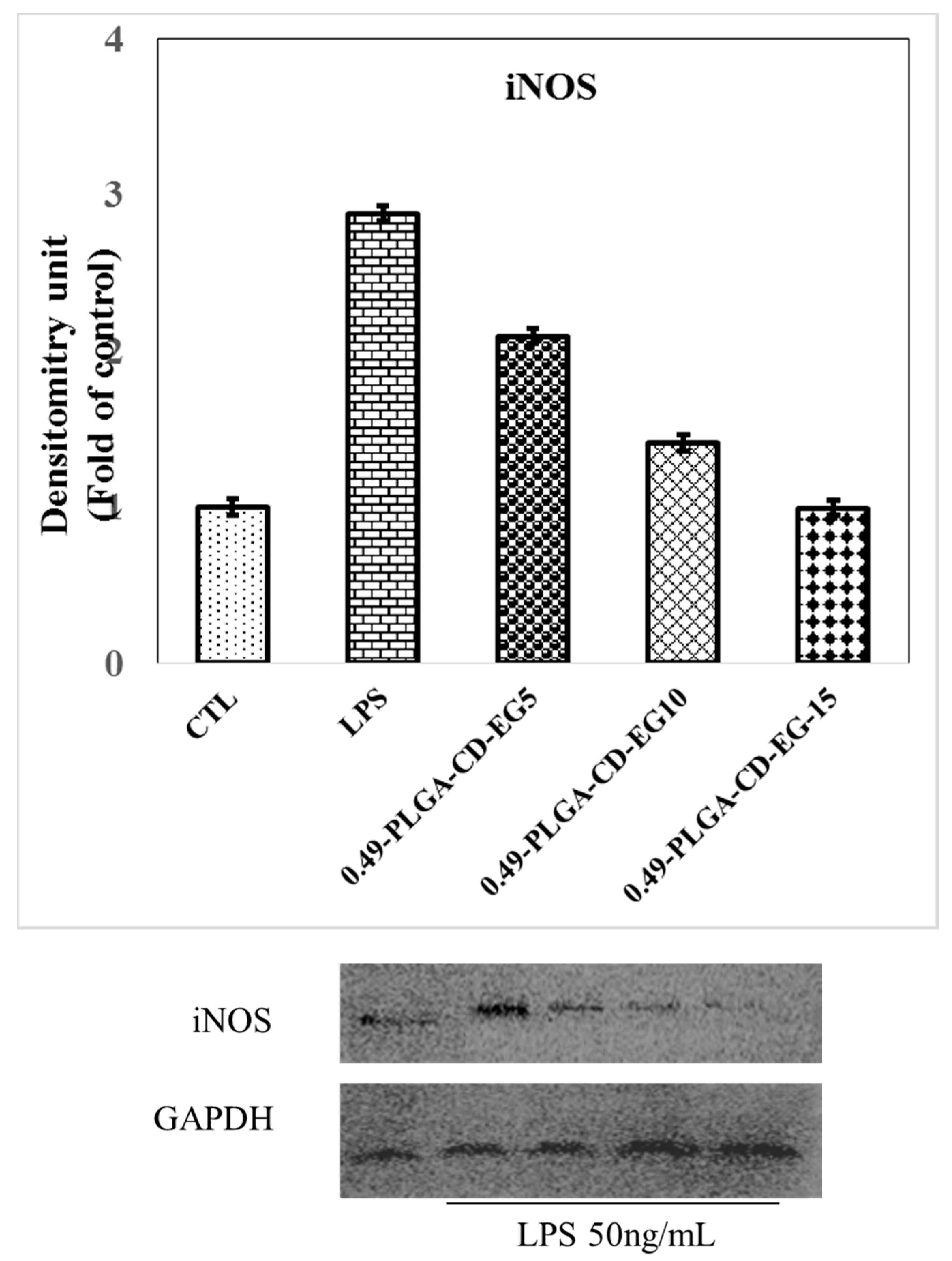
3.9. Western Blot of iNOS in BV-2 Microglial Cells
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gan, P.; Zhang, L.; Chen, Y.; Zhang, Y.; Zhang, F.; Zhou, X.; Zhang, X.; Gao, B.; Zhen, X.; Zhang, J.; et al. Anti-inflammatory effects of glaucocalyxin B in microglia cells. J. Pharmacol. Sci. 2015, 128, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C. Microglia and neurodegeneration: The role of systemic inflammation. Glia 2013, 61, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Iwai-Shimada, M.; Takahashi, T.; Kim, M.-S.; Fujimura, M.; Ito, H.; Toyama, T.; Naganuma, A.; Hwang, G.-W. Methylmercury induces the expression of TNF-α selectively in the brain of mice. Sci. Rep. 2016, 6, 38294. [Google Scholar] [CrossRef] [PubMed]
- Voloboueva, L.A.; Emery, J.F.; Sun, X.; Giffard, R.G. Inflammatory response of microglial BV-2 cells includes a glycolytic shift and is modulated by mitochondrial glucose-regulated protein 75/mortalin. FEBS Lett. 2013, 587, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, B.; Sonobe, Y.; Horiuchi, H.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Oligomeric amyloid β induces IL-1β processing via production of ROS: Implication in Alzheimer’s disease. Cell Death Dis. 2013, 4, e975. [Google Scholar] [CrossRef] [PubMed]
- Guadagno, J.; Swan, P.; Shaikh, R.; Cregan, S.P. Microglia-derived IL-1β triggers p53-mediated cell cycle arrest and apoptosis in neural precursor cells. Cell Death Dis. 2015, 6, e1779. [Google Scholar] [CrossRef] [PubMed]
- Taka, E.; Mazzio, E.; Goodman, C.; Reeams, R.; Soliman, K. Anti- inflammatory effects of thymoquinone in LPS- stimulated BV-2 murine microglia cells. FASEB J. 2014, 28 (Suppl. 1), 730.2. [Google Scholar]
- Sierra, A.; Navascués, J.; Cuadros, M.A.; Calvente, R.; Martín-Oliva, D.; Ferrer-Martín, R.M.; Martín-Estebané, M.; Carrasco, M.; Marín-Teva, J.L. Expression of Inducible Nitric Oxide Synthase (iNOS) in Microglia of the Developing Quail Retina. PLoS ONE 2014, 9, e106048. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-C.; Wu, M.-S.; Lin, H.-Y.; Yang, L.-Y.; Chen, Y.-H.; Chen, Y.-C. Reactive Oxygen Species-Dependent Nitric Oxide Production in Reciprocal Interactions of Glioma and Microglial Cells. J. Cell. Physiol. 