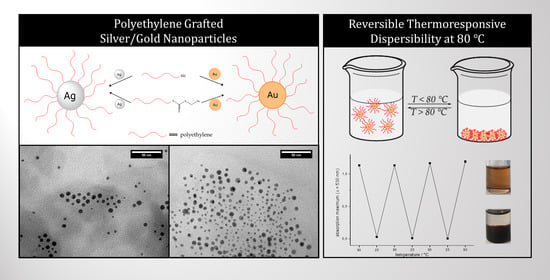
Polyethylene-Grafted Gold and Silver Nanoparticles Using Catalyzed Chain Growth (CCG)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Synthesis of (C5Me5)2NdCl2Li(OEt)2
2.2.2. Synthesis of bis(benzylsulfinyl thiocarbonyl)disulfide
2.2.3. Synthesis of TOAB-Capped Gold Nanoparticles
2.2.4. Synthesis of Silver Nanoparticles
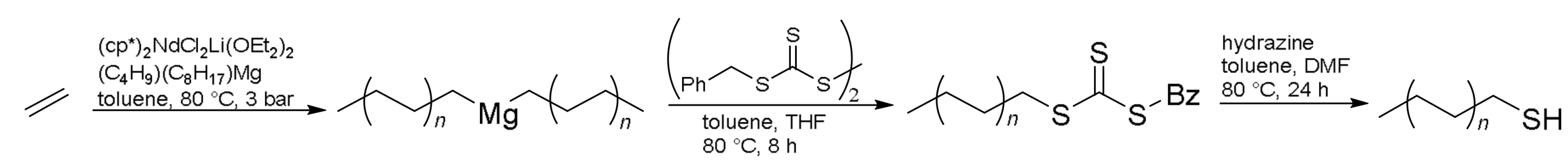
2.2.5. Synthesis of Trithiocarbonate Terminated Polyethylene
2.2.6. Synthesis of Polyethylenethiol
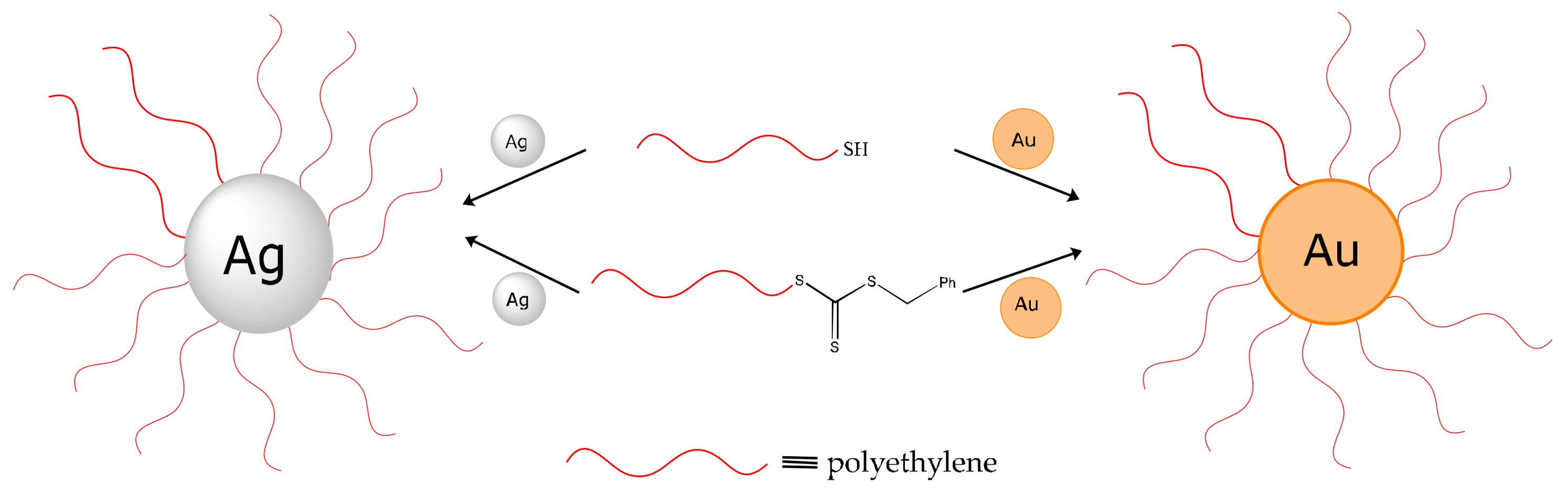
2.2.7. Preparation of Core-Shell Particles
2.3. Methods
2.3.1. Transmission Electron Microscopy (TEM)
2.3.2. High Temperature Size Exclusion Chromatography (HT-SEC)
2.3.3. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.3.4. Dynamic Light Scattering (DLS)
2.3.5. UV/visible Spectroscopy
3. Results and Discussion
3.1. Synthesis
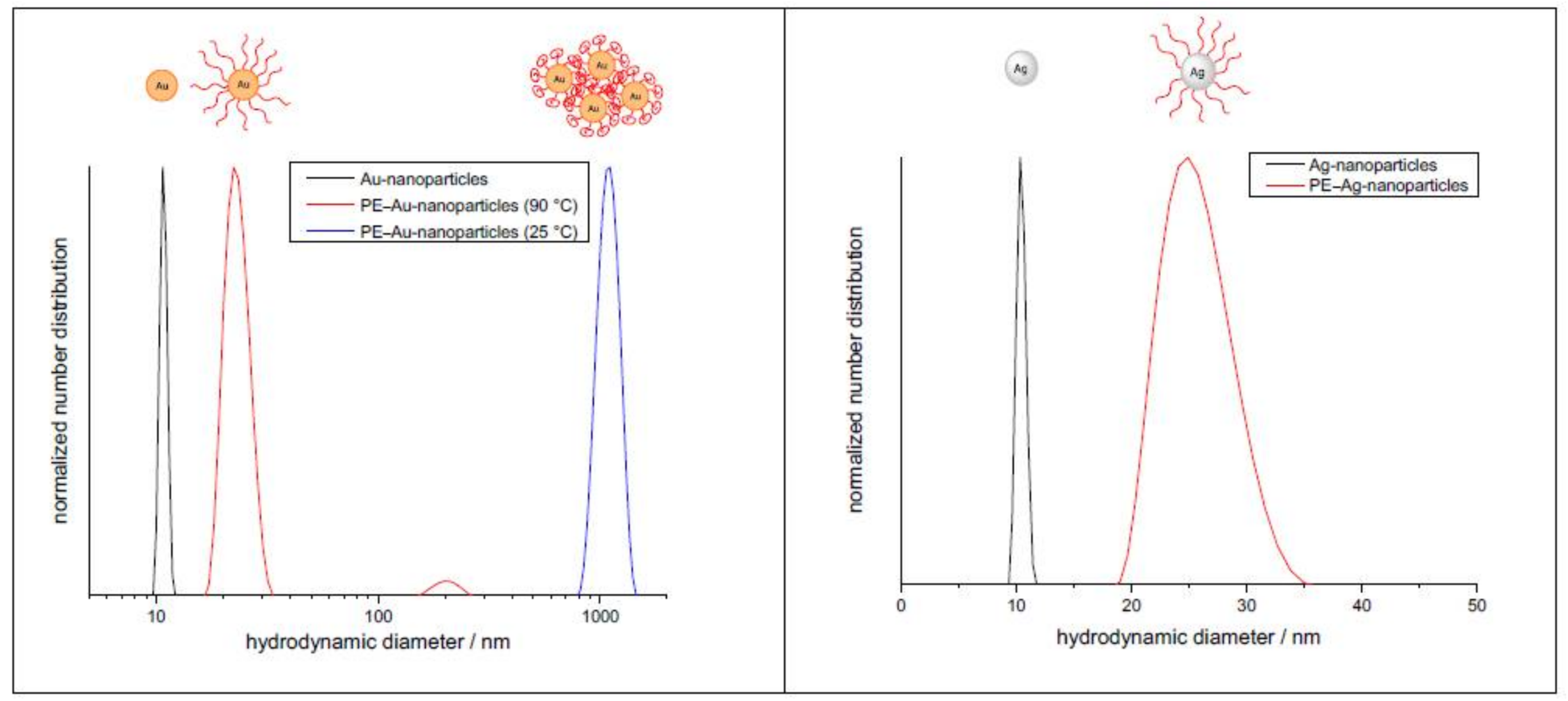
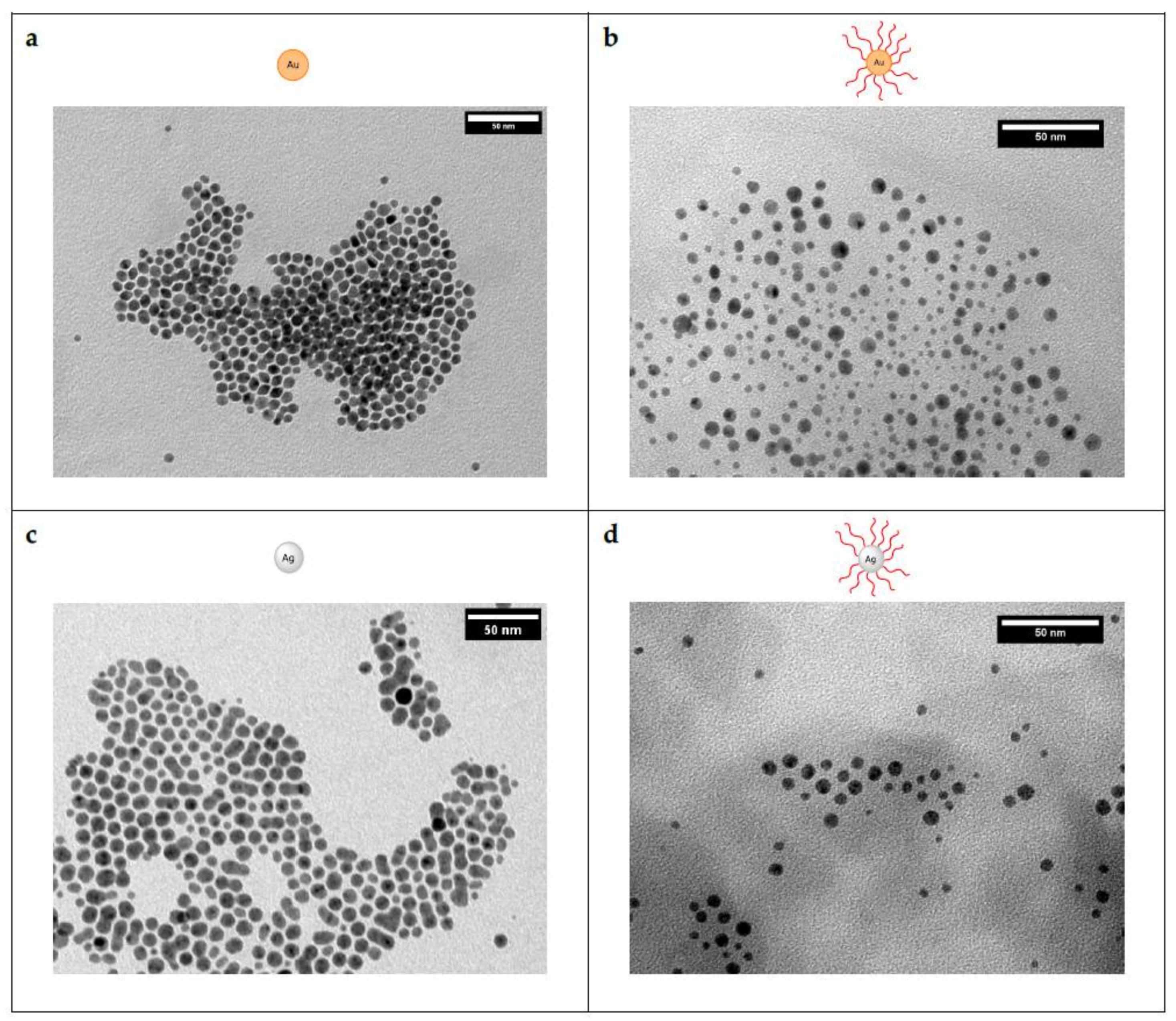
3.2. Characterization of the Core–Shell Nanohybrids
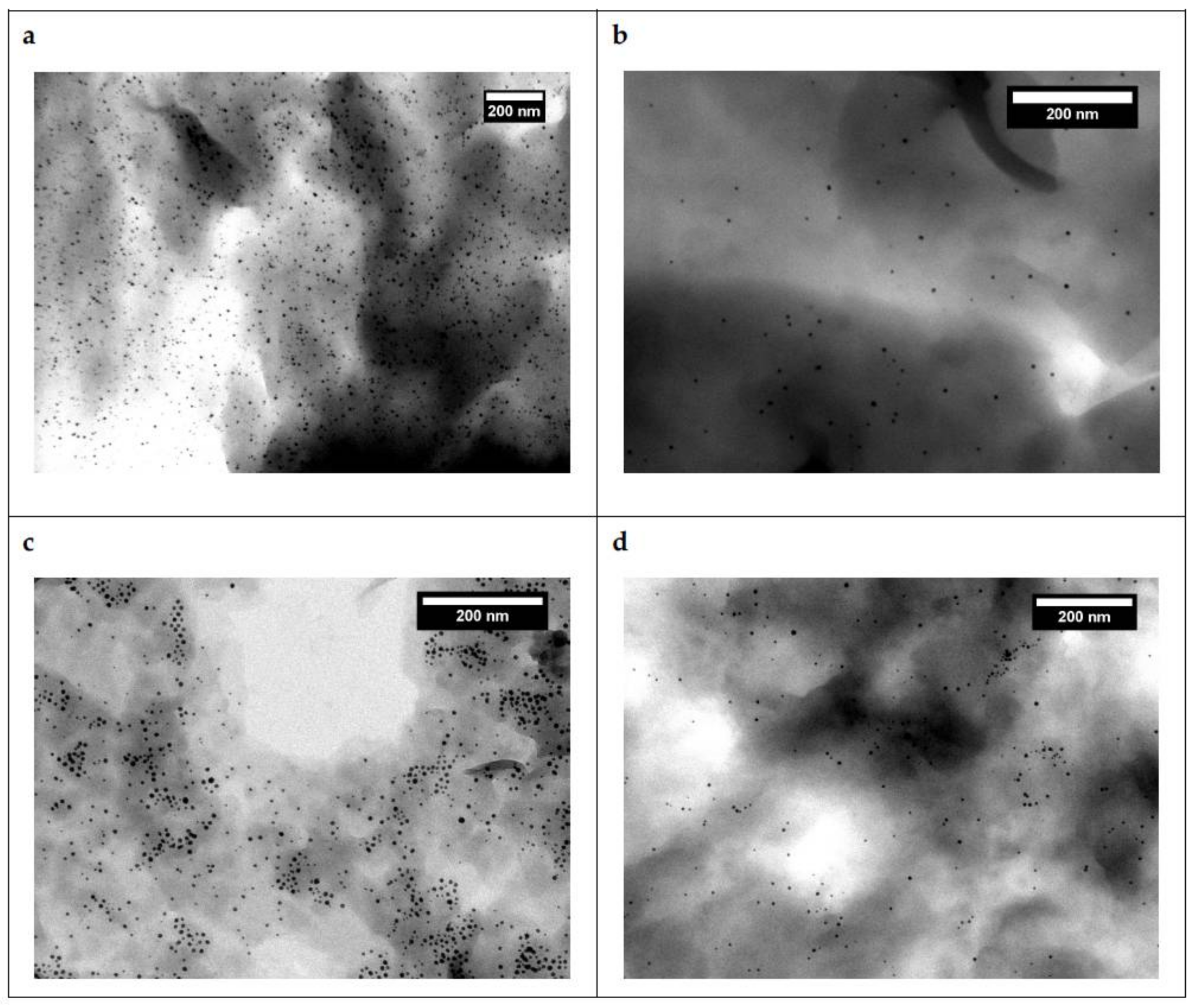
3.3. Incorporation into a Polyethylene Matrix
