3D Porous Gelatin/PVA Hydrogel as Meniscus Substitute Using Alginate Micro-Particles as Porogens
Abstract
:1. Introduction
2. Materials and Methods
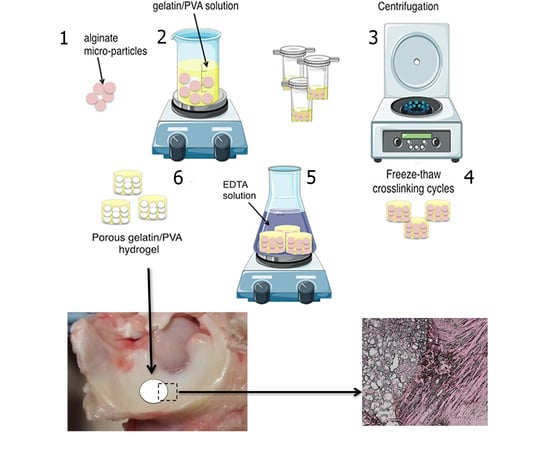
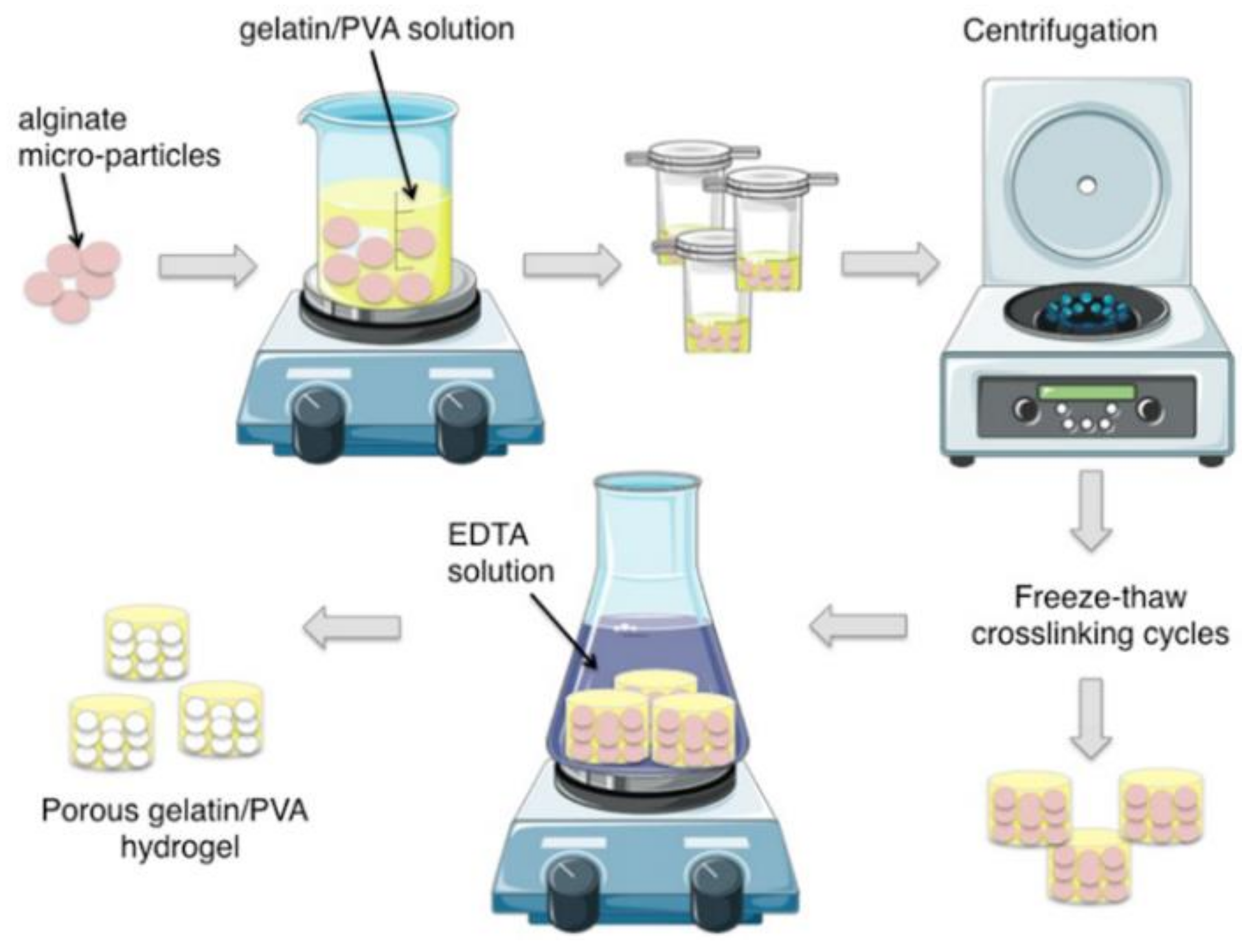
2.1. Fabrication of Alginate Micro-Particles
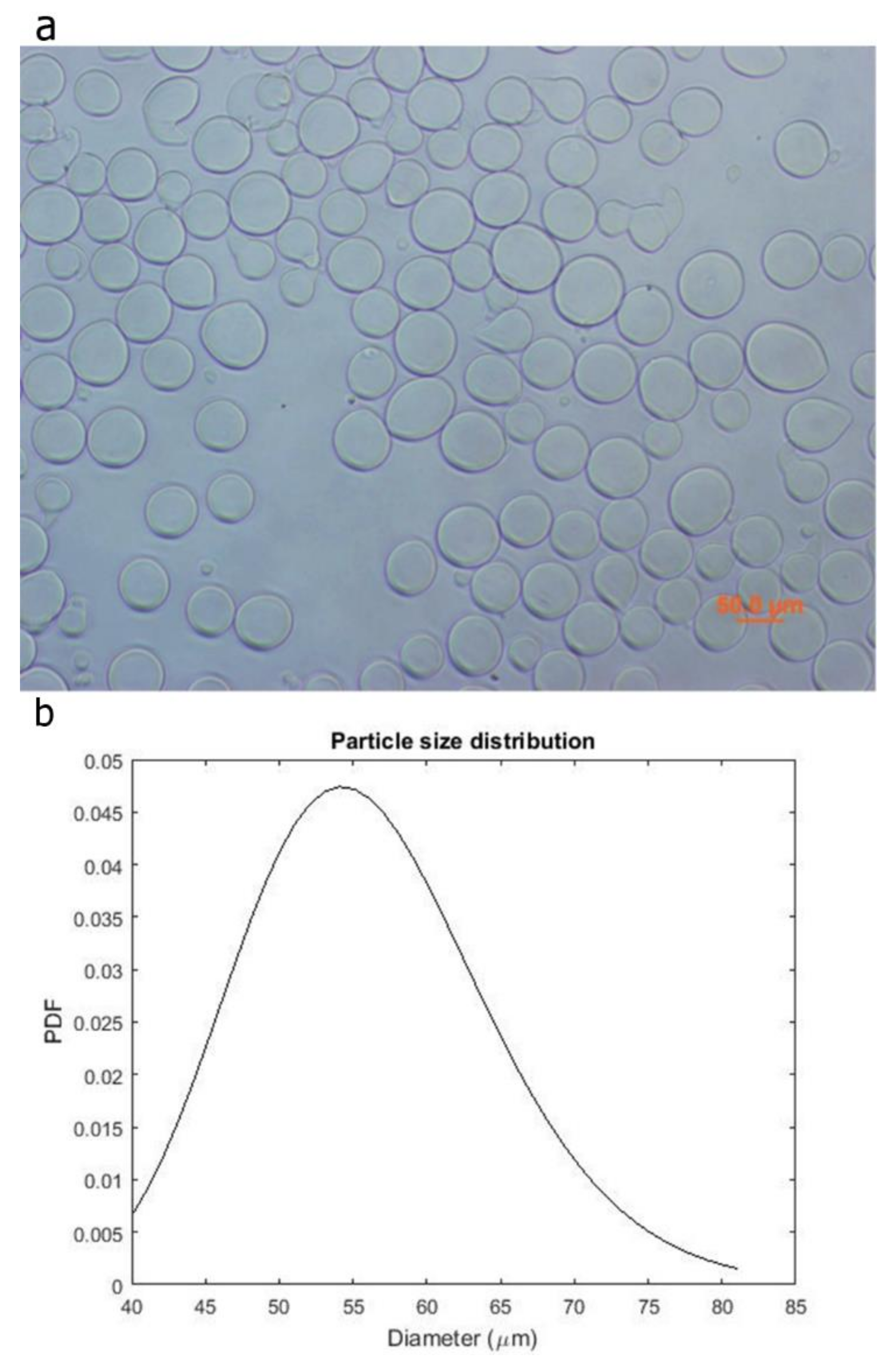
2.2. Micro-Particles Characterization
2.3. Preparation of Hydrogels
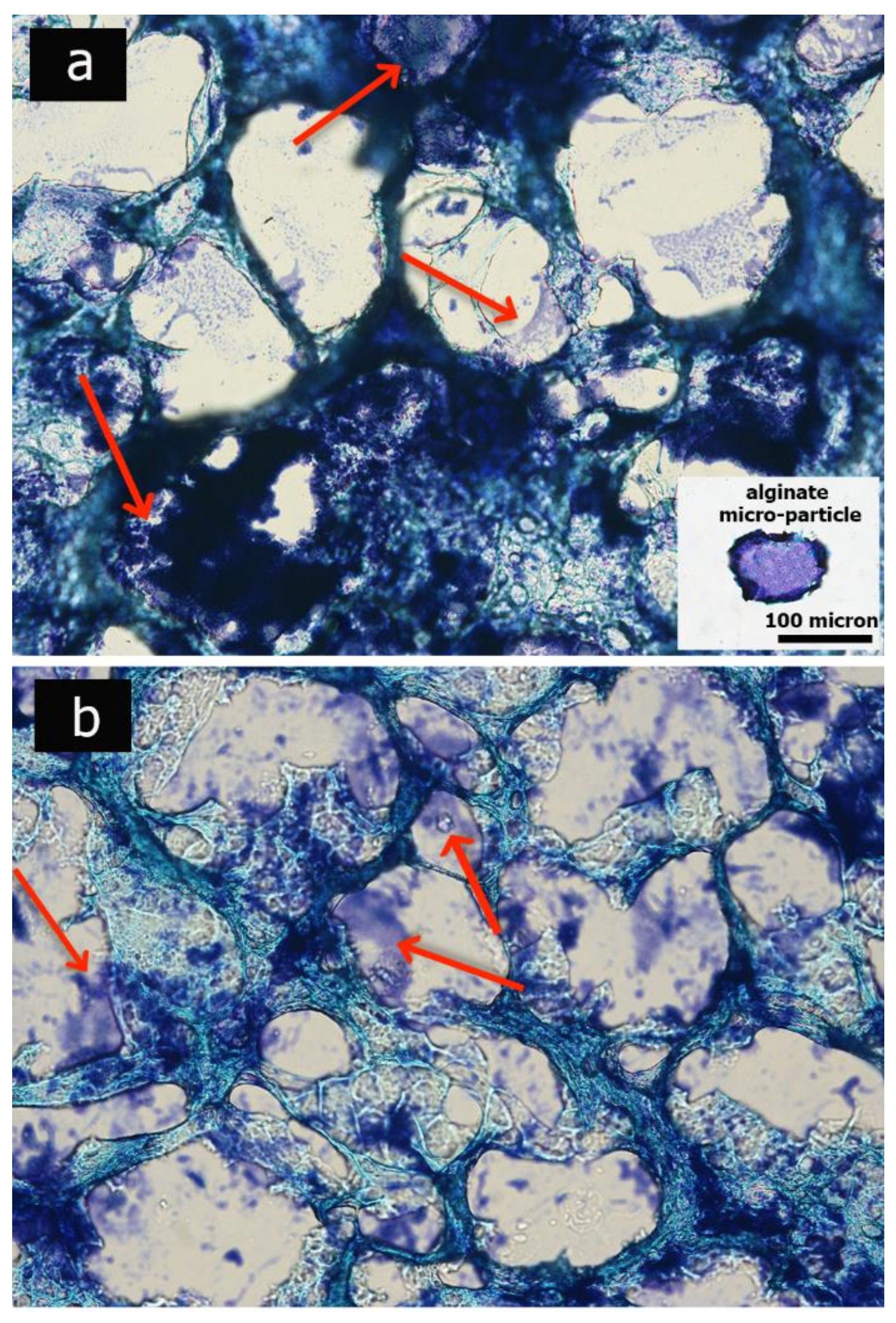
2.4. Morphological Analysis of Hydrogel
2.5. Chemical Characterization
2.6. Analysis of Swelling
2.7. Mechanical Characterization
2.8. Cell Culture
2.9. Meniscal Integration Model System Ex Vivo
3. Results
3.1. Micro-Particles Characterization
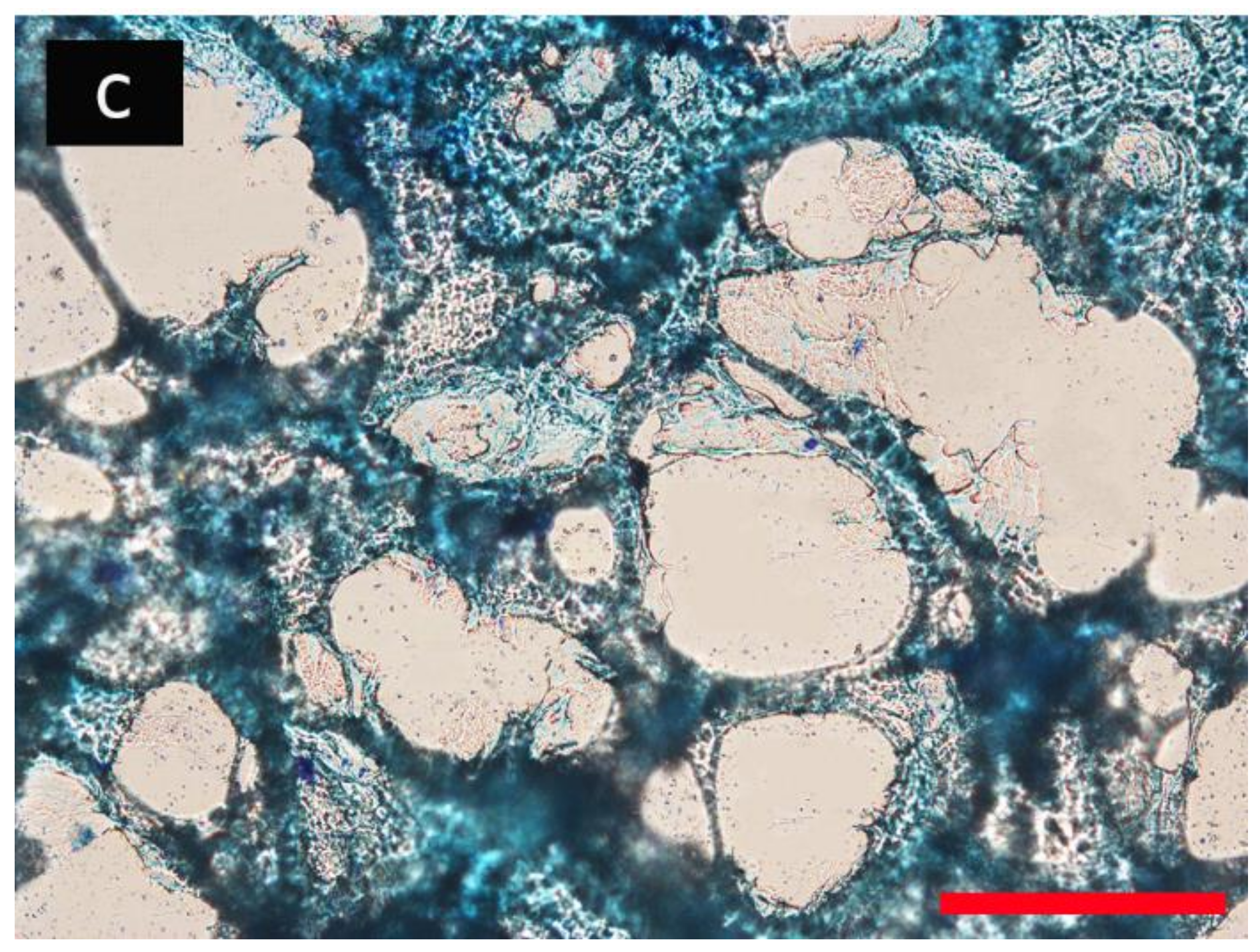
3.2. Porous Hydrogel Morphological Characterization
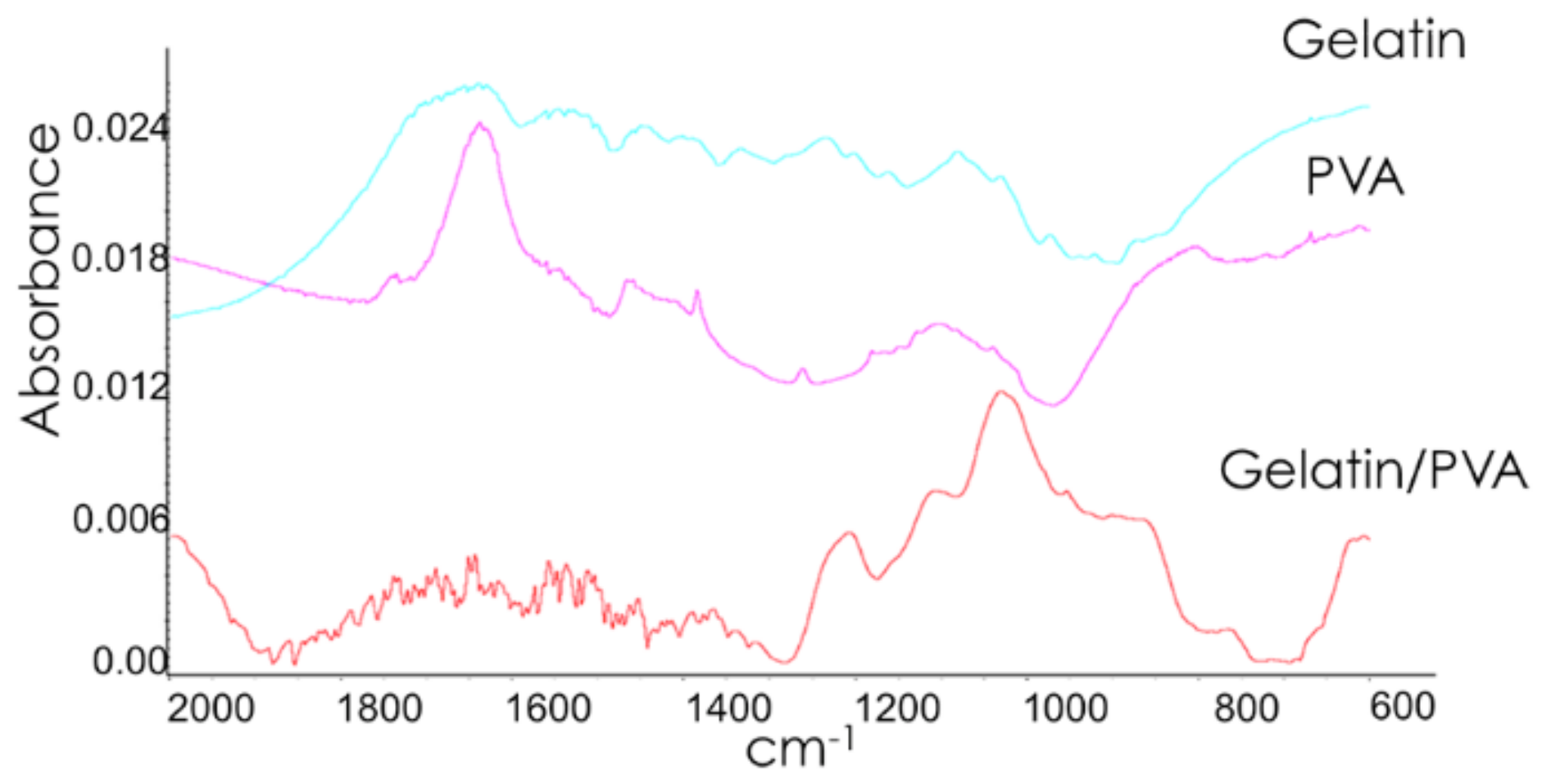
3.3. Chemical Characterization
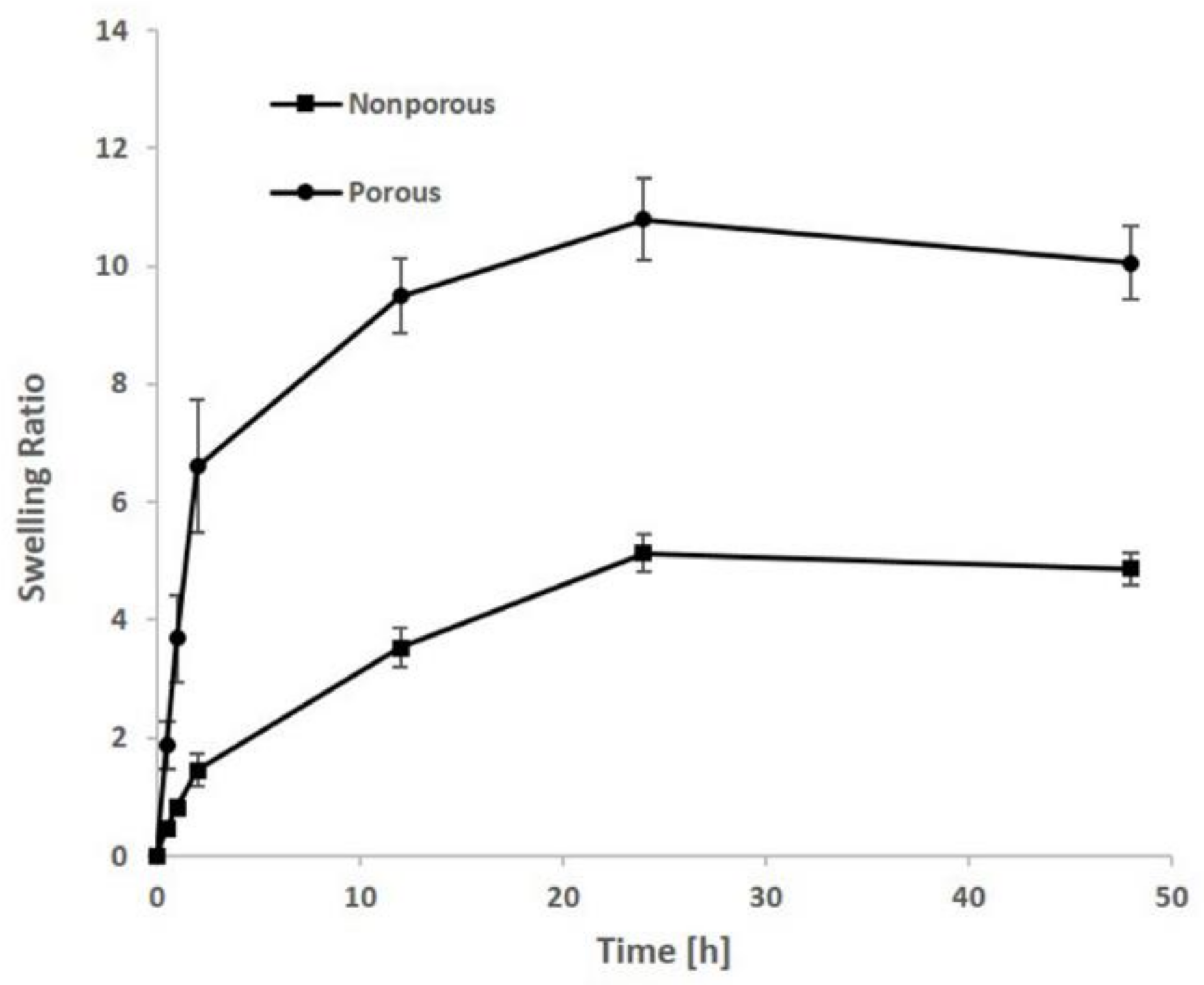
3.4. Swelling
3.5. Mechanical Properties
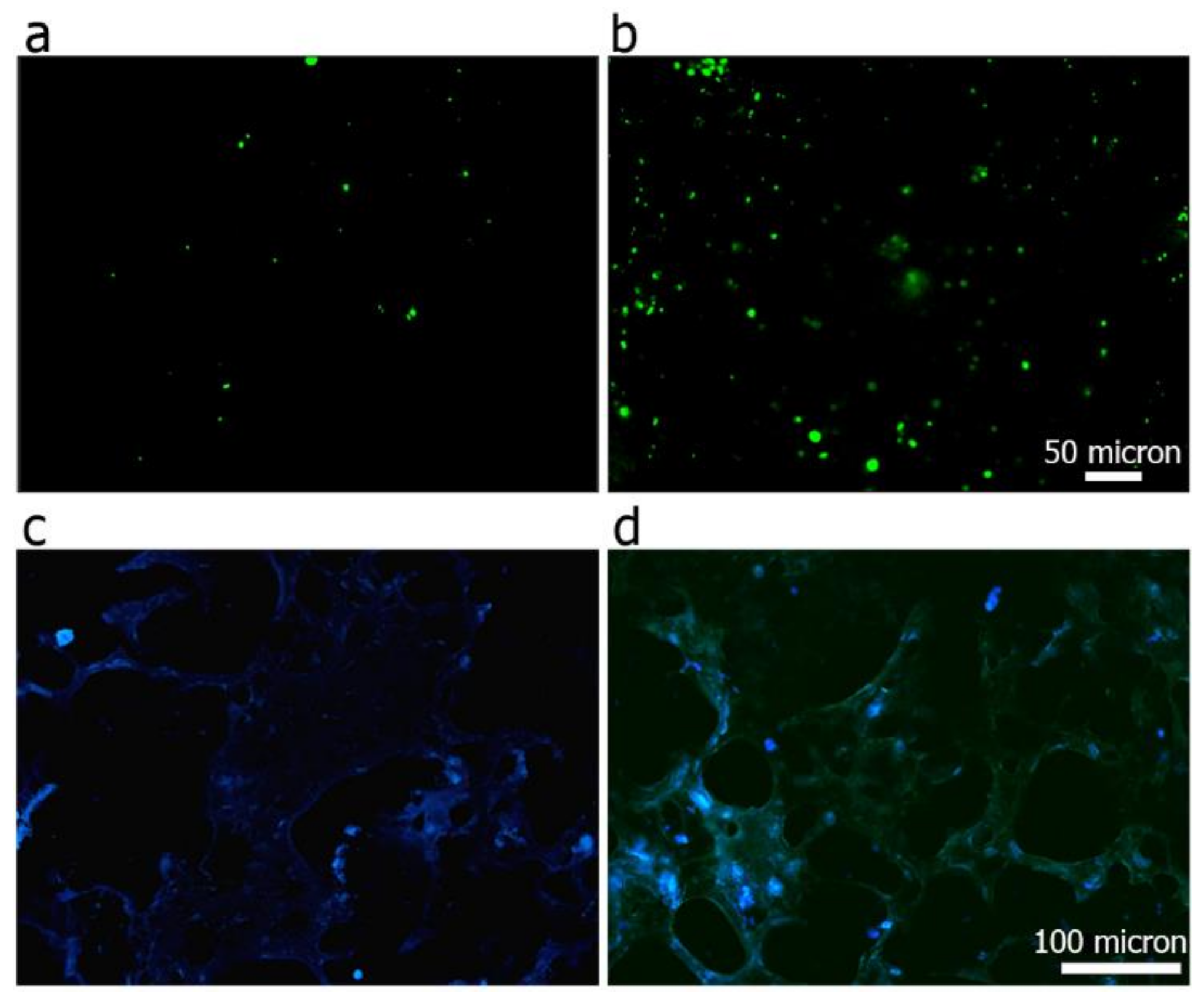
3.6. Cell Viability and Adhesion Analysis
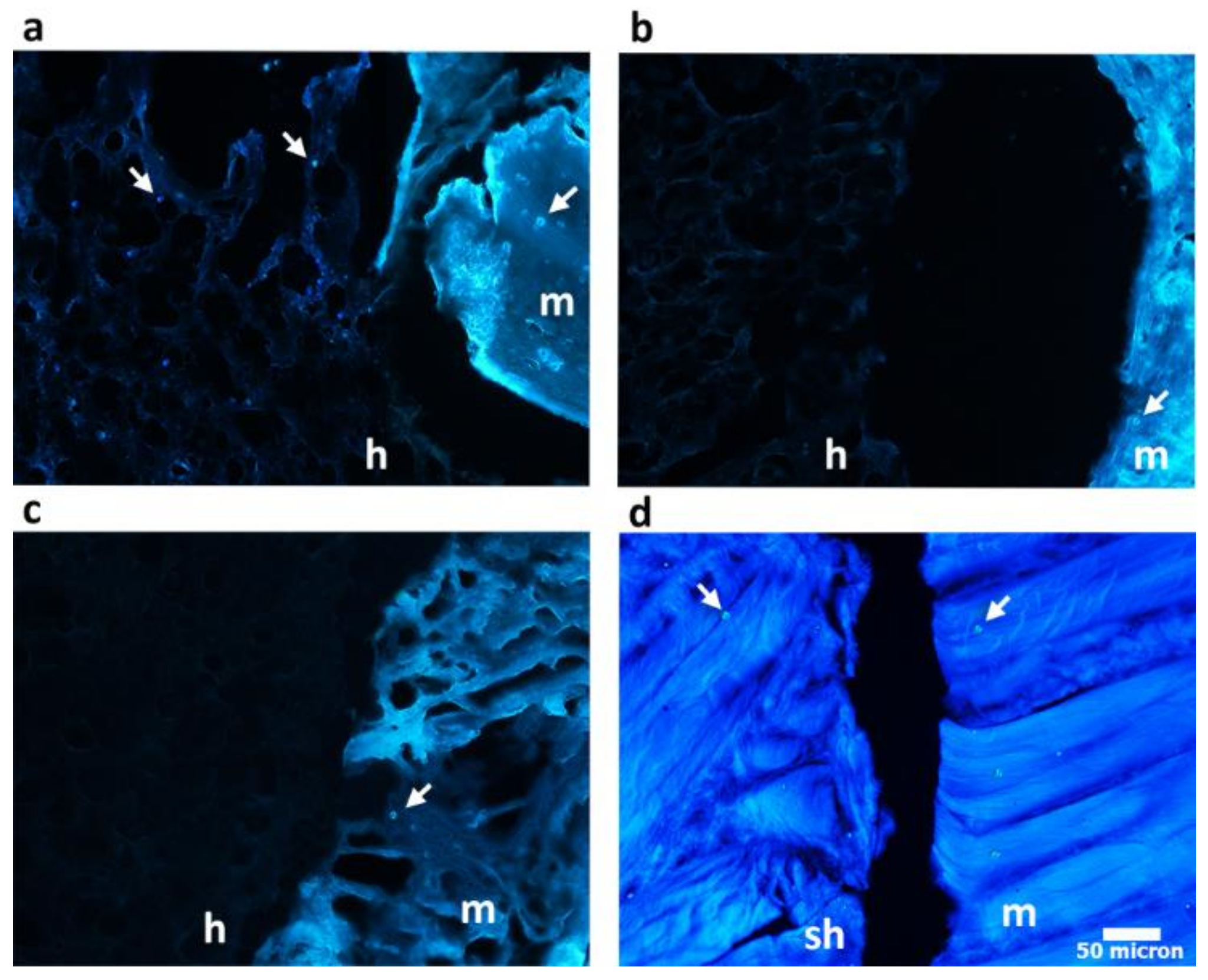
3.7. Hydrogel Integration in a Ex Vivo Animal Model
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rnjak-Kovacina, J.; Wray, L.S.; Burke, K.A.; Torregrosa, T.; Golinski, J.M.; Huang, W.; Kaplan, D.L. Lyophilized silk sponges: A versatile biomaterial platform for soft tissue engineering. ACS Biomater. Sci. Eng. 2015, 1, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.A.; Court-Brown, C.M. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 2008, 39, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Greis, P.E.; Bardana, D.D.; Holmstrom, M.C.; Burks, R.T. Meniscal injury: I. Basic science and evaluation. J. Am. Acad. Orthop. Surg. 2002, 10, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Brindle, T.; Nyland, J.; Johnson, D.L. The meniscus: Review of basic principles with application to surgery and rehabilitation. J. Athl. Train. 2001, 36, 160. [Google Scholar] [PubMed]
