Recent Developments in Graphene/Polymer Nanocomposites for Application in Polymer Solar Cells
Abstract
:1. Introduction
2. Graphene and its Derivatives: Synthesis and Properties
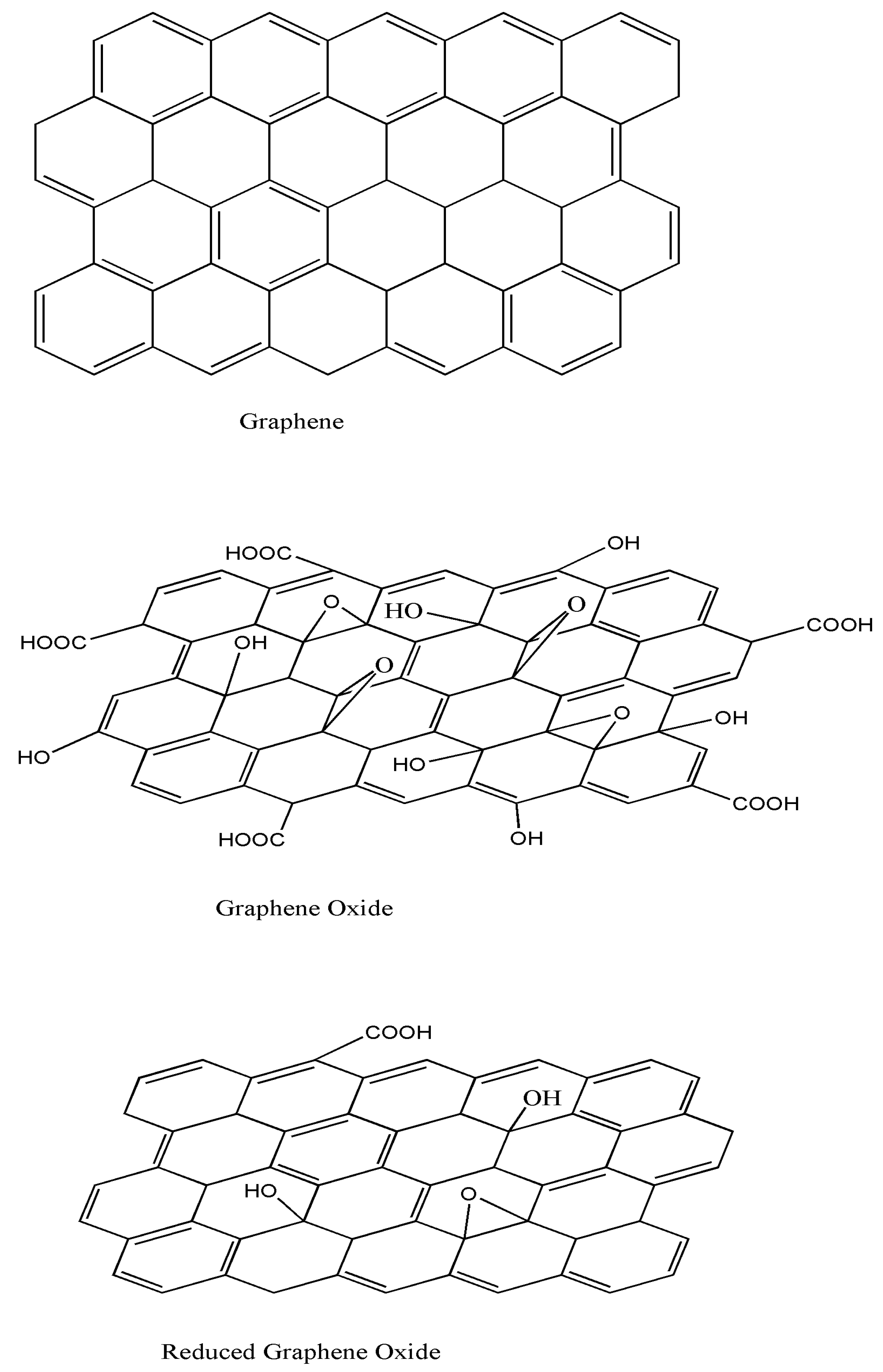
2.1. Synthesis and Properties of Graphene
2.2. Synthesis and Properties of Graphene Oxide
2.3. Synthesis and Properties of Reduced Graphene Oxide
3. Preparation of Graphene/Polymer Nanocomposites
3.1. Non-Covalent Approaches
3.2. Covalent Functionalization
4. Graphene/Polymer Nanocomposites in Solar Cells
4.1. Graphene/Polymer Nanocomposites as Transparent Conductive Electrodes
4.2. Graphene/Polymer Nanocomposites as Active Layers
4.3. Graphene/Polymer Nanocomposites as Interfacial Layers
5. Conclusions and Outlook
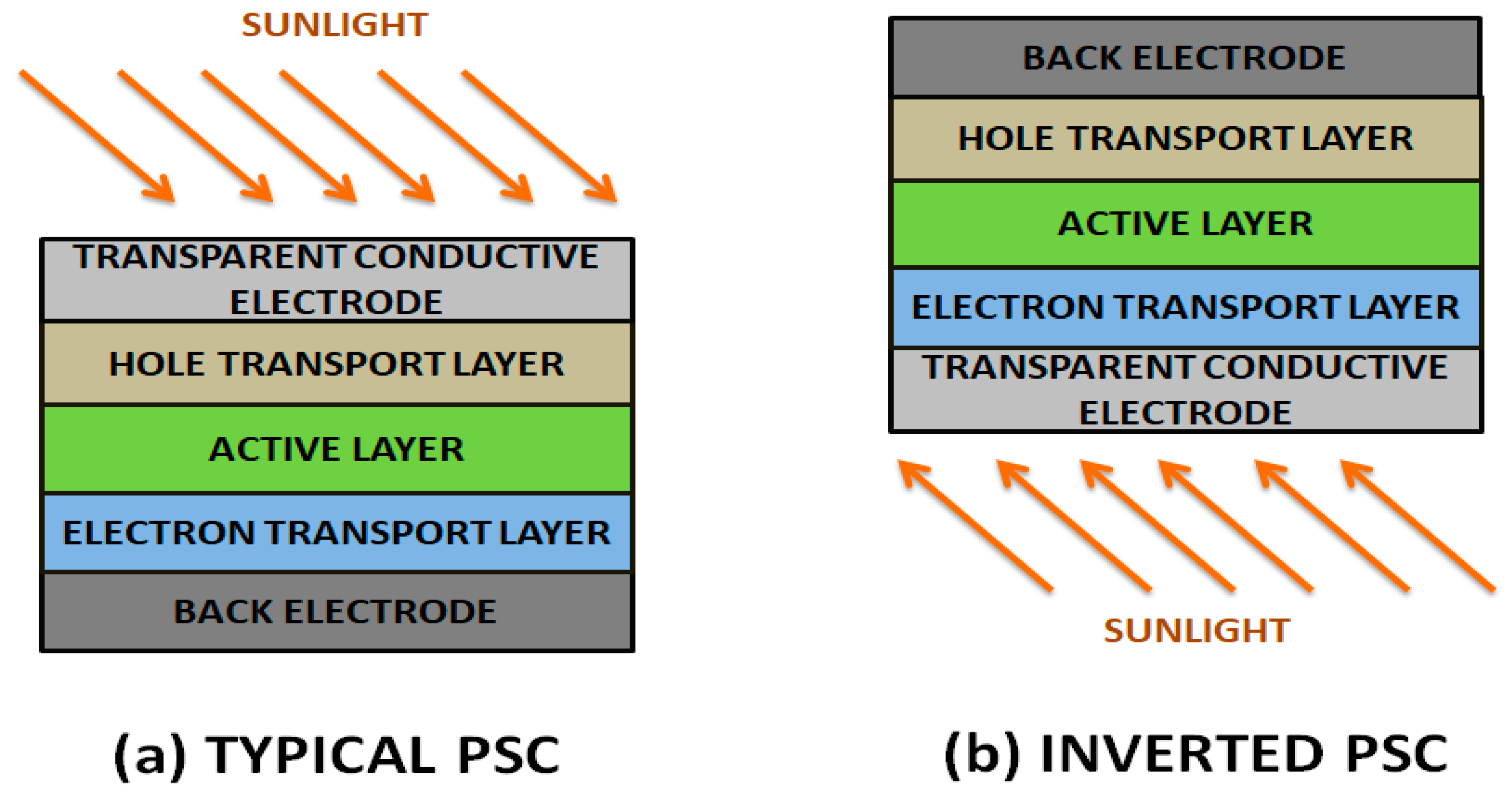
- (a)
- The functionalization and ultrasonication processes applied to G prior to and during the fabrication of polymer nanocomposites leads to a strong reduction in electrical conductivity. Hence, the conductivity of chemically-derived G is considerably lower than that of ideal G. Consequently, G/polymer nanocomposites present poor electrical properties and do not satisfy the requirements for TCEs and counter electrodes in solar cells.
- (b)
- Novel fabrication techniques to synthesize high-quality G thin films with tailored morphology and electronic properties are required, since the performance of G-based PSCs is directly related to the G characteristics: purity, quality, band-gap, structural morphology, etc. In the case of TCEs, solution-processable films with a good balance between high transparency and low sheet resistance are needed. More importantly, techniques that allow the synthesis of G at a large scale and at relatively low cost are highly desirable. Even though a lot of efforts have been made in this direction, the current methods are seriously limited by their low efficiencies, which should be addressed for commercial applications.
- (c)
- The real specific surface area of G-based materials is much lower than the theoretical predictions owing to the strong agglomeration of the G sheets via π–π stacking interactions, and blending with polymers makes the problem even worse. Thus, new approaches to exfoliate the G sheets and keep a large specific surface area when blended with polymers are required. Although significant success has been attained via the addition of stabilizers such as surfactants, these frequently have detrimental effects on final device performance. Hence, novel synthetic routes are sought.
- (d)
- Novel doping or functionalization strategies compatible with the fabrication process of PSCs have to be explored to attain high charge-carrier densities, better stability and tunable energy levels in G-based materials.
- (e)
- The performance of G/polymer nanocomposites in PSCs is well below that reported for traditional materials. Thus, the composition and morphologies of the nanocomposites and the structure of the corresponding PSCs need further optimization. In this regard, developing new types of G-based materials such as GQDs is an interesting alternative. Owing to quantum confinement and the edge effect, the GQDs can exhibit better properties than pristine G, which could allow for the fabrication of PSCs with better PCE.