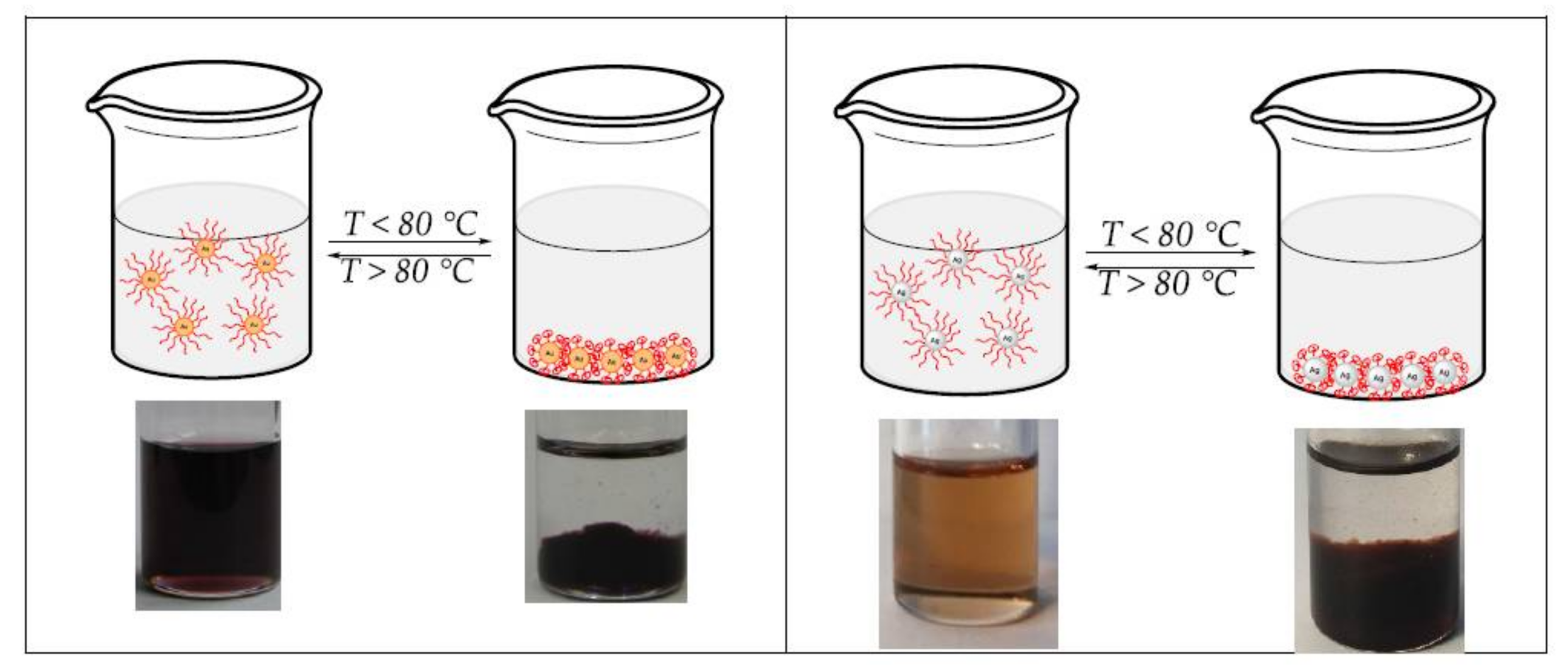
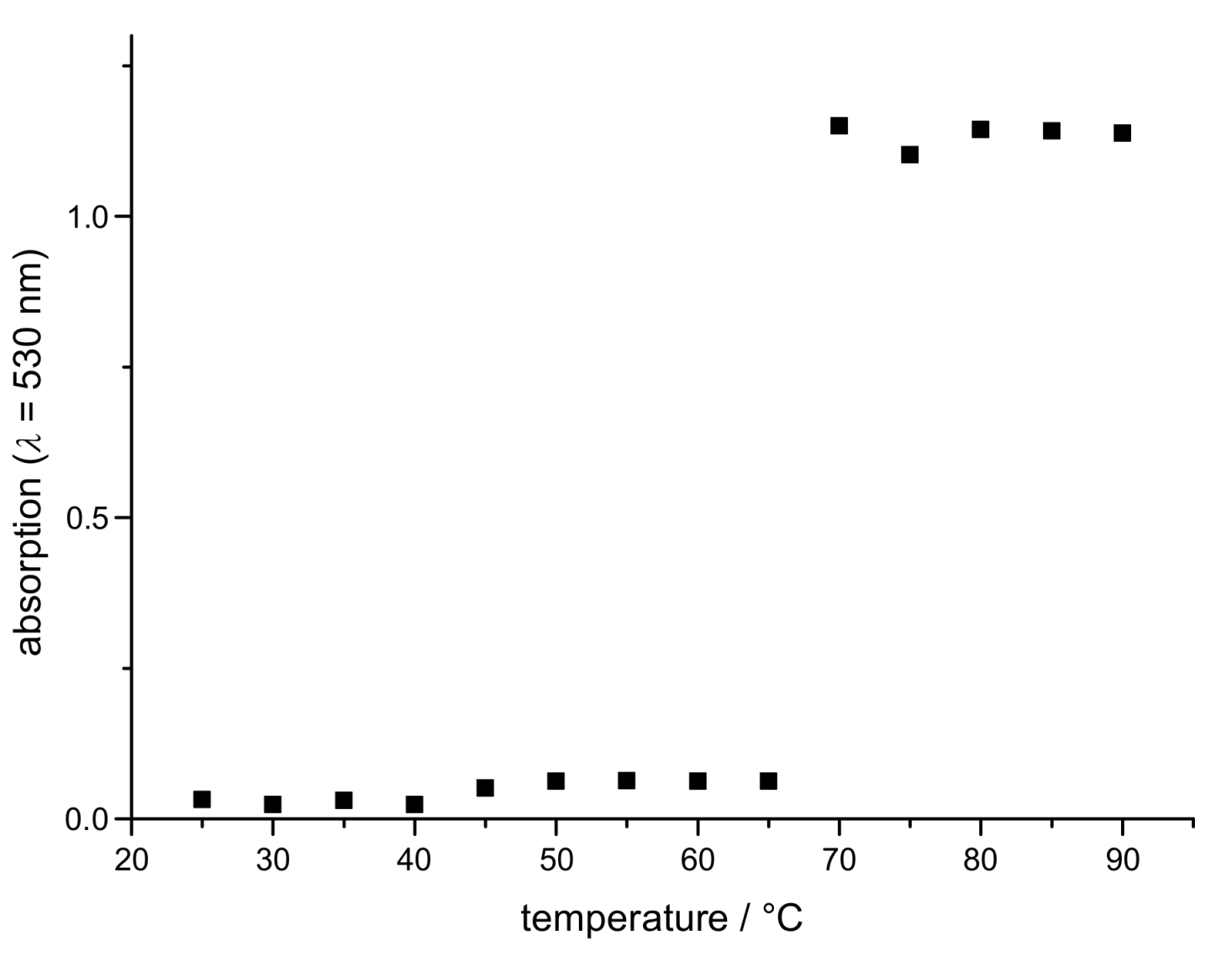
3.4. Thermoresponsive Behavior of the Cores–Shell-Nanoparticles
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bell, A.T. The impact of nanoscience on heterogeneous catalysis. Science 2003, 299, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Compañó, R.; Hullmann, A. Forecasting the development of nanotechnology with the help of science and technology indicators. Nanotechnology 2002, 13, 243–247. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A Review on Functionalized Gold Nanoparticles for Biosensing Applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Stratakis, M.; Garcia, H. Catalysis by supported gold nanoparticles: Beyond aerobic oxidative processes. Chem. Rev. 2012, 112, 4469–4506. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Van Nguyen, Q.; Le, A. Silver nanoparticles. Adv. Natl. Sci. Nanosci. Nanotechnol. 2013, 4, 33001–33021. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Jouault, N.; Benicewicz, B.; Neely, T. Nanocomposites with Polymer Grafted Nanoparticles. Macromolecules 2013, 46, 3199–3214. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Zdyrko, B.; Luzinov, I. Polymer brushes by the “grafting to” method. Macromol. Rapid Commun. 2011, 32, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Rossner, C.; Roddatis, V.; Vana, P. Gold-Planet–Silver-Satellite Nanostructures Using RAFT Star Polymer. ACS Macro Lett. 2016, 5, 1227–1231. [Google Scholar] [CrossRef]
- Hotchkiss, J.W.; Lowe, A.B.; Boyes, S.G. Surface Modification of Gold Nanorods with Polymers Synthesized by Reversible Addition−Fragmentation Chain Transfer Polymerization. Chem. Mater. 2007, 19, 6–13. [Google Scholar] [CrossRef]
- Lowe, A.B.; Sumerlin, B.S.; Donovan, M.S. Facile Preparation of Transition Metal Nanoparticles Stabilized by Well-Defined (Co)polymers Synthesized via Aqueous Reversible Addition-Fragmentation Chain Transfer Polymerization. J. Am. Chem. Soc. 2002, 124, 11562–11563. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.A.; Tamayo, L.; Páez, M.; Cerda, E.; Azócar, I. Nanocomposites based on polyethylene and nanosilver particles produced by metallocenic “in situ” polymerization. Eur. Polym. J. 2011, 47, 1541–1549. [Google Scholar] [CrossRef]