2014, 229, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, D.; Bai, Y. Microglia-Mediated Inflammation and Neurodegenerative Disease. Mol. Neurobiol. 2016, 53, 6709–6715. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Kang, S.; Son, S.M.; Mook-Jun, I. Microglia Contributes to Plaque Growth by Cell Death Due to Uptake of Amyloid beta in the Brain of Alzheimer’s Disease Mouse Model. Glia 2016, 64, 2274–2290. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Bennett, N.K.; Chmielowski, R.; Abdelhamid, D.S.; Faig, J.J.; Francis, N.L.; Baum, J.; Pang, Z.P.; Uhrich, K.E.; Moghe, P.V. Polymer brain-nanotherapeutics for multipronged inhibition of microglial alpha-synuclein aggregation, activation, and neurotoxicity. Biomaterials 2016, 111, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Miller, O.G.; Behring, J.B.; Siedlak, S.L.; Jiang, S.; Matsui, R.; Bachschmid, M.M.; Zhu, X.; Mieyal, J.J. Upregulation of Glutaredoxin-1 Activates Microglia and Promotes Neurodegeneration: Implications for Parkinson’s Disease. Antioxid. Redox Signal. 2016, 25, 967–982. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R. Inhibition of microglial activation and induction of neurotrophic factors by flavonoids: A potential therapeutic strategy against Parkinson’s disease. Neural Regen. Res. 2015, 10, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Figuera-Losada, M.; Rojas, C.; Slusher, B.S. Inhibition of Microglia Activation as a Phenotypic Assay in Early Drug Discovery. J. Biomol. Screen. 2014, 19, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Elias, R.J.; Vijay-Kumar, M.; Lambert, J.D. (−)-Epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss. Mol. Nutr. Food Res. 2016, 60, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Klaus, S.; Pültz, S.; Thöne-Reineke, C.; Wolfram, S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. Relat. Metab. Disord. 2005, 29, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Min, K.-J.; Kwon, T.K. Anticancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr. Med. Res. 2014, 3, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Tazi, M.; Caution, K.; Ahmed, A.; Kanneganti, A.; Assani, K.; Kopp, B.; Marsh, C.; Dakhlallah, D.; Amer, A.O. Aging is associated with hypermethylation of autophagy genes in macrophages. Epigenetics 2016, 11, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chiou, Y.