Synthesis of a Flexible Freestanding Sulfur/Polyacrylonitrile/Graphene Oxide as the Cathode for Lithium/Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Physical Characterization
2.3. Electrochemical Characterization
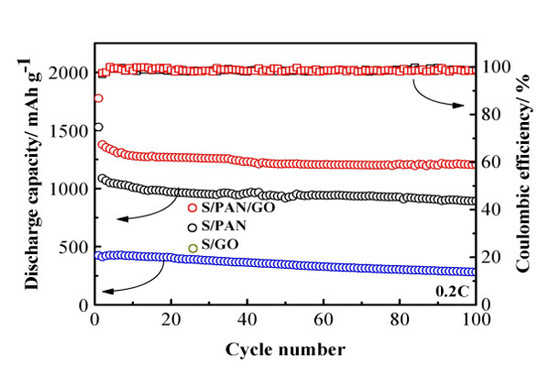
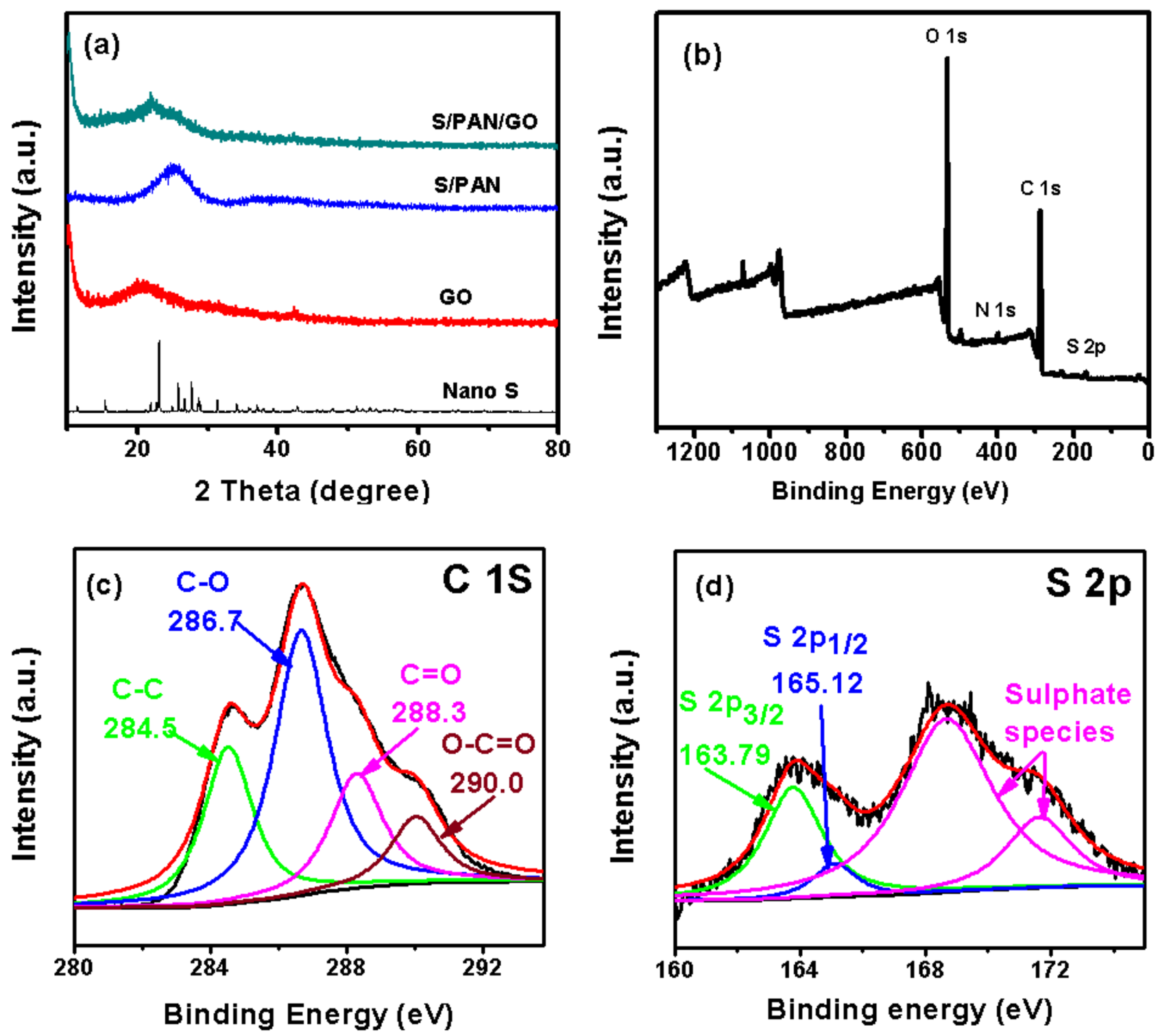
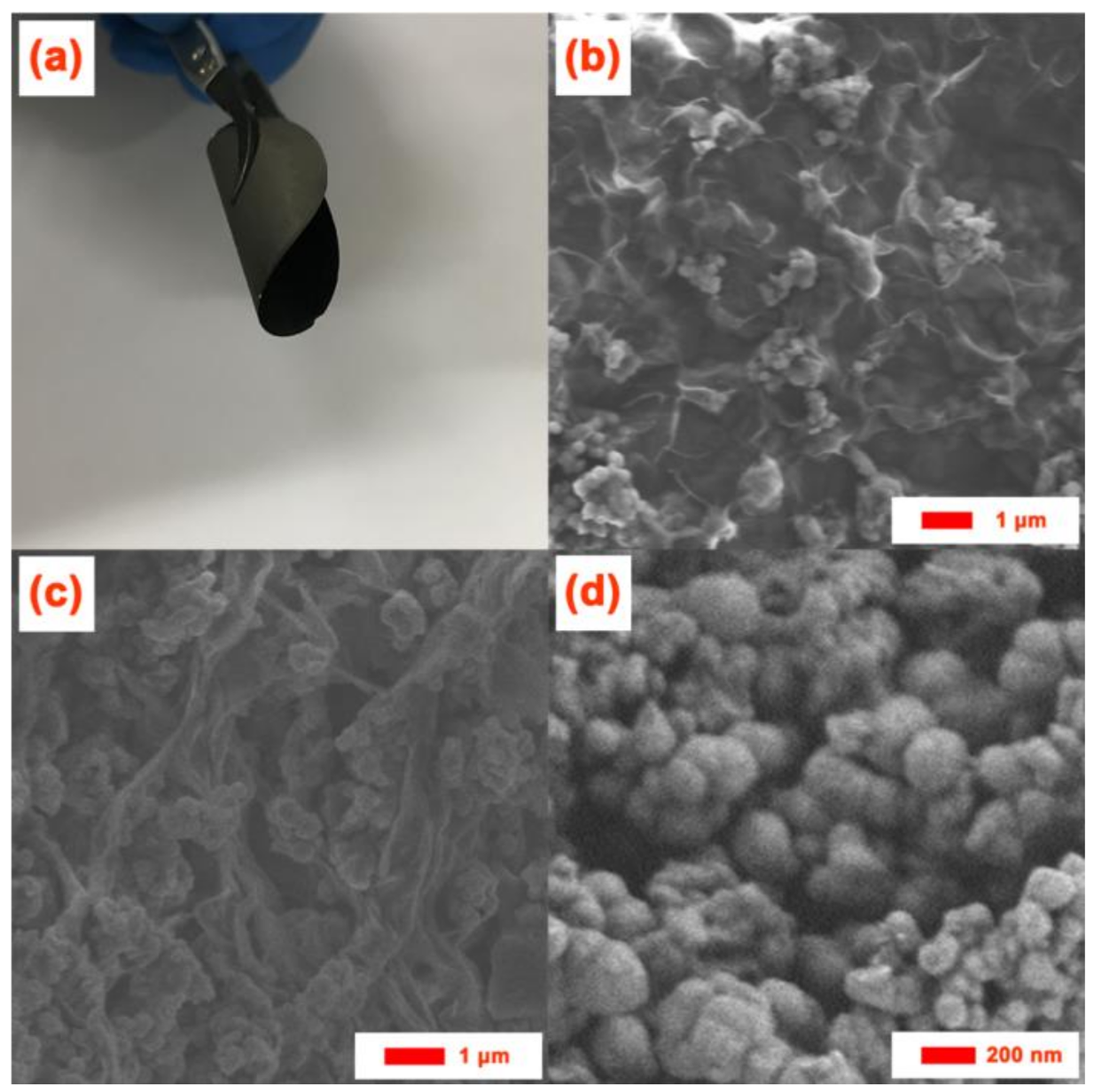
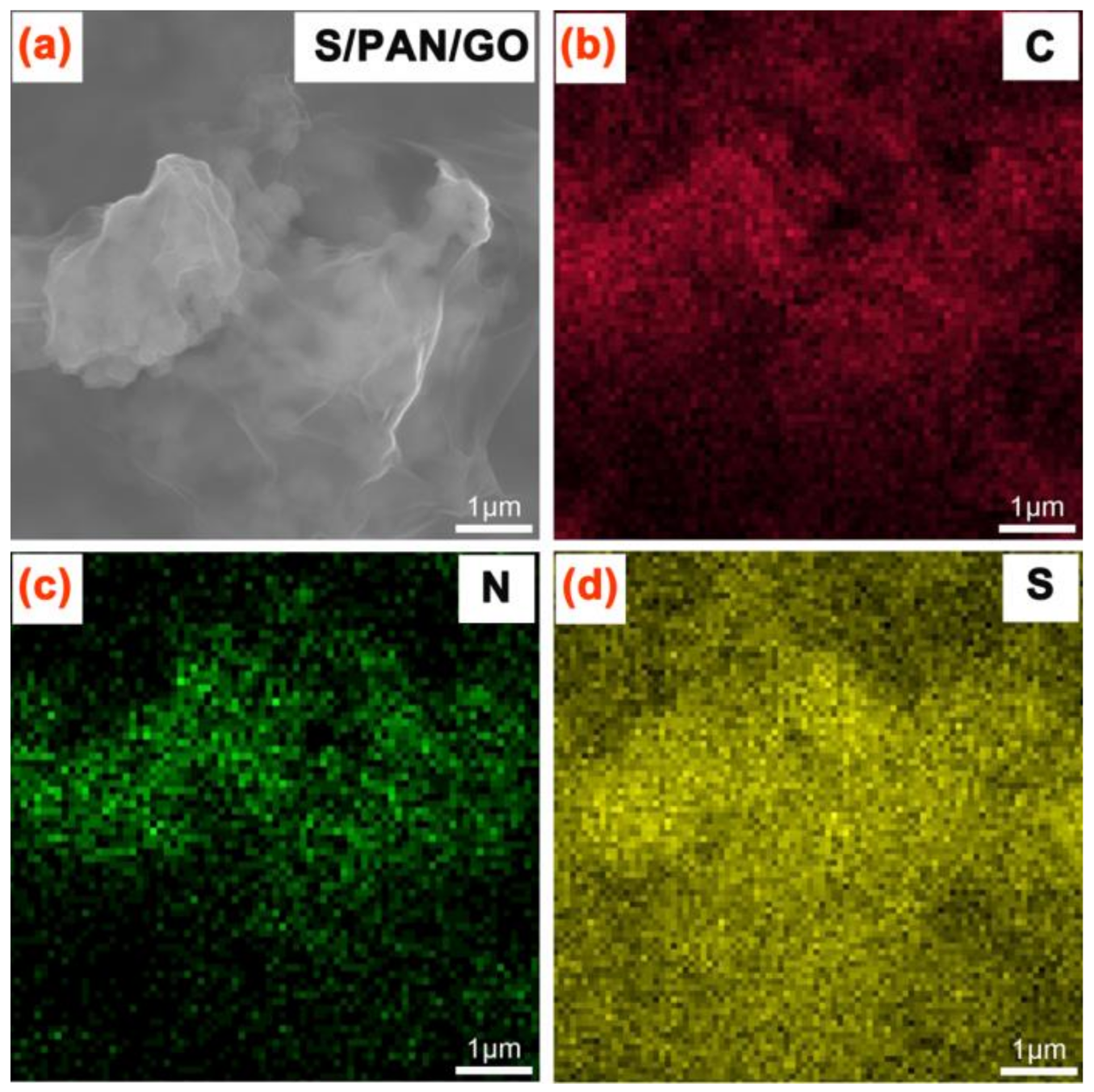
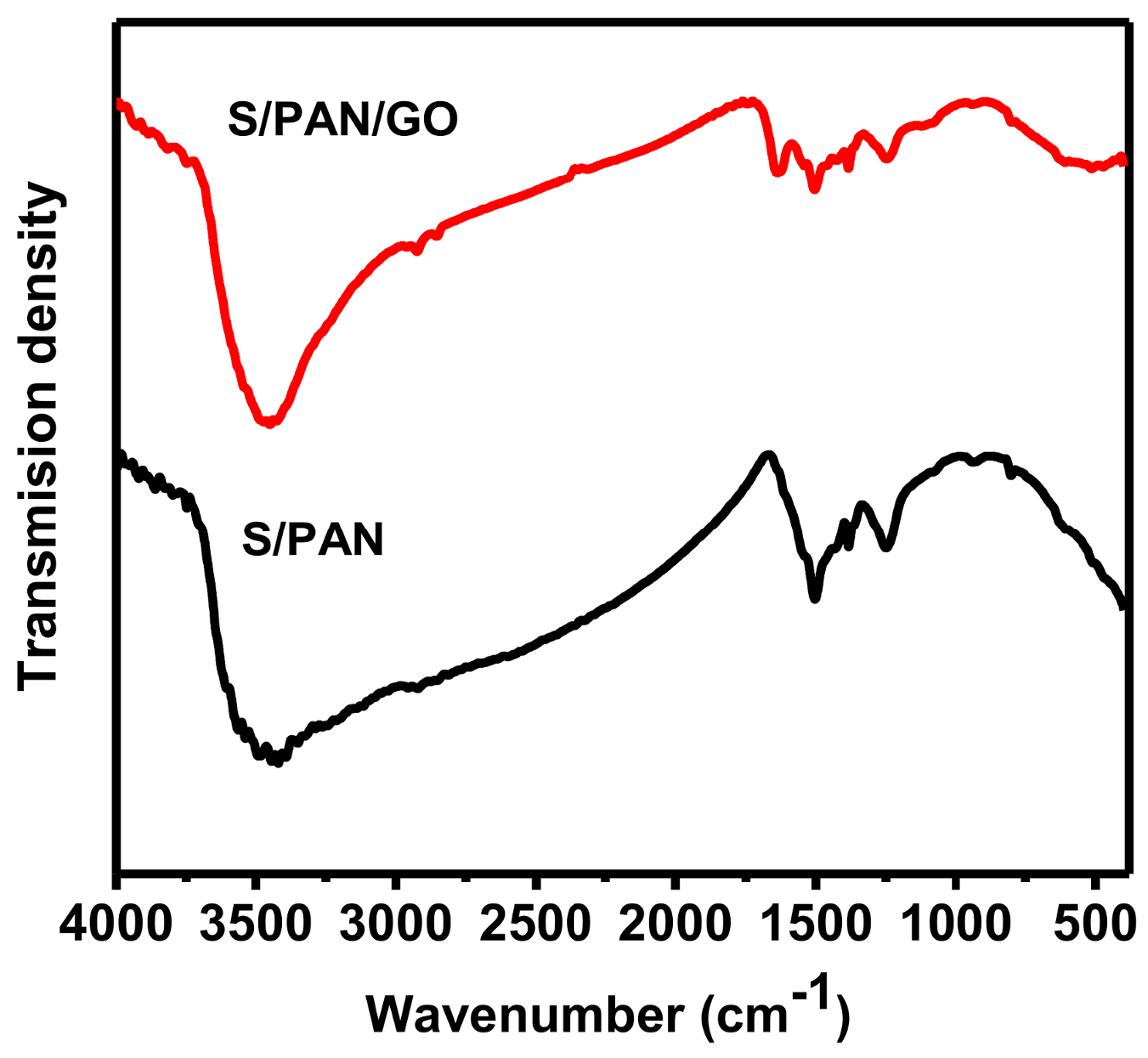
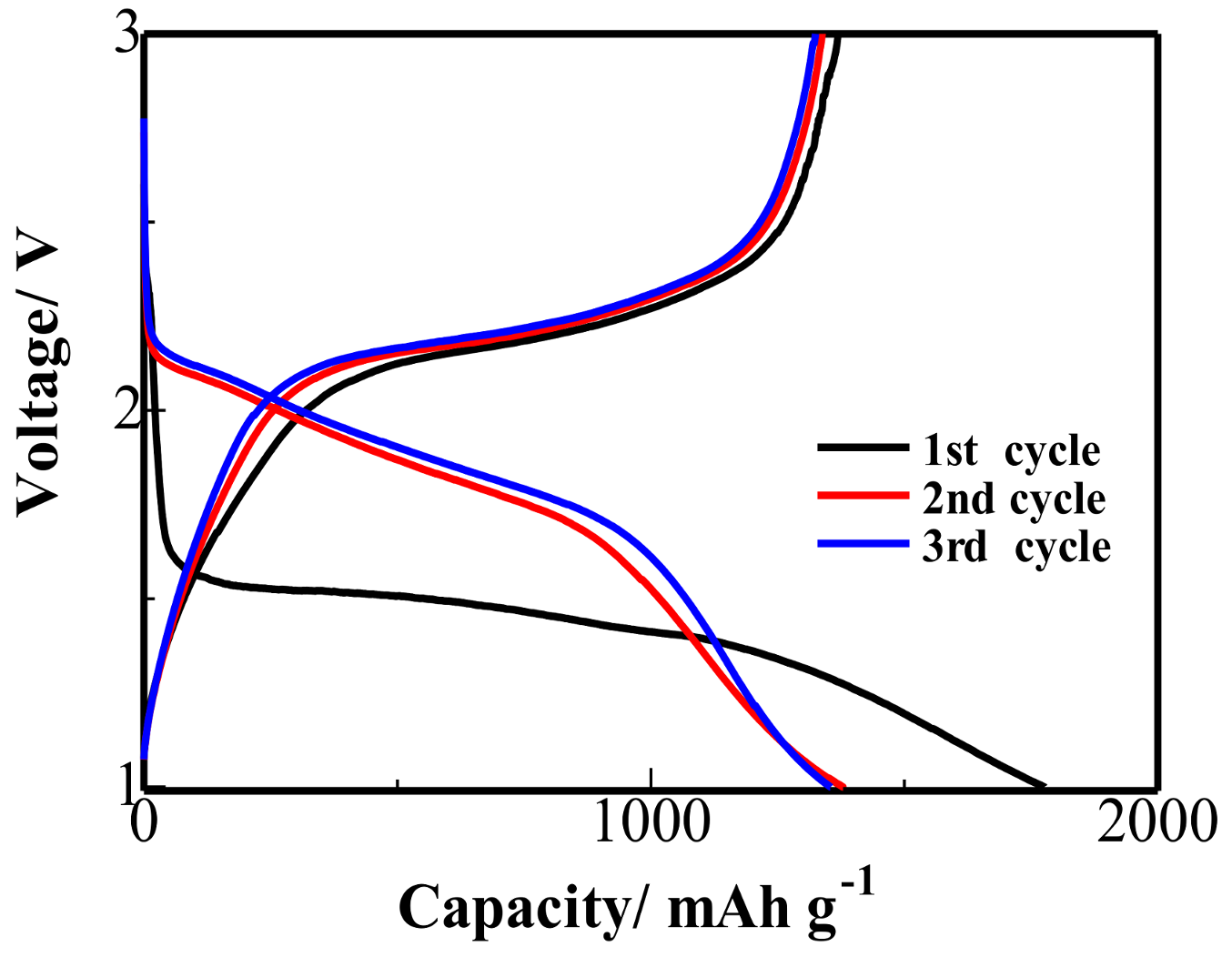
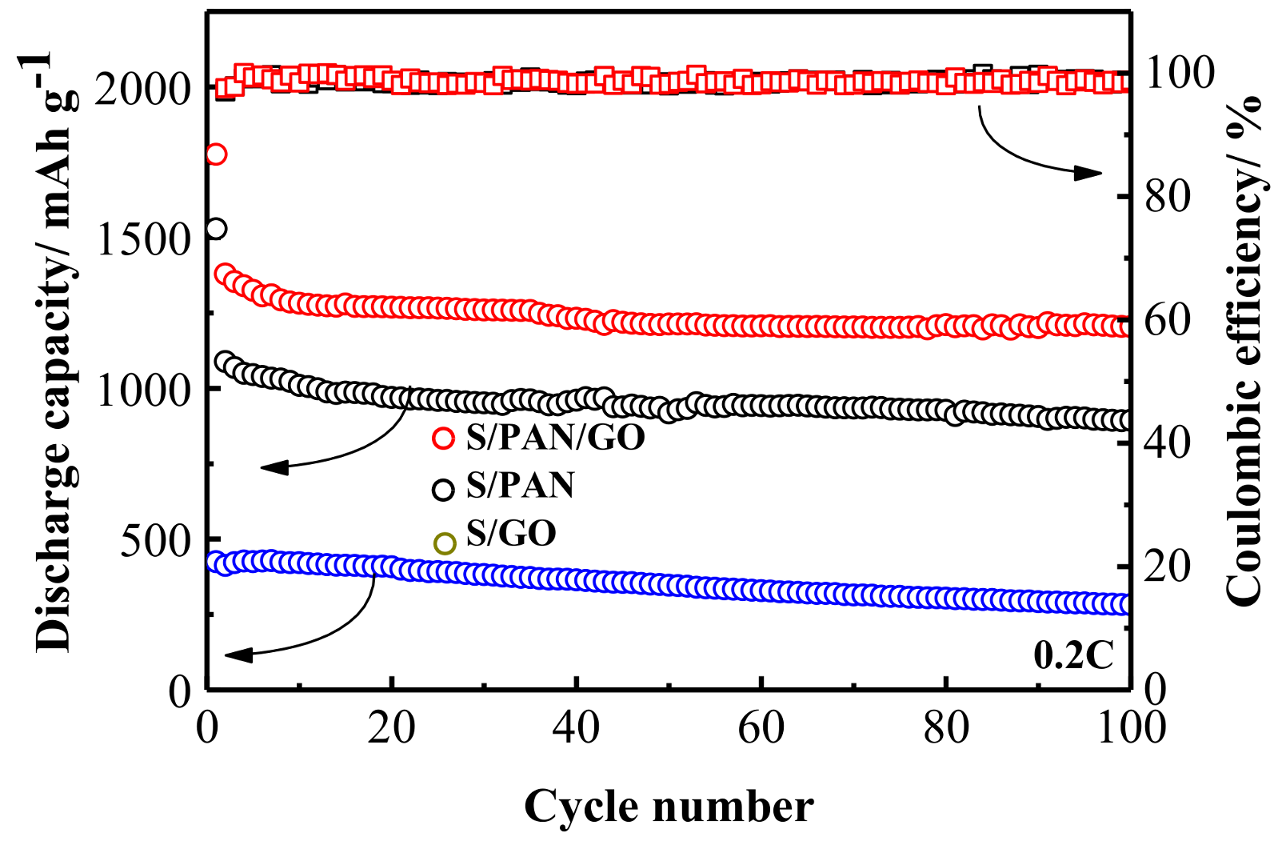
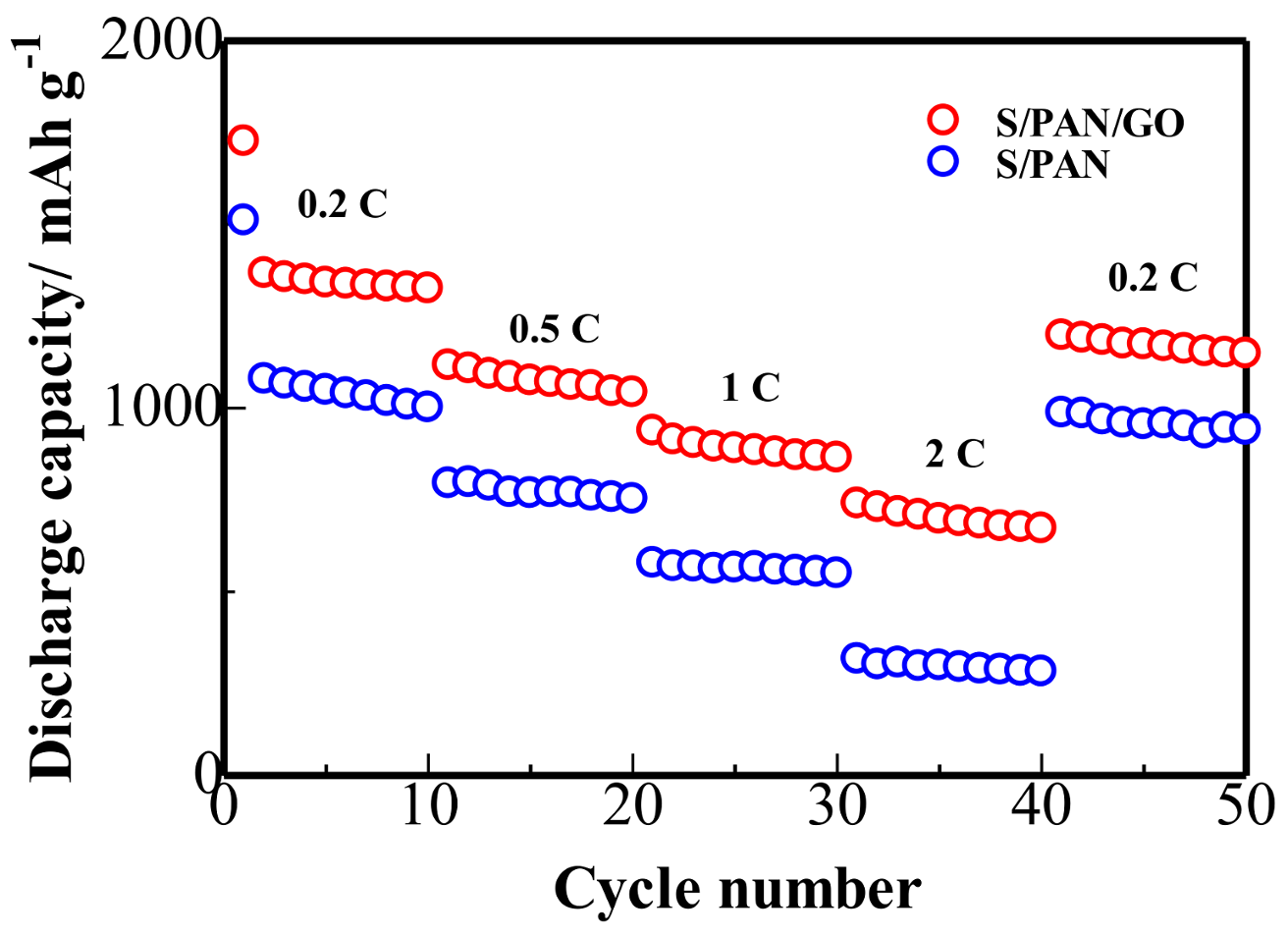
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, X.; Xie, J.; Yang, J.; Zou, Y.; Tang, J.; Wang, S.; Ma, L.; Liao, Q. Improving the performance of lithium–sulfur batteries by graphene coating. J. Power Sources 2013, 243, 993–1000. [Google Scholar] [CrossRef]
- Sohn, H.; Gordin, M.L.; Regula, M.; Kim, D.H.; Jung, Y.S.; Song, J.X.; Wang, D.H. Porous spherical polyacrylonitrile-carbon nanocomposite with high loading of sulfur for lithium–sulfur batteries. J. Power Sources 2016, 302, 70–78. [Google Scholar] [CrossRef]
- Lu, S.; Cheng, Y.; Wu, X.; Liu, J. Significantly Improved Long-Cycle Stability in High-Rate Li–S Batteries Enabled by Coaxial Graphene Wrapping over Sulfur-Coated Carbon Nanofibers. Nano Lett. 2013, 13, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Misra, S.; Yang, Y.; Jackson, A.; Liu, Y.; Wang, H.; Dai, H.; Andrews, J.C.; Cui, Y.; Toney, M.F. In Operando X-ray Diffraction and Transmission X-ray Microscopy of Lithium Sulfur Batteries. J. Am. Chem. Soc. 2012, 134, 6337–6343. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, X.; Zhao, Y.; Tan, T.; Mentbayeva, A.; Bakenov, Z.; Zhang, Y. Enhanced electrochemical performance of sulfur/polyacrylonitrile composite by carbon coating for lithium/sulfur batteries. J. Nanopart. Res. 2017, 19, 348. [Google Scholar] [CrossRef]
- Yin, F.; Liu, X.; Zhang, Y.; Zhao, Y.; Menbayeva, A.; Bakenov, Z.; Wang, X. Well-dispersed sulfur anchored on interconnected polypyrrole nanofiber network as high performance cathode for lithium–sulfur batteries. Solid State Sci. 2017, 66, 44–49. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Bakenov, Z.; Konarov, A.; Chen, P. Preparation of novel network nanostructured sulfur composite cathode with enhanced stable cycle performance. J. Power Sources 2014, 270, 326–331. [Google Scholar] [CrossRef]
- Doan, T.N.L.; Ghaznavi, M.; Zhao, Y.; Zhang, Y.; Konarov, A.; Sadhu, M.; Tangirala, R.; Chen, P. Binding mechanism of sulfur and dehydrogenated polyacrylonitrile in sulfur/polymer composite cathode. J. Power Sources 2013, 241, 61–69. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.J.; Shi, Y.N.; Zheng, M.S.; Dong, Q.F. Preparation and performance of a core–shell carbon/sulfur material for lithium/sulfur battery. Electrochim. Acta 2010, 55, 7010–7015. [Google Scholar] [CrossRef]
- Wei, W.; Wang, J.; Zhou, L.; Yang, J.; Schumann, B.; Nuli, Y. CNT enhanced sulfur composite cathode material for high rate lithium battery. Electrochem. Commun. 2011, 13, 399–402. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, H.; Qiu, X.; Chen, L.; Zhu, W. Improvement of cycle property of sulfur-coated multi-walled carbon nanotubes composite cathode for lithium/sulfur batteries. J. Power Sources 2009, 189, 1141–1146. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Y.W.; Hu, X.G.; Zhang, C.F. Novel nanosized adsorbing sulfur composite cathode materials for the advanced secondary lithium batteries. Electrochim. Acta 2006, 51, 1330–1335. [Google Scholar] [CrossRef]
- Li, J.; Li, K.; Li, M.; Gosselink, D.; Zhang, Y.; Chen, P. A sulfur–polyacrylonitrile/graphene composite cathode for lithium batteries with excellent cyclability. J. Power Sources 2014, 252, 107–112. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-wrapped sulfur particles as a rechargeable lithium–sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 2011, 11, 2644–2647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Bakenov, Z.; Babaa, M.R.; Konarov, A.; Ding, C.; Chen, P. Effect of Graphene on Sulfur/Polyacrylonitrile Nanocomposite Cathode in High Performance Lithium/Sulfur Batteries. J. Electrochem. Soc. 2013, 160, A1194–A1198. [Google Scholar] [CrossRef]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene Oxide as a Sulfur Immobilizer in High Performance Lithium/Sulfur Cells. J. Am. Chem. Soc. 2017, 133, 18522–18525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Huang, L.; Sun, L.C.; Xie, S.Y.; Xu, G.L.; Chen, S.R.; Xu, Y.F.; Li, J.T.; Chou, S.L.; Dou, S.X.; et al. Facile synthesis of a interleaved expanded graphite-embedded sulphur nanocomposite as cathode of Li–S batteries with excellent lithium storage performance. J. Mater. Chem. 2012, 22, 4744–4750. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Dong, O.S.; Park, H.; Lee, Y.G.; Kim, K.M.; Han, T.H. Direct hybridization of tin oxide/graphene nanocomposites for highly efficient lithium-ion battery anodes. J. Electroceram. 2014, 33, 195–201. [Google Scholar]