- Tudor, F.; McDermott, I.D.; Myers, P. Meniscal repair: A review of current practice. Orthop. Trauma 2014, 28, 88–96. [Google Scholar] [CrossRef]
- Fairbank, T. Knee joint changes after meniscectomy. Bone Jt. J. 1948, 30, 664–670. [Google Scholar] [CrossRef]
- Allen, P.; Denham, R.; Swan, A. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. Bone Jt. J. 1984, 66, 666–671. [Google Scholar] [CrossRef]
- Jackson, J. Degenerative changes in the knee after meniscectomy. Br. Med. J. 1968, 2, 525. [Google Scholar] [CrossRef] [PubMed]
- McDermott, I.; Amis, A. The consequences of meniscectomy. Bone Jt. J. 2006, 88, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Rongen, J.J.; van Tienen, T.G.; van Bochove, B.; Grijpma, D.W.; Buma, P. Biomaterials in search of a meniscus substitute. Biomaterials 2014, 35, 3527–3540. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Munoz-Pinto, D.; Qu, X.; Hou, Y.; Grunlan, M.A.; Hahn, M.S. Influence of hydrogel mechanical properties and mesh size on vocal fold fibroblast extracellular matrix production and phenotype. Acta Biomater. 2008, 4, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.T.; Choi, G.; Bryk, E.; Vigorita, V. The human knee meniscus: A review with special focus on the collagen meniscal implant. J. Long-Term Effects Med. Implants 2011, 21, 321–337. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Marcheggiani Muccioli, G.M.; Lopomo, N.; Bruni, D.; Giordano, G.; Ravazzolo, G.; Molinari, M.; Marcacci, M. