Preparation of Maleic Anhydride Grafted Polybutene and Its Application in Isotactic Polybutene-1/Microcrystalline Cellulose Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MAPB Preparation
2.3. MAPB Characterization
2.3.1. FTIR Analysis
2.3.2. Elemental Analysis
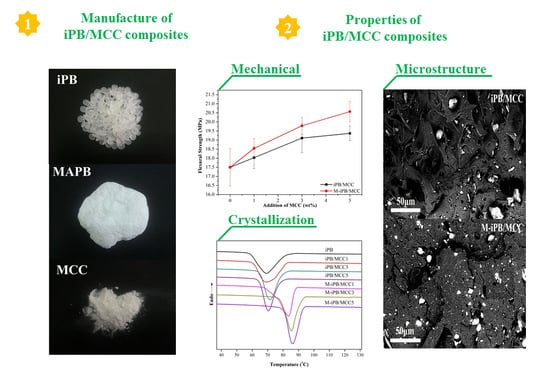
2.4. Preparation of iPB/MCC Composites
2.5. Characterization of iPB/MCC Composites
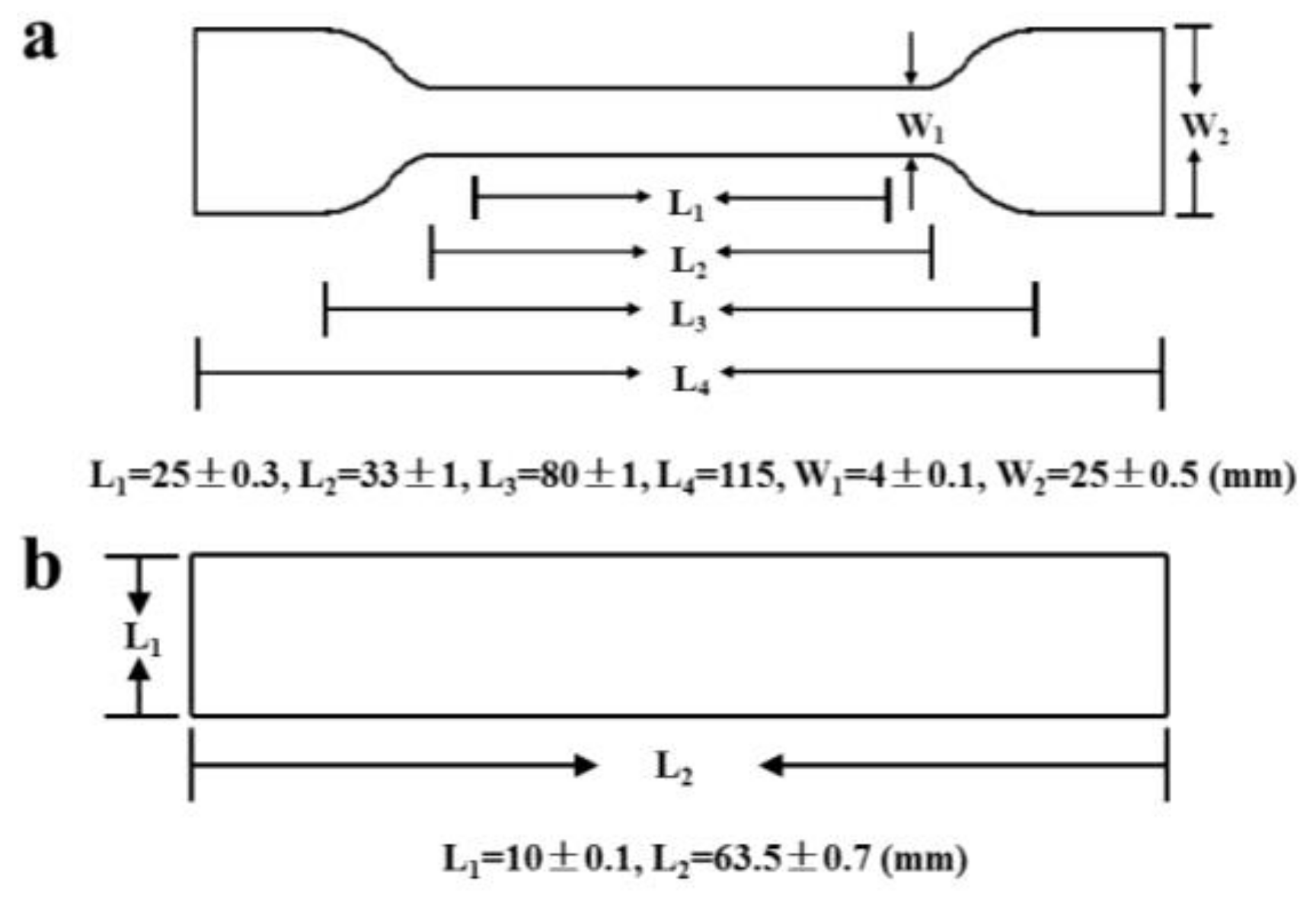
2.5.1. Mechanical Properties
2.5.2. Heat-Distortion Temperature
2.5.3. Scanning Electron Microscopy
2.5.4. Thermogravimetric Analysis
2.5.5. Differential Scanning Calorimetry Analysis
3. Results and Discussion
3.1. FTIR Spectra of iPB and MAPB
3.2. Elemental Analysis of iPB and MAPB
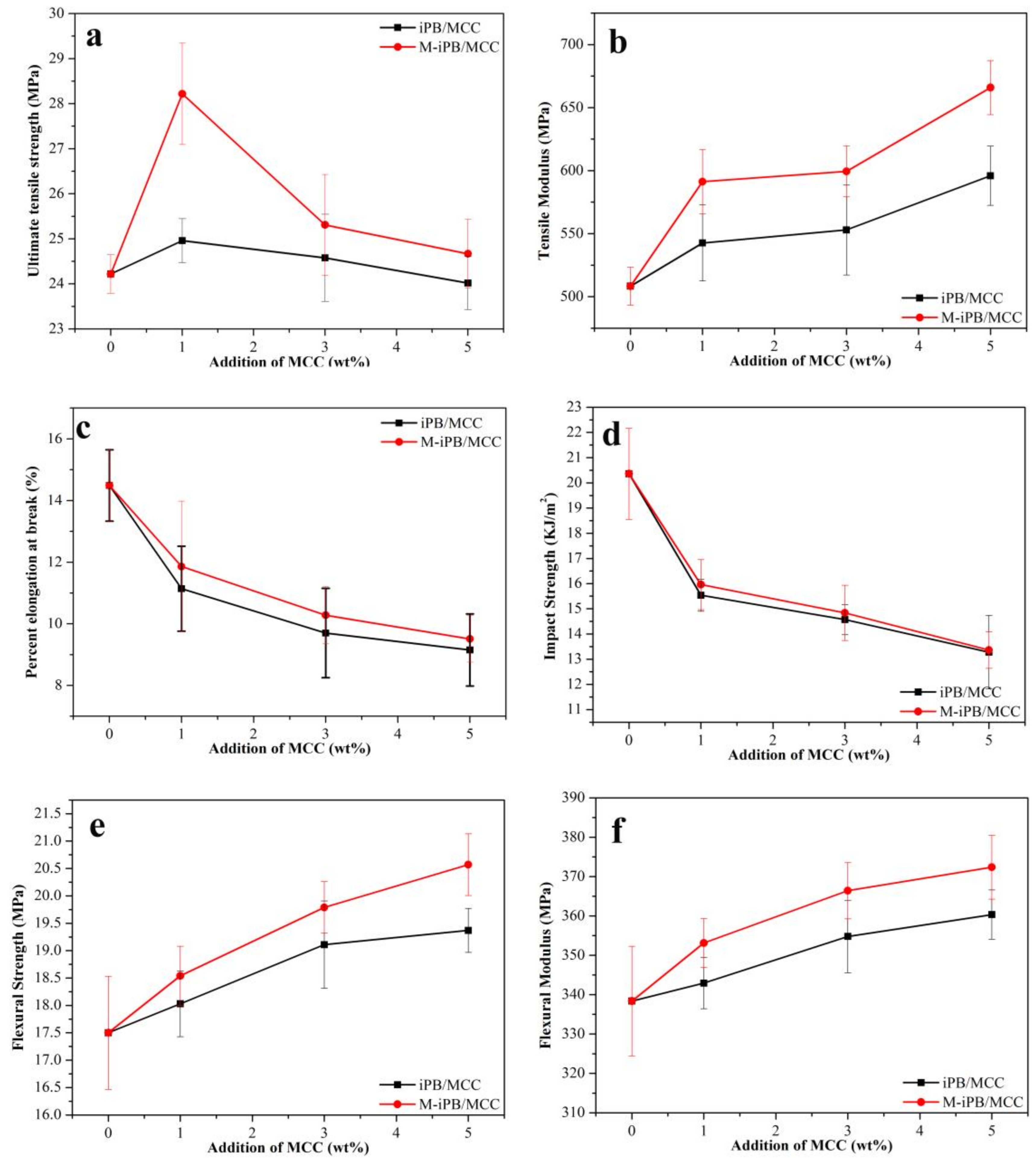
3.3. Mechanical Properties of iPB/MCC Composites
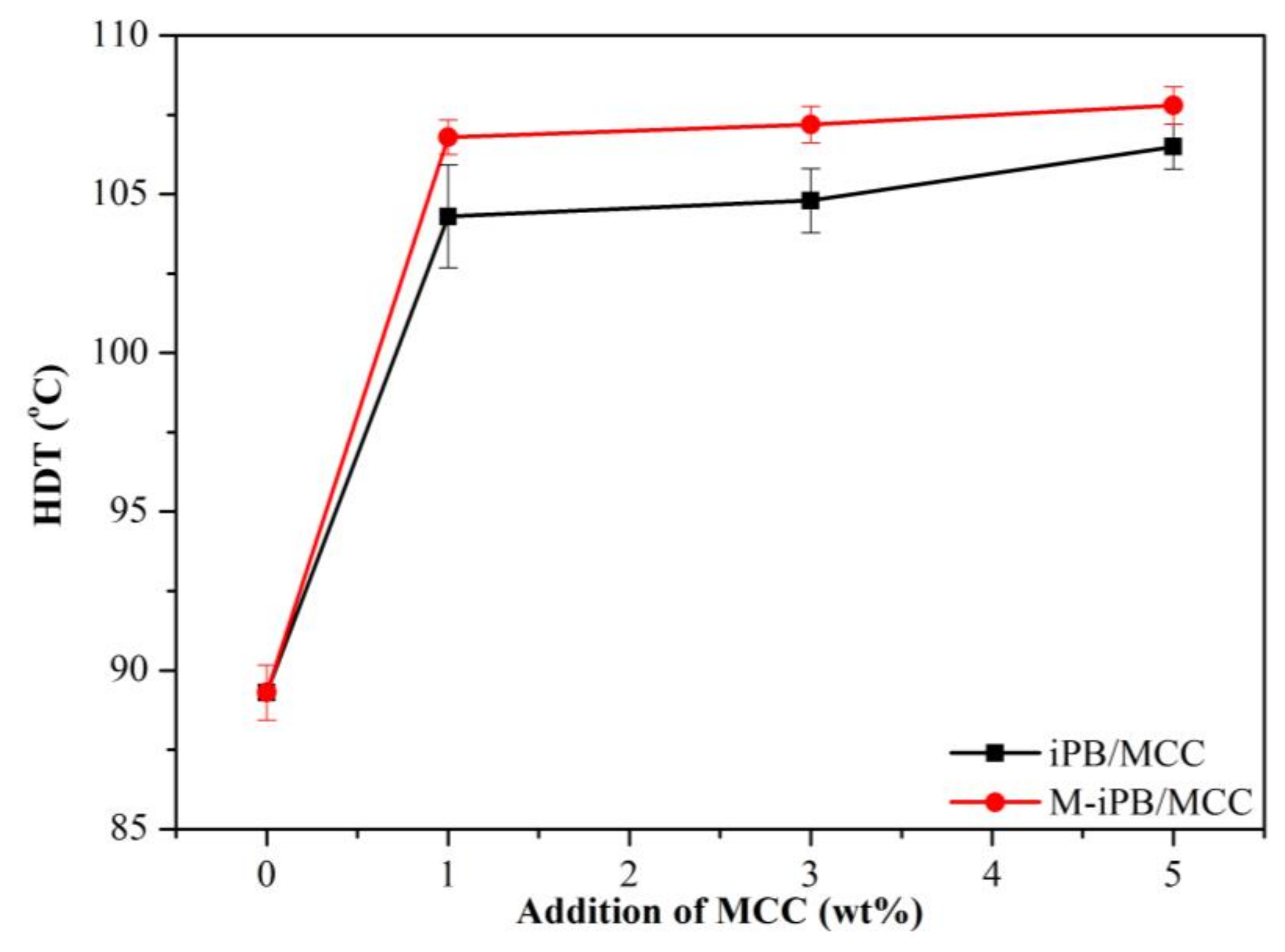
3.4. HDT of iPB/MCC Composites
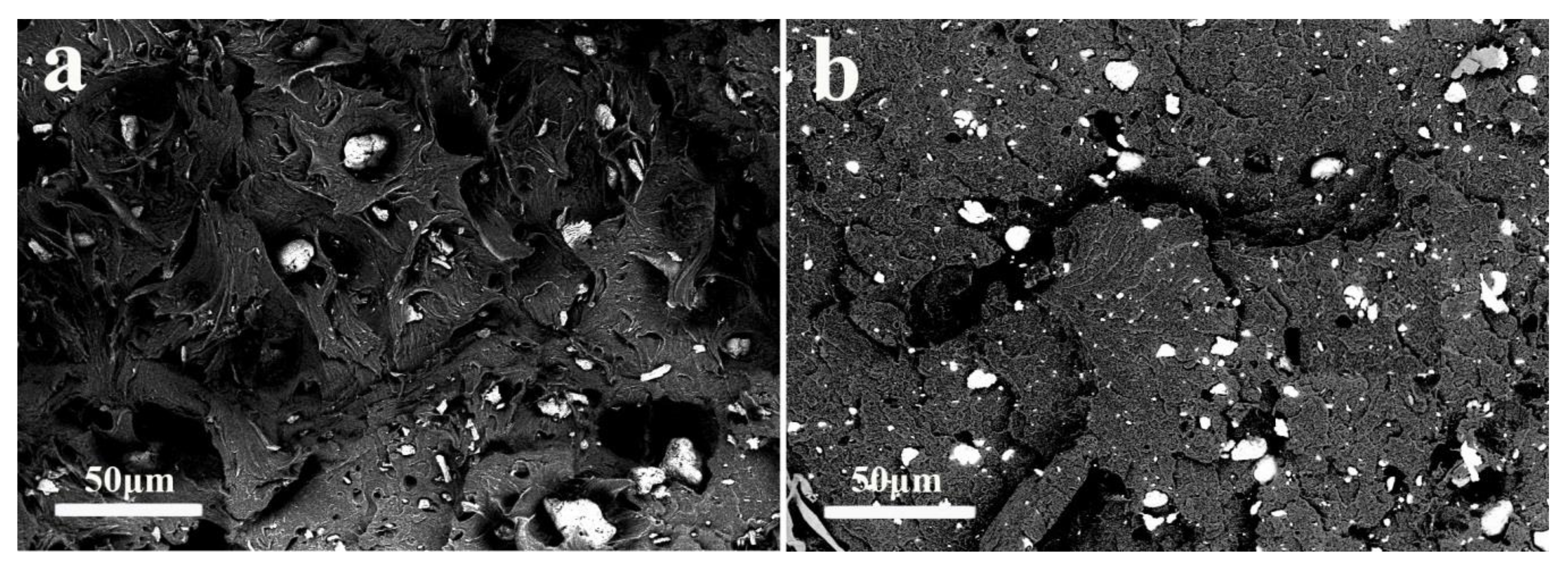
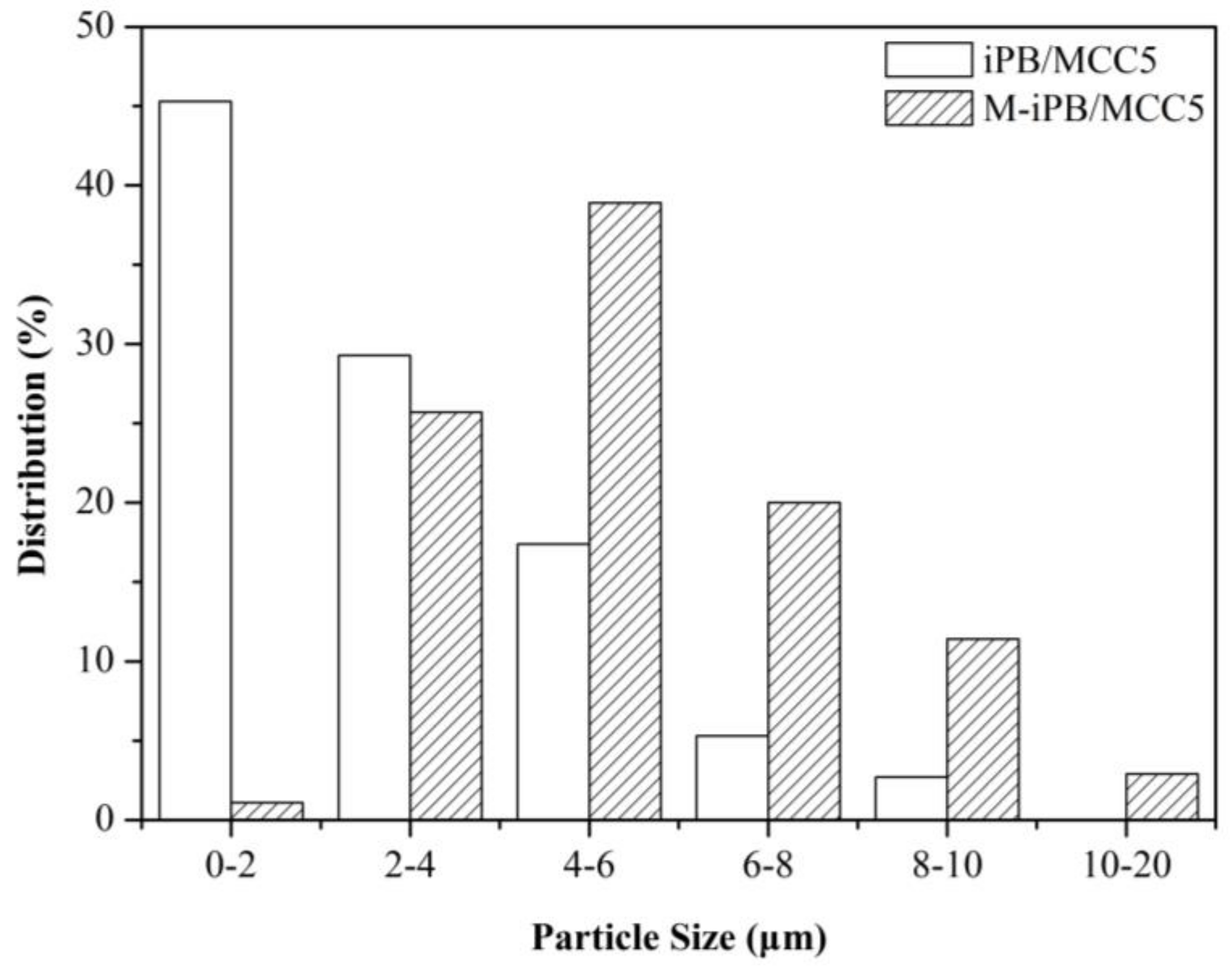
3.5. SEM Micrograph of iPB/MCC Composites
3.6. DTG of iPB/MCC Composites
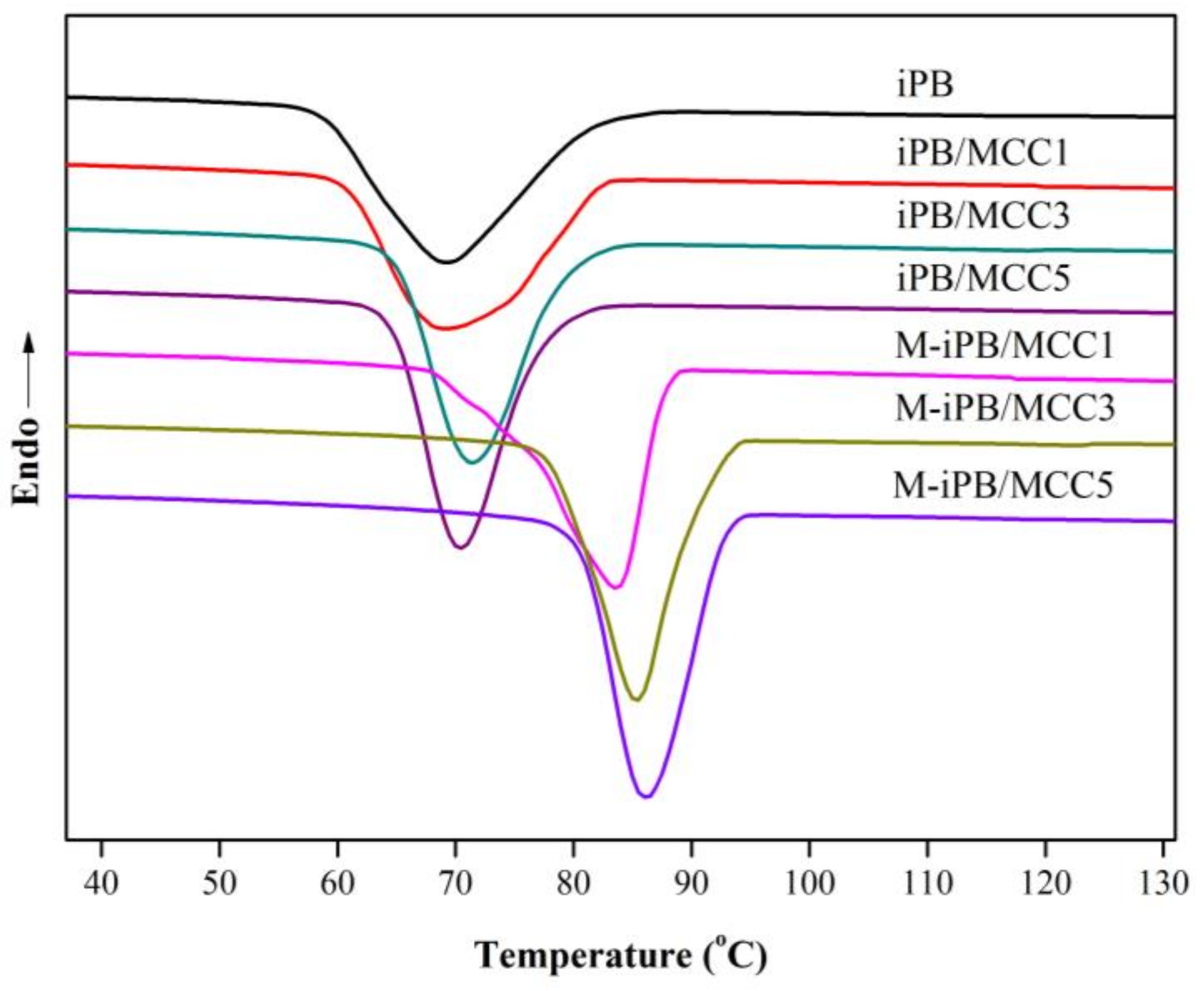