- Chen, H.; Wang, C.; Dai, Y.; Qiu, S.; Yang, J.; Lu, W.; Chen, L. Rational Design of Cathode Structure for High Rate Performance Lithium–Sulfur Batteries. Nano Lett. 2015, 15, 5443–5448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yin, F.; Zhang, Y.; Zhang, C.; Mentbayeva, A.; Umirov, N.; Xie, H.; Bakenov, Z. A Free-standing Sulfur/Nitrogen Doped Carbon Nanotube Electrode for High Performance Lithium/Sulfur Batteries. Nanoscale Res. Lett. 2015, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Qie, L.; Manthiram, A. A Facile Layer-by-Layer Approach for High-Areal-Capacity Sulfur Cathodes. Adv. Mater. 2015, 27, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Pan, F.; Li, W.; Jiang, Y.; Zhong, X.; Yu, Y. Free-standing porous carbon nanofibers-sulfur composite for flexible Li–S battery cathode. Nanoscale 2014, 6, 9579–9587. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Pu, X.; Li, X.; Liu, M.; Li, M.; Feng, N.; Dou, S. High areal capacity of Li–S batteries enabled by freestanding CNF/rGO electrode with high loading of lithium polysulfide. Electrochim. Acta 2017, 241, 406–413. [Google Scholar] [CrossRef]
- Fu, K.; Li, Y.; Dirican, M.; Chen, C.; Lu, Y.; Zhu, J.; Li, Y.; Cao, L.; Bradford, P.D.; Zhang, X. Sulfur gradient-distributed CNF composite: A self-inhibiting cathode for binder-free lithium–sulfur batteries. Chem. Commun. 2014, 50, 10277–10280. [Google Scholar] [CrossRef] [PubMed]