- Dirix, Y.; Darribère, C.; Heffels, W.; Bastiaansen, C. Optically anisotropic polyethylene–gold nanocomposites. Appl. Opt. 1999, 38, 6581–6586. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Petrie, K.; Kontopoulou, M.; Ye, Z. Tuning structural parameters of polyethylene brushes on silica nanoparticles in surface-initiated ethylene “living” polymerization and effects on silica dispersion in a polyolefin matrix. Polym. Chem. 2013, 4, 1381–1395. [Google Scholar] [CrossRef]
- Wang, L.; Feng, L.; Xie, T. Novel magnetic polyethylene nanocomposites produced by supported nanometre magnetic Ziegler-Natta catalyst. Polym. Int. 2000, 49, 184–188. [Google Scholar] [CrossRef]
- Lin, Y.; Jin, J.; Song, M. Preparation and characterisation of covalent polymer functionalized graphene oxide. J. Mater. Chem. 2011, 21, 3455–3461. [Google Scholar] [CrossRef]
- Bieligmeyer, M.; Taheri, S.M.; German, I. Completely miscible polyethylene nanocomposites. J. Am. Chem. Soc. 2012, 134, 18157–18160. [Google Scholar] [CrossRef] [PubMed]
- Damiron, D.; Mazzolini, J.; Cousin, F.; Boisson, C. Poly(ethylene) brushes grafted to silicon substrates. Polym. Chem. 2012, 3, 1838–1845. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Q.; Li, H.; Hu, Y. Polyethylene-Modified Nano Silica and Its Fine Dispersion in Polyethylene. Ind. Eng. Chem. Res. 2017, 56, 5892–5898. [Google Scholar] [CrossRef]
- Gibson, V.C. Chemistry. Shuttling polyolefins to a new materials dimension. Science 2006, 312, 703–704. [Google Scholar] [CrossRef] [PubMed]
- Briquel, R.; Mazzolini, J.; Bris, T.L. Polyethylene building blocks by catalyzed chain growth and efficient end functionalization strategies, including click chemistry. Angew. Chem. Int. Ed. 2008, 47, 9311–9313. [Google Scholar] [CrossRef] [PubMed]
- Mazzolini, J.; Espinosa, E.; D’Agosto, F.; Boisson, C. Catalyzed chain growth (CCG) on a main group metal. Polym. Chem. 2010, 1, 793–800. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Dong, J.Y.; Hu, Y. Facile synthesis of chain end functionalized polyethylenes via epoxide ring-opening and thiol–ene addition click chemistry. Polym. Chem. 2014, 5, 105–115. [Google Scholar] [CrossRef]