3.7. Non-Isothermal Crystallization of iPB/MCC Composites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shieh, Y.T.; Lee, M.S.; Chen, S.A. Crystallization behavior, crystal transformation, and morphology of polypropylene/polybutene-1 blends. Polymer 2001, 42, 4439–4448. [Google Scholar] [CrossRef]
- Alfadhel, K.; Al-Mulla, A.; Al-Busairi, B. Development and characterization of novel polybutylene nanocomposites. J. Compos. Mater. 2016, 51, 95–108. [Google Scholar] [CrossRef]
- Ji, Y.; Su, F.; Cui, K. Mixing Assisted Direct Formation of Isotactic Poly(1-butene) Form I′ Crystals from Blend Melt of Isotactic Poly(1-butene)/Polypropylene. Macromolecule 2016, 49, 266–270. [Google Scholar] [CrossRef]
- Cavallo, D.; Gardella, L.; Portale, G.; Müller, A.J.; Alfonso, G.C. Kinetics of cross-nucleation in isotactic poly(1-butene). Macromolecules 2014, 47, 870–873. [Google Scholar] [CrossRef]
- Giavarini, C.; Filippis, P.D.; Santarelli, M.L.; Scarsella, M. Production of stable polypropylene-modified bitumens. Fuel 1996, 75, 681–686. [Google Scholar] [CrossRef]