- (f)
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brabec, C.J.; Sariciftci, N.S.; Hummelen, J.C. Plastic solar cells. Adv. Funct. Mater. 2001, 11, 15–26. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C.-S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.; Norrman, K.; Krebs, F.C. Stability/degradation of polymer solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 686–714. [Google Scholar] [CrossRef]
- Thompson, B.C.; Fréchet, J.M. Polymer-Fullerene Composite Solar Cells. Angew. Chem. Int. Ed. Engl. 2007, 47, 58–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Hou, J.; Zhang, S.; Liang, Y.; Yang, G.; Yang, Y.; Yu, L.; Wu, Y.; Li, G. Polymer solar cells with enhanced open-circuit voltage and efficiency. Nat. Photonics 2009, 3, 649–653. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-fullerene bulk-heterojuntion solar cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Green, M.A. Solar cell fill factors: General graph and empirical expressions. Solid State Electron. 1981, 24, 788–789. [Google Scholar] [CrossRef]
- Luo, J.; Wu, H.; He, C.; Li, A.; Yang, W.; Cao, Y. Enhanced open-circuit voltage in polymer solar cells. Appl. Phys. Lett. 2009, 95, 043301. [Google Scholar] [CrossRef]
- De Kok, M.M.; Buechel, M.; Vulto, S.I.E.; van de Weijer, P.; Meulenkamp, E.A.; de Winter, S.H.P.M.; Mank, A.J.G.; Vorstenbosch, H.J.M.; Weijtens, C.H.L.; van Elsbergen, V. Modification of PEDOT:PSS as Hole Injection Layer in Polymer LEDs. Phys. Status Solidi A Appl. Res. 2004, 201, 1342–1359. [Google Scholar] [CrossRef]
- Yun, J.-M.; Yeo, J.-S.; Kim, J.; Jeong, H.-G.; Kim, D.-Y.; Noh, Y.-J.; Kim, S.-S.; Ku, B.-C.; Na, S.-I. Solution-processable reduced graphene oxide as a novel alternative to PEDOT:PSS hole transport layers for highly efficient and stable polymer solar cells. Adv. Mater. 2011, 23, 4923–4928. [Google Scholar] [CrossRef] [PubMed]
- Taleghani, H.G.; Aleahmad, M.; Eisazadeh, H. Preparation and Characterization of Polyaniline Nanoparticles Using Various Solutions. World Appl. Sci. J. 2009, 6, 1607–1611. [Google Scholar]
- Walker, B.; Kim, C.; Nguyen, T.Q. Small molecule solution-processed bulk heterojunction Solar Cells. Chem. Mater. 2011, 23, 470–482. [Google Scholar] [CrossRef]
- Das, S.; Keum, J.K.; Browning, J.F.; Gu, G.; Yang, B.; Dyck, O.; Chen, W.; Chen, J.; Ivanov, I.N.; Hong, K.; et al. Correlating high power conversion efficiency of PTB7:PC71BM inverted organic solar cells with nanoscale structures. Nanoscale 2015, 14, 15576–15583. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Dou, L.; Yoshimura, K.; Kato, T.; Ohya, K.; Moriarty, T.; Emery, K.; Chen, C.-C.; Gao, J.; Li, G.; Yang, Y. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 2013, 4, 1446. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, S.; Yang, J.; Zhong, Y.; Qian, M.; Li, C.; Zhang, Z.; Xing, G.; Tao, Y.; Li, Y.; Huang, W. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Npj Flex. Electron. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Wan, Q.; Guo, X.; Wang, Z.; Li, W.; Guo, B.; Ma, W.; Zhang, M.; Li, Y. 10.8% efficiency polymer solar cells based on PTB-Th and PC71BM via binary solvent additive treatment. Adv. Funct. Mater. 2016, 26, 6635–6640. [Google Scholar] [CrossRef]