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: A minimum 10-year follow-up study. Am. J. Sports Med. 2011, 39, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Fisher, O.Z.; Khademhosseini, A.; Langer, R.; Peppas, N.A. Bioinspired materials for controlling stem cell fate. Acc. Chem. Res. 2010, 43, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, A.M.; Kloxin, C.J.; Bowman, C.N.; Anseth, K.S. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv. Mater. 2010, 22, 3484–3494. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Marrella, A.; Lagazzo, A.; Barberis, F.; Catelani, T.; Quarto, R.; Scaglione, S. Enhanced mechanical performances and bioactivity of cell laden-graphene oxide/alginate hydrogels open new scenario for articular tissue engineering applications. Carbon 2017, 115, 608–616. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual polyvinyl alcohol associated with poly (d,l-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Miao, T.; Miller, E.J.; McKenzie, C.; Oldinski, R.A. Physically crosslinked polyvinyl alcohol and gelatin interpenetrating polymer network theta-gels for cartilage regeneration. J. Mater. Chem. B 2015, 3, 9242–9249. [Google Scholar] [CrossRef]
- Ng, K.W.; Torzilli, P.A.; Warren, R.F.; Maher, S.A. Characterization of a macroporous polyvinyl alcohol scaffold for the repair of focal articular cartilage defects. J. Tissue Eng. Regen. Med. 2014, 8, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, S.R.; Peppast, N.A. Poly(vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer 1992, 33, 3932–3936. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and morphology of freeze/thawed pva hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Toguchida, J.; Oka, M. Development of an artificial meniscus using polyvinyl alcohol-hydrogel for early return to, and continuance of, athletic life in sportspersons with severe meniscus injury. I: Mechanical evaluation. Knee 2003, 10, 47–51. [Google Scholar] [CrossRef]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. Mechanical evaluation of poly (vinyl alcohol)-based fibrous composites as biomaterials for meniscal tissue replacement. Acta Biomater. 2010, 6, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Coluccino, L.; Gottardi, R.; Ayadi, F.; Athanassiou, A.; Tuan, R.S.; Ceseracciu, L. A porous polyvinyl alcohol-based hydrogel for knee meniscus functional repair. ACS Biomater. Sci. Eng. 2018. [Google Scholar] [CrossRef]
- Kobayashi, M.; Chang, Y.-S.; Oka, M. A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-h) artificial meniscus. Biomaterials 2005, 26, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.T.; Robertson, W.; Potter, H.G.; Deng, X.-H.; Turner, A.S.; Lyman, S.; Warren, R.F.; Rodeo, S.A. Hydrogel meniscal replacement in the sheep knee. Am. J. Sports Med. 2007, 35, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Nuttelman, C.R.; Mortisen, D.J.; Henry, S.M.; Anseth, K.S. Attachment of fibronectin to poly (vinyl alcohol) hydrogels promotes nih3t3 cell adhesion, proliferation, and migration. J. Biomed. Mater. Res. 2001, 57, 217–223. [Google Scholar] [CrossRef]