- Nase, M.; Androsch, R.; Langer, B.; Baumann, H.J.; Grellmann, W. Effect of polymorphism of isotactic polybutene-1 on peel behavior of polyethylene/polybutene-1 peel systems. J. Appl. Polym. Sci. 2008, 107, 3111–3118. [Google Scholar] [CrossRef]
- Qiu, W.; Endo, T.; Hirotsu, T. Interfacial interaction, morphology, and tensile properties of a composite of highly crystalline cellulose and maleated polypropylene. J. Appl. Polym. Sci. 2010, 102, 3830–3841. [Google Scholar] [CrossRef]
- Iervolino, R.; Somma, E.; Nobile, M.R. The role of multi-walled carbon nanotubes in shear enhanced crystallization of isotactic poly(1-butene). J. Therm. Anal. Calorim. 2009, 98, 611–622. [Google Scholar] [CrossRef]
- Li, S.C.; Hu, H. l.; Zeng, W. Preparation and Properties of PB-1/PP Blends. Polym. Plast. Tech. Eng. 2012, 51, 744–749. [Google Scholar] [CrossRef]
- Liu, W.; Fei, M.E.; Ban, Y.; Jia, A.; Qiu, R. Preparation and evaluation of green composites from microcrystalline cellulose and a soybean-oil derivative. Polymers 2017, 9, 541. [Google Scholar] [CrossRef]