- Chen, J.-D.; Cui, C.; Li, Y.-Q.; Zhou, L.; Ou, Q.-D.; Li, C.; Li, Y.; Tang, J.-X. Single junction polymer solar cells exceeding 10% power conversion efficiency. Adv. Mater. 2014, 27, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-F.; Zhang, Z.-Y.; Yuan, Z.-K.; Li, J.; Xiao, X.-F.; Hong, W.; Chen, X.-D.; Yu, D.-S. Graphene-based materials for polymer solar cells. Chin. Chem. Lett. 2016, 27, 1259–1270. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Chi, H.; Liu, Y.; Hou, C.L.; Fang, D. Recent development of graphene materials applied in polymer solar cell. Renew. Sustain. Energy Rev. 2015, 43, 973–980. [Google Scholar] [CrossRef]
- Wallace, P.R. The band theory of graphite. Phys. Rev. 1947, 71, 622–634. [Google Scholar] [CrossRef]
- Chang, H.; Wu, H. Graphene-Based Nanomaterials: Synthesis, Properties, and Optical and Optoelectronic Applications. Adv. Funct. Mater. 2013, 23, 1984–1997. [Google Scholar] [CrossRef]
- Weiss, N.O.; Zhou, H.; Liao, L.; Liu, Y.; Jiang, S.; Huang, Y.; Duan, X. Graphene: An Emerging Electronic Material. Adv. Mater. 2012, 24, 5782–5825. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M.; Gomez-Fatou, M.A.; Ania, F.; Flores, A. Nanoindentation in polymer nanocomposites. Prog. Mater. Sci. 2015, 67, 1–94. [Google Scholar] [CrossRef]
- Nair, R.R.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Kim, J.K. Synthesis, Structure and Properties of Grpahene and Graphene Oxide. In Graphene for Transparent Conductors. Synthesis, Properties and Applications; Springer: New York, NY, USA, 2015; pp. 29–94. ISBN 978-1493927685. [Google Scholar]
- Wu, J.; Becerril, H.A.; Bao, Z.; Liu, Z.; Chen, Y.; Peumans, P. Organic solar cells with solution processed graphene transparent electrodes. Appl. Phys. Lett. 2008, 92, 263302. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(Propylene Fumarate)/Polyethylene Glycol-Modified Graphene Oxide Biocomposites for Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17902–17914. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-Y.; Lu, A.-Y.; Xu, Y.; Chen, F.-R.; Khlobystov, A.N.; Li, L.-J. High-quality thin graphene films from fast electrochemical exfoliation. ACS Nano 2011, 5, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Chen, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Cote, L.J.; Cruz-Silva, R.; Huang, J. Flash reduction and patterning of graphene oxide and its polymer composite. J. Am. Chem. Soc. 2009, 131, 11027–11032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, L.; Wei, S.; He, Y.; Xia, H.; Chen, Q.; Sun, H.