- Koyano, T.; Minoura, N.; Nagura, M.; Kobayashi, K.i. Attachment and growth of cultured fibroblast cells on pva/chitosan-blended hydrogels. J. Biomed. Mater. Res. 1998, 39, 486–490. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. AAPS PharmSciTech 2007, 8, E142–E146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Geever, L.M.; Kennedy, J.E.; Higginbotham, C.L.; Cahill, P.A.; McGuinness, G.B. Thermal behavior and mechanical properties of physically crosslinked pva/gelatin hydrogels. J. Mech. Behav. Biomed. Mater. 2010, 3, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Linh, N.T.; Lee, B.T. Electrospinning of polyvinyl alcohol/gelatin nanofiber composites and cross-linking for bone tissue engineering application. J. Biomater. Appl. 2012, 27, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Reignier, J.; Huneault, M.A. Preparation of interconnected poly (ε-caprolactone) porous scaffolds by a combination of polymer and salt particulate leaching. Polymer 2006, 47, 4703–4717. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Ding, J. In vitro degradation of three-dimensional porous poly (d,l-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 2004, 25, 5821–5830. [Google Scholar] [CrossRef] [PubMed]
- Marrella, A.; Aiello, M.; Quarto, R.; Scaglione, S. Chemical and morphological gradient scaffolds to mimic hierarchically complex tissues: From theoretical modeling to their fabrication. Biotechnol. Bioeng. 2016, 113, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Draghi, L.; Resta, S.; Pirozzolo, M.; Tanzi, M. Microspheres leaching for scaffold porosity control. J. Mater. Sci. Mater. Med. 2005, 16, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.J.; Kim, J.H.; Park, T.G. Dexamethasone-releasing biodegradable polymer scaffolds fabricated by a gas-foaming/salt-leaching method. Biomaterials 2003, 24, 2323–2329. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. J. Biomed. Mater. Res. Part A 2006, 78, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gong, Y.; Gao, C. Microstructure and mechanical properties of poly (l-lactide) scaffolds fabricated by gelatin particle leaching method. J. Appl. Polym. Sci. 2005, 98, 1373–1379. [Google Scholar] [CrossRef]
- Thu, B.; Bruheim, P.; Espevik, T.; Smidsrød, O.; Soon-Shiong, P.; Skjåk-Bræk, G. Alginate polycation microcapsules: Ii. Some functional properties. Biomaterials 1996, 17, 1069–1079. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/pva hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Arndt, K.; Richter, A.; Ludwig, S.; Zimmermann, J.; Kressler, J.; Kuckling, D.; Adler, H. Poly (vinyl alcohol)/poly (acrylic acid) hydrogels: Ft-ir spectroscopic characterization of crosslinking reaction and work at transition point. Acta Polym. 1999, 50, 383–390. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Chia, H.N.; Hull, M. Compressive moduli of the human medial meniscus in the axial and radial directions at equilibrium and at a physiological strain rate. J. Orthop. Res. 2008, 26, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Gu, H.-O.; Kobayashi, M.; Oka, M. Comparison of the bony ingrowth into an osteochondral defect and an artificial osteochondral composite device in load-bearing joints. Knee 1998, 5, 205–213. [Google Scholar] [CrossRef]
- Maher, S.; Doty, S.; Torzilli, P.; Thornton, S.; Lowman, A.; Thomas, J.; Warren, R.; Wright, T.; Myers, E. Nondegradable hydrogels for the treatment of focal cartilage defects. J. Biomed. Mater. Res. Part A 2007, 83, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Li, K.; Chen, Z.; Liao, Y.; Yang, L.; Liu, C.; Ding, W. Repair