- Rashid, M.; Gafur, M.A.; Sharafat, M.K.; Minami, H.; Miah, M.A.J.; Ahmad, H. Biocompatible microcrystalline cellulose particles from cotton wool and magnetization via a simple in situ co-precipitation method. Carbohydr. Polym. 2017, 170, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yi, S.; He, J.; Wang, H.; Fang, Y.; Wang, Q. Preparation and properties of a novel microcrystalline cellulose-filled composites based on polyamide 6/high-density polyethylene. Materials 2017, 10, 808. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chang, P.R.; Yu, J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr. Polym. 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Roy, K.; Potiyaraj, P. Development of high performance microcrystalline cellulose based natural rubber composites using maleated natural rubber as compatibilizer. Cellulose 2018, 25, 1077–1087. [Google Scholar] [CrossRef]
- Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R.; Montero, B. Processing and characterization of polyols plasticized-starch reinforced with microcrystalline cellulose. Carbohydr. Polym. 2016, 149, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Kiziltas, A.; Gardner, D.J.; Han, Y.; Yang, H.S. Mechanical Properties of microcrystalline cellulose (MCC) filled engineering thermoplastic composites. J. Polym. Environ. 2014, 22, 365–372. [Google Scholar] [CrossRef]
- Singh, A.A.; Palsule, S. Coconut fiber reinforced chemically functionalized high-density polyethylene (CHF/CF-HDPE) composites by palsule process. J. Compos. Mater. 2014, 48, 3673–3684. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.R.; Huang, C.; Xiong, L.; Luo, J.; Chen, X.D. Study on non-isothermal crystallization behavior of isotactic polypropylene/bacterial cellulose composites. Rsc. Adv. 2017, 7, 42113–42122. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.; Zhang, L.; Pan, L.; Tian, Z.; Pang, S.; Xu, N.; Lin, Q. Mechanochemistry: A novel approach to graft polypropylene with dual monomers (PP-g-(MAH-co-St)). Polym. Bull. 2015, 72, 1949–1960. [Google Scholar]
- Shi, D.; Yang, J.; Yao, Z.; Yong, W.; Huang, H.; Wu, J.; Yin, J.; Costa, G. Functionalization of isotactic polypropylene with maleic anhydride by reactive extrusion: Mechanism of melt grafting. Polymer 2001, 42, 5549–5557. [Google Scholar] [CrossRef]