-S.; Pan, M.-H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Doorn, K.J.; Lucassen, P.J.; Boddeke, H.W.; Prins, M.; Berendse, H.W.; Drukarch, B.; van Dam, A. Emerging roles of microglial activation and non-motor symptoms in Parkinson’s disease. Prog. Neurobiol. 2012, 98, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.K.; Koppula, S.; Suk, K. Inhibitors of Microglial Neurotoxicity: Focus on Natural Products. Molecules 2011, 16, 1021–1043. [Google Scholar] [CrossRef] [PubMed]
- Gosslau, A.; Jao, D.L.E.; Huang, M.; Ho, C.; Evans, D.; Rawson, N.E.; Chen, K.Y. Effects of the black tea polyphenol theaflavin-2 on apoptotic and inflammatory pathways in vitro and in vivo. Mol. Nutr. Food Res. 2011, 55, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Fujimura, Y.; Hagihara, T.; Sasaki, M.; Yukihira, D.; Nagao, T.; Miura, D.; Yamaguchi, S.; Saito, K.; Tanaka, H.; et al. In situ label-free imaging for visualizing the biotransformation of a bioactive polyphenol. Sci. Rep. 2013, 3, 2805. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.Y.; Ou, N.; Lu, Q.-B. Antioxidant Induces DNA Damage, Cell Death and Mutagenicity in Human Lung and Skin Normal Cells. Sci. Rep. 2013, 3, 3169. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Yada, S.; Terakawa, M. Biodegradability of poly(lactic-co-glycolic acid) after femtosecond laser irradiation. Sci. Rep. 2016, 6, 27884. [Google Scholar] [CrossRef] [PubMed]
- Andreas, K.; Zehbe, R.; Kazubek, M.; Grzeschik, K.; Sternberg, N.; Bäumler, H.; Schubert, H.; Sittinger, M.; Ringe, J. Biodegradable insulin-loaded PLGA microspheres fabricated by three different emulsification techniques: Investigation for cartilage tissue engineering. Acta Biomater. 2011, 7, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zeng, X.; Liu, M.; Deng, Y.; He, N. Current Progress in Gene Delivery Technology Based on Chemical Methods and Nano-carriers. Theranostics 2014, 4, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Aldayel, A.M.; Naguib, Y.W.; O’Mary, H.L.; Li, X.; Niu, M.; Ruwona, T.B.; Cui, Z. Acid-Sensitive Sheddable PEGylated PLGA Nanoparticles Increase the Delivery of TNF-alpha siRNA in Chronic Inflammation Sites. Mol. Ther. Nucleic Acids 2016, 5, e340. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Yang, H.N.; Yi, S.W.; Kim, J.; Park, K. Neoangiogenesis of human mesenchymal stem cells transfected with peptide-loaded and gene-coated PLGA nanoparticles. Biomaterials 2016, 76, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Sun, W.; Zhang, M.; Zheng, Y.; Shen, H.; Yang, J.; Zhu, X. FBS or BSA inhibits EGCG induced cell death through covalent binding and the reduction of intracellular ROS production. BioMed Res. Int. 2016, 2016, 5013409. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gu, L. Fabrication of Self-Assembled (−)-Epigallocatechin Gallate (EGCG) Ovalbumin−Dextran Conjugate Nanoparticles and Their Transport across Monolayers of Human Intestinal Epithelial Caco-2 Cells. J. Agric. Food Chem. 2014, 62, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Ishizu, T.; Hirata, C.; Yamamoto, H.; Harano, K. Structure and intramolecular flexibility of β-cyclodextrin complex with (−)-epigallocatechin gallate in aqueous solvent. Magn. Reson. Chem. 2006, 44, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Folch-Cano, C.; Jullian, C.; Speisky, H.; Olea-Azar, C. Antioxidant activity of inclusion complexes of tea catechins with β-cyclodextrins by ORAC assays. Food Res. Int. 2010, 43, 2039–2044. [Google Scholar] [CrossRef]
- Chen, C.; Hsieh, M.; Ho, Y.; Huang, C.; Lee, J.; Yang, C.; Chang, Y. Enhancement of catechin skin permeation via a newly fabricated mPEG-PCL-graft-2-hydroxycellulose membrane. J. Membr. Sci. 2011, 371, 134–140. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Bhatnagar, P.; Singh, M.; Mishra, S.; Kumar, P.; Shukla, Y.; Gupta, K.C. Synthesis of PLGA nanoparticles of tea polyphenols and their strong in vivo protective effect against chemically induced DNA damage. Int. J. Nanomed. 2013, 8, 1451–1462. [Google Scholar]
- Ackerman, K.; Fiddler, J.; Soh, T.; Clarke, S. BV-2 Microglial Cells Used in a Model of Neuroinflammation. FASEB J. 2015, 29 (Suppl. 1), 608.2. [Google Scholar]
- Snitsarev, V.; Young, M.N.; Miller, R.M.S.; Rotella, D.P. The Spectral Properties of (−)-Epigallocatechin 3-OGallate (EGCG) Fluorescence in Different Solvents: Dependence on Solvent Polarity. PLoS ONE 2013, 8, e79834. [Google Scholar] [CrossRef] [PubMed]