- Swiderska-Mocek, A.; Rudnicka, E. Lithium–sulphur battery with activated carbon cloth-sulphur cathode and ionic liquid as electrolyte. J. Power Sources 2015, 273, 162–167. [Google Scholar] [CrossRef]
- Zhu, L.; Peng, H.; Liang, J.; Huang, J.; Chen, C.; Guo, X.; Zhu, W.; Li, P.; Zhang, Q. Interconnected carbon nanotube/graphene nanosphere scaffolds as free-standing paper electrode for high-rate and ultra-stable lithium–sulfur batteries. Nano Energy 2015, 11, 746–755. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, S.; Wu, X.; Liu, J. Flexible carbon nanotube-graphene/sulfur composite film: Free standing cathode for high-performance lithium/sulfur batteries. J. Phys. Chem. 2015, 119, 10288–10294. [Google Scholar] [CrossRef]
- Sun, L.; Li, M.; Jiang, Y.; Kong, W.; Jiang, K.; Wang, J.; Fan, S. Sulfur Nanocrystals Confined in Carbon Nanotube Network As a Binder-Free Electrode for High-Performance Lithium Sulfur Batteries. Nano Lett. 2014, 14, 4044–4049. [Google Scholar] [CrossRef] [PubMed]
- Konarov, A.; Gosselink, D.; Doan, T.N.L.; Zhang, Y.; Zhao, Y.; Chen, P. Simple, scalable, and economical preparation of sulfur–PAN composite cathodes for Li/S batteries. J. Power Sources 2014, 259, 183–187. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Chauhan, G.S.; Ahn, J.H. Nanostructured nitrogen-doped mesoporous carbon derived from polyacrylonitrile for advanced lithium sulfur batteries. Appl. Surf. Sci. 2016, 380, 151–158. [Google Scholar] [CrossRef]