- Ghosh, P.; Chattopadhyay, B.; Sen, A.K. Modification of low density polyethylene (LDPE) by graft copolymerization with some acrylic monomers. Polymer 1998, 39, 193–201. [Google Scholar] [CrossRef]
- Madrid, J.F.; Lopez, G.E.P.; Abad, L.V. Application of full-factorial design in the synthesis of polypropylene-g-poly(glycidyl methacrylate) functional material for metal ion adsorption. Radiat. Phys. Chem. 2017, 136, 54–63. [Google Scholar] [CrossRef]
- Zuiderduin, W.C.J.; Westzaan, C.; Huétink, J.; Gaymans, R.J. Toughening of polypropylene with calcium carbonate particles. Polymer 2003, 44, 261–275. [Google Scholar] [CrossRef]
- Wang, B.; Yang, D.; Zhang, H.R.; Huang, C.; Xiong, L.; Luo, J.; Chen, X.D. Preparation of Esterified Bacterial Cellulose for Improved Mechanical Properties and the Microstructure of Isotactic Polypropylene/Bacterial Cellulose Composites. Polymers 2016, 8, 129. [Google Scholar] [CrossRef]
- Kakroodi, A.R.; Leduc, S.; Rodrigue, D. Effect of hybridization and compatibilization on the mechanical properties of recycled polypropylene/hemp composites. J. Appl. Polym. Sci. 2012, 124, 2494–2500. [Google Scholar] [CrossRef]