- Ishizu, T.; Kajitani, S.; Tsutsumi, H.; Sato, T.; Yamamoto, H.; Hirata, C. Configurational Studies of Complexes of Tea Catechins with Caffeine and Various Cyclodextrins. Planta Med. 2011, 77, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Dua, M.; Zhang, M.; Wang, P.; Bao, S.; Fu, Y.; Yao, J. Facile and green fabrication of small, mono-disperse and size-controlled noble metal nanoparticles embedded in water-stable polyvinyl alcohol nanofibers: High sensitive, flexible and reliable materials for biosensors. Sens. Actuators B Chem. 2013, 185, 608–619. [Google Scholar] [CrossRef]
- Wang, M.; Lu, X.; Yin, X.; Tong, Y.; Peng, W.; Wu, L.; Li, H.; Yang, Y.; Gu, J.; Xiao, T.; et al. Synchrotron radiation-based Fourier-transform infrared spectromicroscopy for characterization of the protein/peptide distribution in single microspheres. Acta Pharm. Sin. B 2015, 5, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Jin, Z.; Xu, X. Inclusion complex of astaxanthin with hydroxypropyl–cyclodextrin: UV, FTIR, 1H NMR and molecular modeling studies. Carbohydr. Polym. 2012, 89, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Pho, Q.; Hsieh, M.; Wu, X.; Chin, T. Synthesis of PLGA microparticles of EGCG by a doulble emulsion-solvent technique and their anti-inflammatory effect to activated BV-2 cells by lipopolysaccharide. In Proceedings of the 10th World Biomaterials Congress Frontiers in Bioengineering and Biotechnology, Montréal, QC, Canada, 17 May–22 May 2016. [Google Scholar]
- García, A.; Leonardi, D.; Lamas, M.C. Promising applications in drug delivery systems of a novel β-cyclodextrin derivative obtained by green synthesis. Bioorg. Med. Chem. Lett. 2016, 26, 602–608. [Google Scholar] [CrossRef] [PubMed]
- McCall, R.L.; Sirianni, R.W. PLGA Nanoparticles Formed by Single- or Double-emulsion with Vitamin E-TPGS. J. Vis. Exp. 2013, e51015. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly latic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; II, A.L.M.; Waletzki, B.E.; Mamo, T.K.; Yaszemski, M.J.; Lu, L. Hydrolysable core crosslinked particles for receptor-mediated pH-sensitive anticancer drug delivery. New J. Chem. 2015, 39, 8840–8847. [Google Scholar] [CrossRef] [PubMed]
- Sandra, C.S.; Fernaeus, Z.; Part, K.; Reis, K.; Land, T. LPS- induced iNOS expression in BV-2 cells is suppressed by an oxidative mechanism acting on the JNK pathway-A potential role for neuroprotection. Brain Res. 2010, 1322, 1–7. [Google Scholar]