-B.; Xiao, F.-S. Direct imprinting of microcircuits on graphene oxides film by femtosecond laser reduction. Nano Today 2010, 5, 15–20. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimmey, E.J.; Stach, E.A.; Piner, R.D.; Nquyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Thirumalaikumar, M. Methods of enhancement of reactivity and selectivity of sodium borohydride for applications in organic chemistry. J. Organomet. Chem. 2000, 609, 137–151. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Díez-Pascual, A.M.; Lázaro, E.; Vera, S.; Gómez-Fatou, M.A. Chemical sensors based on polymer composites with carbon nanotubes and graphene: The role of the polymer. J. Mater. Chem. A 2014, 2, 14289–14328. [Google Scholar] [CrossRef]
- Wang, H.L.; Hao, Q.L.; Xia, X.F.; Wang, Z.J.; Tian, J.; Zhu, J.H.; Tang, C.; Wang, X. In Situ Fabrication of Nanoscale Graphene Oxide/Polyaniline Composite and its Electrochemical Properties. Adv. Mater. Res. 2010, 148–149, 1547–1550. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Shi, G. Functional composite materials based on chemically converted graphene. Adv. Mater. 2011, 23, 1089–1115. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M. Tissue Engineering Bionanocomposites based on Poly(propylene fumarate). Polymers 2017, 9, 260. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, S.; Salim, T.; Wu, S.; Huang, X.; He, Q.; Lang, Y.M.; Zhan, H. Organic Photovoltaic Devices Using Highly Flexible Reduced Graphene Oxide Films as Transparent Electrodes. ACS Nano 2010, 4, 5263–5268. [Google Scholar] [CrossRef] [PubMed]
- Konios, D.; Petridis, C.; Kakavelakis, G.; Sygletou, M.; Savva, K.; Stratakis, E.; Kymakis, E. Reduced graphene oxide micromesh electrodes for large area, flexible, organic photovoltaic devices. Adv. Funct. Mater. 2015, 15, 2213–2221. [Google Scholar] [CrossRef]
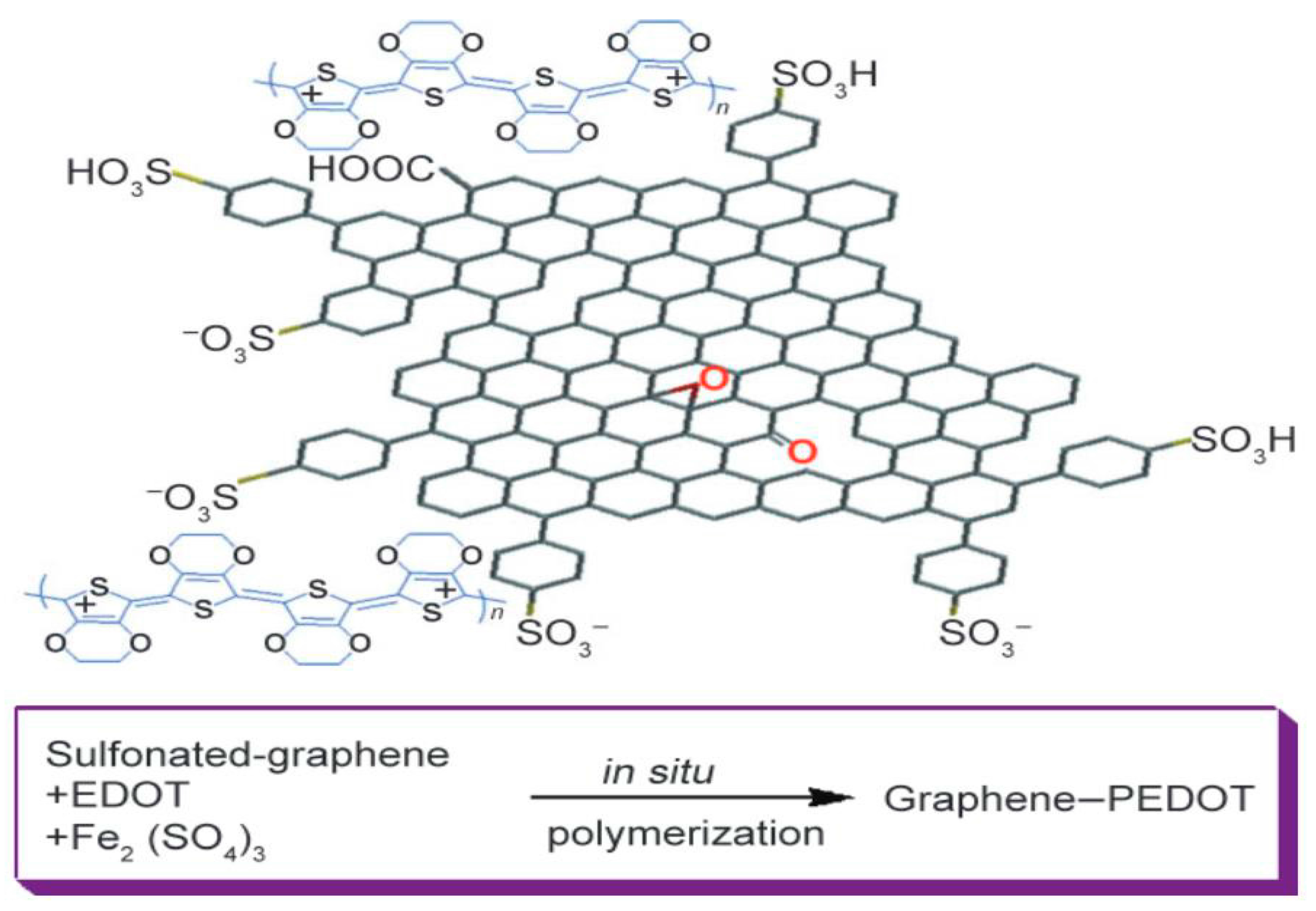
- Xu, Y.; Wang, Y.; Liang, J.; Huang, Y.; Ma, Y.; Wan, X.; Chen, Y. A hybrid material of graphene and poly (3,4-ethyldioxythiophene) with high conductivity, flexibility, and transparency. Nano Res. 2009, 2, 343–348. [Google Scholar] [CrossRef]
- Chang, H.; Wang, G.; Yang, A.; Tao, X.; Liu, X. A Transparent, Flexible, Low-Temperature, and Solution-Processible Graphene Composite Electrode. Adv. Funct. Mater. 2010, 20, 2893–2902. [Google Scholar] [CrossRef]
- Jo, K.; Lee, T.; Choi, H.J.; Park, J.H.; Lee, D.J.; Lee, D.W.; Kim, B.-S. Stable aqueous dispersion of reduced graphene nanosheets via non-covalent functionalization with conducting polymers and application in transparent electrodes. Langmuir 2011, 27, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.F.; Matos, C.F.; Gonçalves, L.C.; Salvatierra, R.V.; Cava, C.E.; Zarbin, A.J.G.; Roman, L.S. Water based, solution-processable, transparent and flexible graphene oxide composite as electrodes in organic solar cell application. J. Phys. D Appl. Phys. 2016, 49, 105106. [Google Scholar] [CrossRef]