- Karmarkar, A.; Chauhan, S.S.; Modak, J.M.; Chanda, M. Mechanical properties of wood–fiber reinforced polypropylene composites: Effect of a novel compatibilizer with isocyanate functional group. Compos. Part A 2007, 38, 227–233. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Cheng, L.; Qu, W. Mechanical properties of poly(butylene succinate) (pbs) biocomposites reinforced with surface modified jute fibre. Compos. Part A Appl. Sci. Manuf. 2009, 40, 669–674. [Google Scholar] [CrossRef]
- He, L.; Li, W.; Chen, D.; Zhou, D.; Lu, G.; Yuan, J. Effects of amino silicone oil modification on properties of ramie fiber and ramie fiber/polypropylene composites. Mater. Des. 2015, 77, 142–148. [Google Scholar] [CrossRef]
- Ashori, A.; Nourbakhsh, A. Performance properties of microcrystalline cellulose as a reinforcing agent in wood plastic composites. Compos. Part B Eng. 2010, 41, 578–581. [Google Scholar] [CrossRef]


| Sample | iPB (g) | MCC (g) | MAPB (g) |
|---|---|---|---|
| iPB | 1000 | - | - |
| iPB/MCC1 | 1000 | 10 | - |
| iPB/MCC3 | 1000 | 30 | - |
| iPB/MCC5 | 1000 | 50 | - |