- Cheng, Y.-T.; Wu, C.-H.; Ho, C.-Y.; Yen, G.-C. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo. J. Nutr. Biochem. 2013, 24, 475–483. [Google Scholar] [CrossRef] [PubMed]
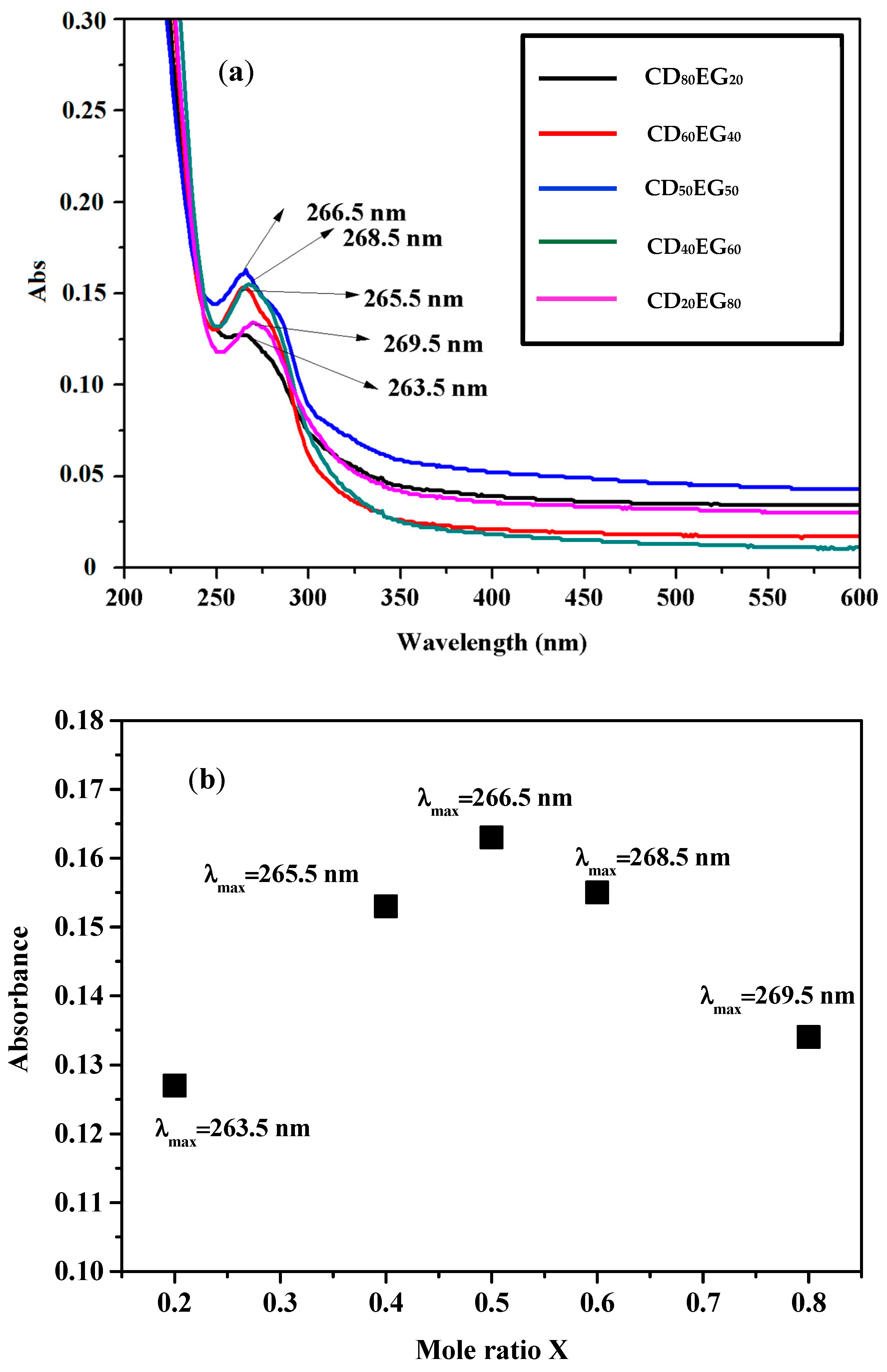


| Abbreviation | EG (µM) | β-CD (µM) |
|---|---|---|
| CD80EG20 | 80 | 20 |
| CD60EG40 | 60 | 40 |
| CD50EG50 | 50 | 50 |
| CD40EG60 | 40 | 60 |
| CD20EG80 | 20 | 80 |
| Feeding CD-EG, BSA-EG or EG (mg) | PLGA Copolymers | |||
|---|---|---|---|---|
| PLGA0.85 | PLGA0.85 | PLGA0.53 | PLGA0.49 | |
| 0 | N/A | N/A | N/A | PLGA0.49-(CD-EG)0 |
| 1 | PLGA0.85-EG1 | PLGA0.85-(BSA-EG)1 | PLGA0.53-(CD-EG)1 | PLGA0.49-(CD-EG)1 |
| 5 | PLGA0.85-EG5 | PLGA0.85-(BSA-EG)5 | PLGA0.53-(CD-EG)5 | PLGA0.49-(CD-EG)5 |
| 10 | PLGA0.85-EG10 | PLGA0.85-(BSA-EG)10 | PLGA0.53-(CD-EG)10 | PLGA0.49-(CD-EG)10 |
| 15 | PLGA0.85-EG15 | PLGA0.85-(BSA-EG)15 | PLGA0.53-(CD-EG)15 | PLGA0.49-(CD-EG)15 |
| Formulation | Feeding of CD-EG, BSA-EG or EG | |||
|---|---|---|---|---|
| 1 mg | 5 mg | 10 mg | 15 mg | |
| PLGA0.85EG | 0.26 | 0.32 | 1.35 | 2.06 |
| PLGA0.85-(BSA-EG) | 1.07 | 1.82 | 2.65 | 3.24 |
| PLGA0.53-(CD-EG) | 2.64 | 3.65 | 8.02 | 9.69 |
| PLGA0.49-(CD-EG) | 2.68 | 3.81 | 9.12 | 16.34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-Y.; Pho, Q.-H.; Wu, X.-Y.; Chin, T.-Y.; Chen, C.-M.; Fang, P.-H.; Lin, Y.-C.; Hsieh, M.-F. PLGA Microspheres Loaded with β-Cyclodextrin Complexes of Epigallocatechin-3-Gallate for the Anti-Inflammatory Properties in Activated Microglial Cells. Polymers 2018, 10, 519. https://doi.org/10.3390/polym10050519
Cheng C-Y, Pho Q-H, Wu X-Y, Chin T-Y, Chen C-M, Fang P-H, Lin Y-C, Hsieh M-F. PLGA Microspheres Loaded with β-Cyclodextrin Complexes of Epigallocatechin-3-Gallate for the Anti-Inflammatory Properties in Activated Microglial Cells. Polymers. 2018; 10(5):519. https://doi.org/10.3390/polym10050519
Chicago/Turabian StyleCheng, Chun-Yuan, Quoc-Hue Pho, Xiao-Yu Wu, Ting-Yu Chin, Chien-Min Chen, Peng-Hsiang Fang, Yung-Chang Lin, and Ming-Fa Hsieh. 2018. "PLGA Microspheres Loaded with β-Cyclodextrin Complexes of Epigallocatechin-3-Gallate for the Anti-Inflammatory Properties in Activated Microglial Cells" Polymers 10, no. 5: 519. https://doi.org/10.3390/polym10050519