- Tilley, T.D.; Andersen, R.A. Pentamethylcyclopentadienyl derivatives of the trivalent lanthanide elements neodymium, samarium, and ytterbium. Inorg. Chem. 1981, 20, 3267–3270. [Google Scholar] [CrossRef]
- Weber, W.G.; McLeary, J.B.; Sanderson, R.D. Facile preparation of bis(thiocarbonyl)disulfides via elimination. Tetrahedron Lett. 2006, 47, 4771–4774. [Google Scholar] [CrossRef]
- Rossner, C.; Ebeling, B.; Vana, P. Spherical Gold-Nanoparticle Assemblies with Tunable Interparticle Distances Mediated by Multifunctional RAFT Polymers. ACS Macro Lett. 2013, 2, 1073–1076. [Google Scholar] [CrossRef]
- Hiramatsu, H.; Osterloh, F.E. A Simple Large-Scale Synthesis of Nearly Monodisperse Gold and Silver Nanoparticles with Adjustable Sizes and with Exchangeable Surfactants. Chem. Mater. 2004, 16, 2509–2511. [Google Scholar] [CrossRef]
- Lopez, R.G.; Boisson, C.; D’Agosto, F. New Functional Polyolefins. Macromol. Rapid Commun. 2006, 27, 173–181. [Google Scholar] [CrossRef]