- Carrasco-Valenzuela, L.; Zaragoza-Contreras, E.A.; Vega-Rios, A. Synthesis of graphene oxide/poly(3,4-ethylenedioxythiophene) composites by Fenton’s reagent. Polymer 2017, 130, 124–134. [Google Scholar] [CrossRef]
- La Notte, L.; Bianco, G.V.; Palma, A.L.; Carlo, A.D.; Bruno, G.; Reale, A. Sprayed organic photovoltaic cells and mini-modules based on chemical vapor deposited graphene as transparent conductive electrode. Carbon 2018, 129, 878–883. [Google Scholar] [CrossRef]
- Soltani-kordshuli, F.; Zabihi, F.; Eslamian, M. Graphene-doped PEDOT:PSS nanocomposite thin films fabricated by conventional and substrate vibration-assisted spray coating (SVASC). JESTECH 2016, 19, 1216–1223. [Google Scholar] [CrossRef]
- Chen, Q.; Zabihi, F.; Eslamian, M. Improved functionality of PEDOT:PSS thin films via graphene doping, fabricated by ultrasonic substrate vibration-assisted spray coating. Synth Met. 2016, 222, 309–317. [Google Scholar] [CrossRef]
- Sookhakian, M.; Amin, Y.M.; Baradaran, S.; Tajabadi, M.T.; Golsheikh, A.M.; Basirun, W.J. A layer-by-layer assembled graphene/zinc sulfide/polypyrrole thin-film electrode via electrophoretic deposition for solar cells. Thin Solid Films 2014, 552, 204–211. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Sun, Z.-H.; Tai, G.; Lau, S.-P.; Yan, F. The Application of Highly Doped Single-Layer Graphene as the Top Electrodes of Semitransparent Organic Solar Cells. ACS Nano 2012, 6, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Yan, F. Package-Free Flexible Organic Solar Cells with Graphene top Electrodes. Adv. Mater. 2013, 25, 4296–4301. [Google Scholar] [CrossRef] [PubMed]
- Ricciardulli, A.G.; Yang, S.; Feng, X.; Blom, P.W.M. Solution-processable high-quality graphene for organic solar cells. ACS Appl. Mater. Interfaces 2017, 9, 25412–25417. [Google Scholar] [CrossRef] [PubMed]
- An, C.J.; Kim, S.J.; Choi, H.O.; Kim, D.W.; Jang, S.W.; Jin, M.L.; Park, J.-M.; Choi, J.K.; Jung, H.-T. Ultraclean transfer of CVD-grown graphene and its application to flexible organic photovoltaic cells. J. Mater. Chem. A 2014, 2, 20474–20480. [Google Scholar] [CrossRef]
- Park, H.; Chang, S.; Smith, M.; Gradečak, S.; Kong, J. Interface engineering of graphene for universal applications as both cathode and anode in organic photovoltaics. Sci. Rep. 2013, 3, 1581. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Chang, S.; Zhou, X.; Kong, J.; Palacios, T.; Gradečak, S. Flexible graphene electrode-based organic photovoltaics with record-high efficiency. Nano Lett. 2014, 14, 5148–5154. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chang, S.; Gradečak, S.; Kong, J. Visibly transparent organic solar cells on flexible substrates with all-graphene electrodes. Adv. Energy Mater. 2016, 6, 1600847. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Huang, Y.; Ma, Y.; Yin, S.; Zhang, X.; Sun, W.; Chen, Y. Organic Photovoltaic Devices Based on a Novel Acceptor Material: Graphene. Adv. Mater. 2008, 20, 3924–3930. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Zhang, X.; Yang, L.; Zhang, N.; Pan, G.; Yin, S.; Chen, Y.; Wei, J. Polymer Photovoltaic Cells Based on Solution-Processable Graphene and P3HT. Adv. Funct. Mater. 2009, 19, 894–904. [Google Scholar] [CrossRef]
- Wang, H.; He, D.; Wang, Y.; Liu, Z.; Wu, H.; Wang, J. Organic Photovoltaic Devices Based on graphene as an electron-acceptor material and P3OT as a donor material. Phys. Status Solidi A 2011, 208, 2339–2343. [Google Scholar] [CrossRef]
- Liu, Z.; He, D.; Wang, Y.; Wu, H.; Wang, J.; Wang, H. Improving photovoltaic properties by incorporating both SPFGraphene and functionalized multiwalled carbon nanotubes. Sol. Energy Mater. Sol. Cells 2010, 94, 2148–2153. [Google Scholar] [CrossRef]
- Yu, D.; Yang, Y.; Durstock, M.; Baek, J.-B.; Dai, L. Soluble P3HT-Grafted Graphene for Efficient Bilayer–Heterojunction Photovoltaic Devices. ACS Nano 2010, 4, 5633–5640. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Park, K.; Durstock, M.; Dai, L. Fullerene-Grafted Graphene for Efficient Bulk Heterojunction Polymer Photovoltaic Devices. J. Phys. Chem. Lett. 2011, 2, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Zhao, Y.; Shi, G.; Deng, L.; Hou, Y.; Qu, L. An Electrochemical Avenue to Green-Luminescent Graphene Quantum Dots as Potential Electron-Acceptors for Photovoltaics. Adv. Mater. 2011, 8, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Chaudhary, N.; Srivastava, R.; Sharma, G.D.; Bhardwaj, R.; Chand, S. Luminescent Graphene Quantum Dots for Organic Photovoltaic Devices. J. Am. Chem. Soc. 2011, 133, 9960–9963. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Kou, L.; Chen, W.; Wu, C.; Guo, T. Enhancing the short-circuit current and power conversion efficiency of polymer solar cells with graphene quantum dots derived from double-walled carbon nanotubes. NPG Asia Mater. 2013, 5, e60. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, M.J.; Kim, S.J.; Wang, D.H.; Cho, S.P.; Bae, S.; Park, J.H.; Hong, B.H. Balancing Light Absorptivity and Carrier Conductivity of Graphene Quantum Dots for High-Efficiency Bulk Heterojunction Solar Cells. ACS Nano 2013, 7, 7207–7212. [Google Scholar] [CrossRef] [PubMed]
- Jun, G.H.; Jin, S.H.; Lee, B.; Kim, B.H.; Chae, W.-S.; Hong, S.H.; Jeon, S. Enhanced conduction and charge-selectivity by N-doped graphene flakes in the active layer of bulk-heterojunction organic solar cells. Energy Environ. Sci. 2013, 6, 3000–3006. [Google Scholar] [CrossRef]
- Lee, R.-H.; Huang, J.-L.; Chi, C.-H. Conjugated polymer-functionalized graphite oxide sheets thin films for enhanced photovoltaic properties of polymer solar cells. J. Polym. Sci. B Polym. Phys. 2013, 51, 137–148. [Google Scholar] [CrossRef]