of avascular meniscal injuries using juvenile meniscal fragments: An in vitro organ culture study. J. Orthop. Res. 2013, 31, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Lippuner, K.; Keel, M.J.; Shintani, N. Novel organ-slice culturing system to simulate meniscal repair: Proof of concept using a synovium-based pool of meniscoprogenitor cells. J. Orthop. Res. 2016, 34, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- O'brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Griffon, D.J.; Sedighi, M.R.; Schaeffer, D.V.; Eurell, J.A.; Johnson, A.L. Chitosan scaffolds: Interconnective pore size and cartilage engineering. Acta Biomater. 2006, 2, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Z.; Jiang, D.; Ding, J.-X.; Wang, S.-J.; Zhang, L.; Zhang, J.-Y.; Qi, Y.-S.; Chen, X.-S.; Yu, J.-K. Role of scaffold mean pore size in meniscus regeneration. Acta Biomater. 2016, 43, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, S.; Ceseracciu, L.; Aiello, M.; Coluccino, L.; Ferrazzo, F.; Giannoni, P.; Quarto, R. A novel scaffold geometry for chondral applications: Theoretical model and in vivo validation. Biotechnol. Bioeng. 2014, 111, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, K.; Zohuriaan-Mehr, M.J. Porous superabsorbent hydrogel composites: Synthesis, morphology and swelling rate. Macromol. Mater. Eng. 2004, 289, 653–661. [Google Scholar] [CrossRef]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng. Part B Rev. 2011, 17, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Anseth, K.S. Hydrogel properties influence ecm production by chondrocytes photoencapsulated in poly (ethylene glycol) hydrogels. J. Biomed. Mater. Res. Part A 2002, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chern, K.Y.; Mow, V.C. Anisotropic viscoelastic shear properties of bovine meniscus. Clin. Orthop. Relat. Res. 1994, 306, 34–45. [Google Scholar]