- Meng, Z.; Xie, Y.; Cai, T.; Sun, Z.; Jiang, K.; Han, W.Q. Graphene-like g-C3N4, nanosheets/sulfur as cathode for lithium–sulfur battery. Electrochim. Acta 2016, 210, 829–836. [Google Scholar] [CrossRef]
- Sun, D.; Ban, R.; Zhang, P.H.; Wu, G.H.; Zhang, J.R.; Zhu, J.J. Hair fiber as a precursor for synthesizing of sulfur and nitrogen-co-doped carbon dots with tunable luminescence properties. Carbon 2013, 64, 424–434. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Q.; Wang, L.; Li, Q.; Peng, X.; Gao, B.; Huo, K.; Chu, P.K. Mesoporous TiO2 Nanocrystals/Graphene as an Efficient Sulfur Host Material for High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2016, 8, 23784–23792. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, Y.; Li, H.; Zhao, Z.; Wu, H.; Hao, C.; Liu, S.; Qiu, J.; Lou, X.W. Enhancing lithium–sulphur battery performance by strongly binding the discharge products on amino-functionalized reduced graphene oxide. Nat. Commun. 2014, 5, 5002–5009. [Google Scholar] [CrossRef] [PubMed]
- Lim, V.W.L.; Li, S.; Kang, E.T.; Neoh, K.G.; Tan, K.L. In situ XPS study of thermally deposited aluminum on chemically synthesized polypyrrole films. Synth. Met. 1999, 106, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Qu, Y.; Lai, Y.; Li, J. Nitrogen-doped graphene/sulfur composite as cathode material forhigh capacity lithium–sulfur batteries. J. Power Sources 2014, 256, 361–368. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Wan, C.; Du, K.; Xie, J.; Xu, N. Sulfur Composite Cathode Materials for Rechargeable Lithium Batteries. Adv. Funct. Mater. 2003, 13, 487–492. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Wang, X.; Zhao, Y.; Tan, T.; Bakenov, Z.; Zhang, Y. Synthesis of a Flexible Freestanding Sulfur/Polyacrylonitrile/Graphene Oxide as the Cathode for Lithium/Sulfur Batteries. Polymers 2018, 10, 399. https://doi.org/10.3390/polym10040399
Peng H, Wang X, Zhao Y, Tan T, Bakenov Z, Zhang Y. Synthesis of a Flexible Freestanding Sulfur/Polyacrylonitrile/Graphene Oxide as the Cathode for Lithium/Sulfur Batteries. Polymers. 2018; 10(4):399. https://doi.org/10.3390/polym10040399
Chicago/Turabian StylePeng, Huifen, Xiaoran Wang, Yan Zhao, Taizhe Tan, Zhumabay Bakenov, and Yongguang Zhang. 2018. "Synthesis of a Flexible Freestanding Sulfur/Polyacrylonitrile/Graphene Oxide as the Cathode for Lithium/Sulfur Batteries" Polymers 10, no. 4: 399. https://doi.org/10.3390/polym10040399