- Shen, W.; Qiu, Q.; Wang, Y.; Miao, M.; Li, B. Hydrazine as a Nucleophile and Antioxidant for Fast Aminolysis of RAFT Polymers in Air. Macromol. Rapid Commun. 2010, 31, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; De, P.; Li, H.; Sumerlin, B.S. Conjugation of RAFT-generated polymers to proteins by two consecutive thiol–ene reactions. Polym. Chem. 2010, 1, 854. [Google Scholar] [CrossRef]
- Kade, M.J.; Burke, D.J.; Hawker, C.J. The power of thiol-ene chemistry. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 743–750. [Google Scholar] [CrossRef]
- Ebeling, B.; Vana, P. RAFT-Polymers with Single and Multiple Trithiocarbonate Groups as Uniform Gold-Nanoparticle Coatings. Macromolecules 2013, 46, 4862–4871. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Peacock, A.J. Handbook of Polyethylene: Structure, Properties and Applications; CRC Press: Boca Raton, FL, USA, 2000; Volume 57. [Google Scholar]
- Benaglia, M.; Puglisi, A.; Cozzi, F. Polymer-supported organic catalysts. Chem. Rev. 2003, 103, 3401–3429. [Google Scholar] [CrossRef] [PubMed]
- Bergbreiter, D.E.; Chandran, R. Polyethylene-bound rhodium(I) hydrogenation catalysts. J. Am. Chem. Soc. 1987, 109, 174–179. [Google Scholar] [CrossRef]