- Giuliani, F. Mechanical Characterization of Bovine Menisci in Swelling Condition. M. D. Thesis, University of Genoa, Genova GE, Italy, 2017. [Google Scholar]

| Hydrogel Sample | 1 Hz | 10 Hz | ||
|---|---|---|---|---|
| E’ (MPa) | Q−1 | E’ (MPa) | Q−1 | |
| Gelatin/PVA porous | 0.24 ± 0.03 * | 0.16 ± 0.01 | 0.25 ± 0.03 | 0.14 ± 0.00 |
| Gelatin/PVA not porous | 5.40 ± 2.80 | 0.06 ± 0.01 | 5.44 ± 2.90 | 0.05 ± 0.01 |
| PVA porous | 0.78 ± 0.13 | 0.09 ± 0.01 | 0.78 ± 0.13 | 0.08 ± 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrella, A.; Lagazzo, A.; Dellacasa, E.; Pasquini, C.; Finocchio, E.; Barberis, F.; Pastorino, L.; Giannoni, P.; Scaglione, S. 3D Porous Gelatin/PVA Hydrogel as Meniscus Substitute Using Alginate Micro-Particles as Porogens. Polymers 2018, 10, 380. https://doi.org/10.3390/polym10040380
Marrella A, Lagazzo A, Dellacasa E, Pasquini C, Finocchio E, Barberis F, Pastorino L, Giannoni P, Scaglione S. 3D Porous Gelatin/PVA Hydrogel as Meniscus Substitute Using Alginate Micro-Particles as Porogens. Polymers. 2018; 10(4):380. https://doi.org/10.3390/polym10040380
Chicago/Turabian StyleMarrella, Alessandra, Alberto Lagazzo, Elena Dellacasa, Camilla Pasquini, Elisabetta Finocchio, Fabrizio Barberis, Laura Pastorino, Paolo Giannoni, and Silvia Scaglione. 2018. "3D Porous Gelatin/PVA Hydrogel as Meniscus Substitute Using Alginate Micro-Particles as Porogens" Polymers 10, no. 4: 380. https://doi.org/10.3390/polym10040380