| M-iPB/MCC1 | 1000 | 10 | 20 |
| M-iPB/MCC3 | 1000 | 30 | 20 |
| M-iPB/MCC5 | 1000 | 50 | 20 |
| Sample | C (%) | H (%) | O (%) |
|---|---|---|---|
| iPB | 85.89 | 14.11 | 0 |
| MAPB | 85.28 | 14.24 | 0.38 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Nie, K.; Xue, X.-r.; Lin, F.-h.; Li, X.-y.; Xue, Y.-b.; Luo, J. Preparation of Maleic Anhydride Grafted Polybutene and Its Application in Isotactic Polybutene-1/Microcrystalline Cellulose Composites. Polymers 2018, 10, 393. https://doi.org/10.3390/polym10040393
Wang B, Nie K, Xue X-r, Lin F-h, Li X-y, Xue Y-b, Luo J. Preparation of Maleic Anhydride Grafted Polybutene and Its Application in Isotactic Polybutene-1/Microcrystalline Cellulose Composites. Polymers. 2018; 10(4):393. https://doi.org/10.3390/polym10040393
Chicago/Turabian StyleWang, Bo, Kai Nie, Xiao-rong Xue, Fu-hua Lin, Xiang-yang Li, Yong-bing Xue, and Jun Luo. 2018. "Preparation of Maleic Anhydride Grafted Polybutene and Its Application in Isotactic Polybutene-1/Microcrystalline Cellulose Composites" Polymers 10, no. 4: 393. https://doi.org/10.3390/polym10040393