- Hobbs, C.; Yang, Y.C.; Ling, J.; Nicola, S.; Su, H.L. Thermomorphic polyethylene-supported olefin metathesis catalysts. Org. Lett. 2011, 13, 3904–3907. [Google Scholar] [CrossRef] [PubMed]
- Bergbreiter, D.E. Using Soluble Polymers to Recover Catalysts and Ligands. Chem. Rev. 2002, 102, 3345–3384. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Zhao, Y.; Granqvist, N.; Tenhu, H. Thermoresponsive Properties of N-Isopropylacrylamide Oligomer Brushes Grafted to Gold Nanoparticles. Macromolecules 2009, 42, 2696–2701. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, J.; Peng, W.; Vana, P. Polyethylene-Grafted Gold and Silver Nanoparticles Using Catalyzed Chain Growth (CCG). Polymers 2018, 10, 407. https://doi.org/10.3390/polym10040407
Wagner J, Peng W, Vana P. Polyethylene-Grafted Gold and Silver Nanoparticles Using Catalyzed Chain Growth (CCG). Polymers. 2018; 10(4):407. https://doi.org/10.3390/polym10040407
Chicago/Turabian StyleWagner, Jannik, Wentao Peng, and Philipp Vana. 2018. "Polyethylene-Grafted Gold and Silver Nanoparticles Using Catalyzed Chain Growth (CCG)" Polymers 10, no. 4: 407. https://doi.org/10.3390/polym10040407