- Rafique, S.; Abdullah, S.M.; Shahid, M.M.; Ansari, M.O.; Sulaiman, K. Significantly improved photovoltaic performance in polymer bulk heterojuntion solar cells with graphene oxide/PEDOT:PSS double decked hole transport layer. Sci. Rep. 2016, 7, 39555. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.-K.; Chen, F.-C. Synergistic Plasmonic Effects of Metal Nanoparticle–Decorated PEGylated Graphene Oxides in Polymer Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 7397–7405. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.-H.; Noh, Y.-J.; Bae, J.-H.; Yu, J.-H.; Hwang, I.-T.; Shin, J.; Shin, K.; Lee, J.-S.; Choi, J.-H.; Na, S.-I. Polyacrylonitrile-grafted reduced graphene oxide hybrid: An all-round and efficient hole-extraction material for organic and inorganic-organic hybrid photovoltaics. Nano Energy 2017, 31, 19–27. [Google Scholar] [CrossRef]
- Hu, A.; Wang, Q.; Chen, L.; Hu, X.; Zhang, Y.; Wu, Y.; Chen, Y. In Situ Formation of ZnO in Graphene: A Facile Way To Produce a Smooth and Highly Conductive Electron Transport Layer for Polymer Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 16078–16085. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Tu, K.-H.; Lin, C.-C.; Chen, C.-W.; Chhowalla, M. Solution-processable graphene oxide as an efficient hole transport layer in polymer solar cells. ACS Nano 2010, 4, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Tai, N.H.; Chueh, S.Y.; Chen, Y.J.; Kuo, W.S.; Chou, T.W.; Hsu, C.S.; Chen, L.J. Synthesis of ethanol-soluble few-layer graphene nanosheets for flexible and transparent conducting composite films. Nanotechnology 2011, 22, 295606. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, Q. Health and Ecosystem Risks of Graphene. Chem. Rev. 2013, 113, 3815–3835. [Google Scholar] [CrossRef] [PubMed]
- Chng, E.L.K.; Pumera, M. The toxicity of graphene oxides: Dependence on the oxidative methods used. Chem.–Eur. J. 2013, 19, 8227–8235. [Google Scholar] [CrossRef] [PubMed]
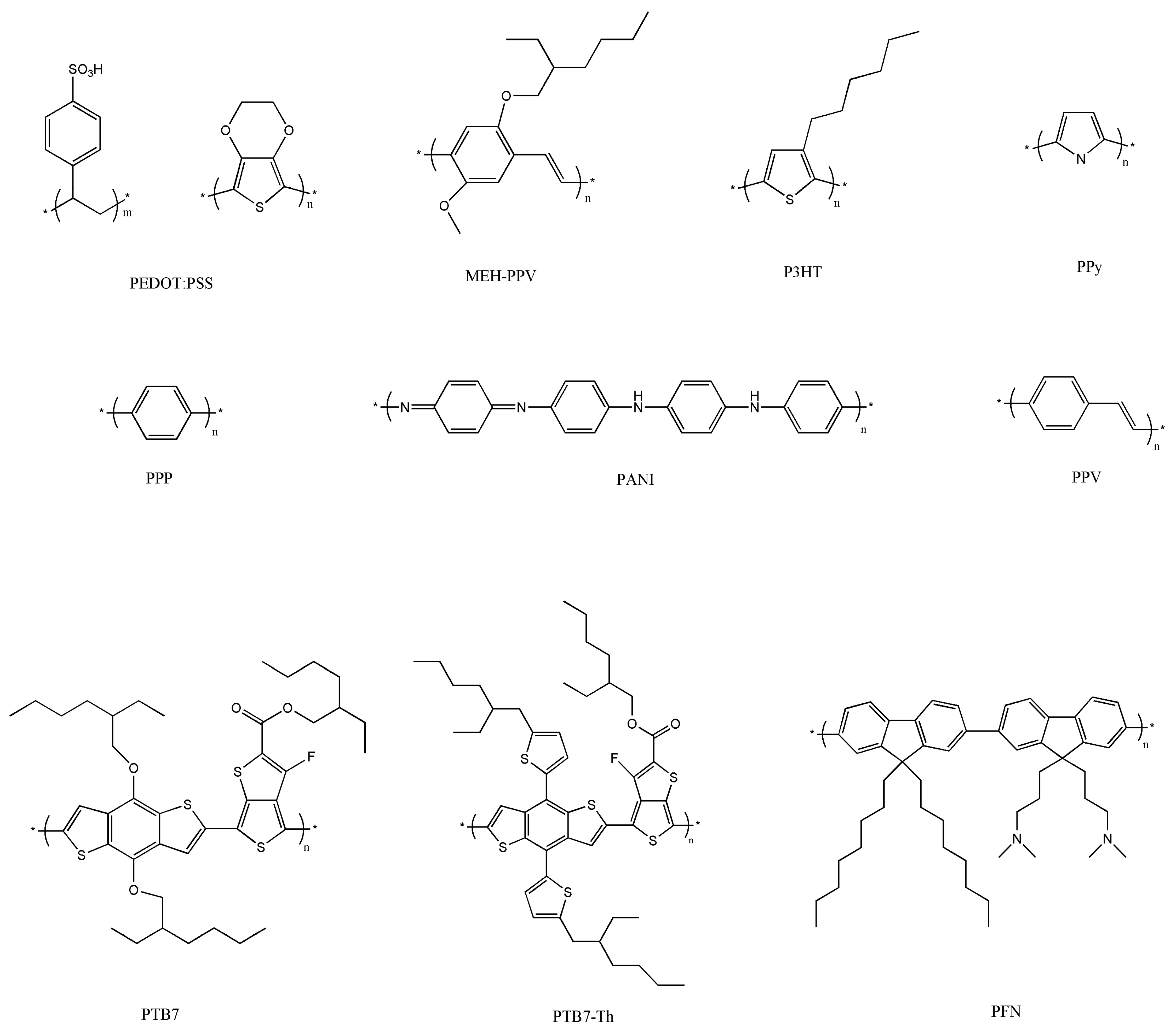

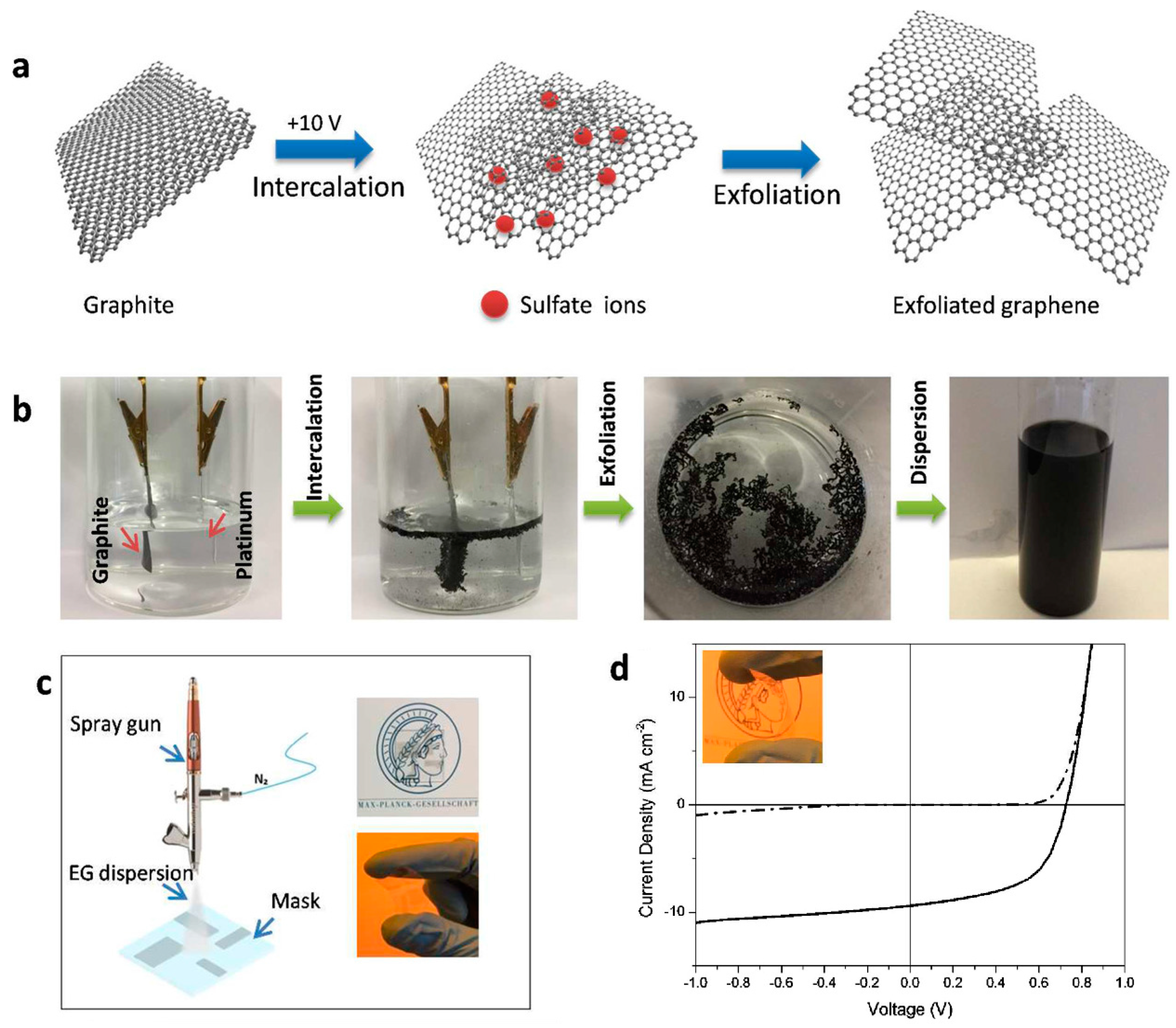



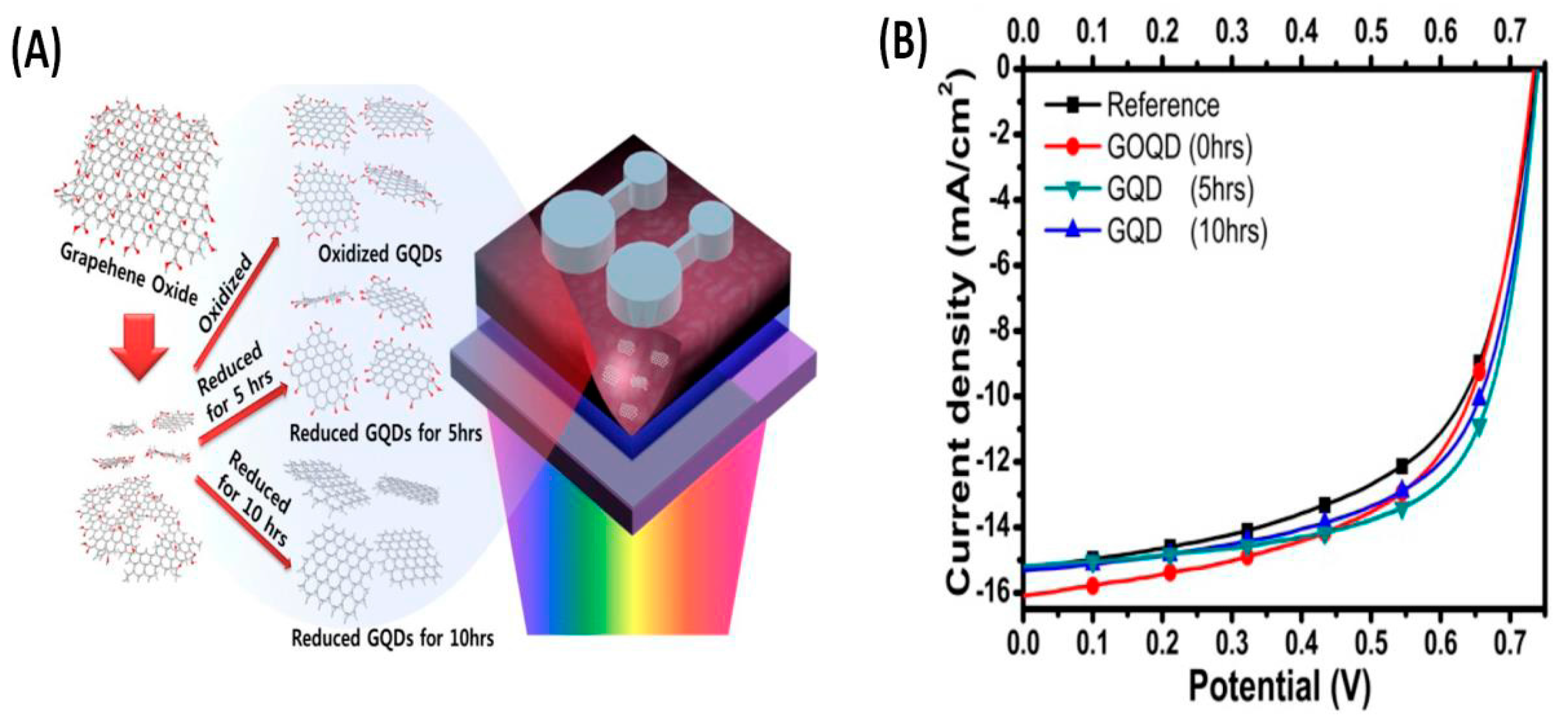

| Composite | Device Structure | Jsc (mA·cm−2) | Voc (V) | FF | PCE (%) | REF |
|---|---|---|---|---|---|---|
| PET/rGO | PET/rGO/PEDOT:PSS/P3HT:PCBM/TiO2/Al | 4.39 | 0.56 | NA | 0.78 | 42 |
| GO:PEDOT:PSS | PET/GO:PEDOT:PSS/F8T2/C60/Al | 2.75 | 0.71 | 0.52 | 1.10 | 47 |
| Au/G/PEDOT:PSS | ITO/ZnO/P3HT:PCBM/PEDOT: PSS/Au/G | 10.58 | 0.59 | 0.43 | 2.7 | 53 |
| G/Au/PEDOT:PSS | G/Au/PEDOT:PSS/P3HT:PCBM/ZnO/Ag/PI | 10.6 | 0.597 | 0.50 | 3.17 | 54 |
| EG/PEDOT:PSS | EG/PEDOT:PSS/PTB7:PC71BM/Ba /Al | 6.53 | 0.707 | 0.56 | 4.23 | 55 |
| PMMA-G | PET/PMMA-G/MoO3/PEDOT:PSS /PCDTBT:PC70BM/Ca/Al | 8.88 | 0.83 | 0.45 | 3.3 | 56 |
| PEN/G | PEN/G/ZnO/PTB7:PC71BM/MoO3/Ag | 14.9 | 0.71 | 0.58 | 6.2 | 58 |
| P3OT:G | ITO/ PEDOT:PSS/P3OT:G/LiF/Al | 4.2 | 0.92 | 0.37 | 1.4 | 60 |
| P3HT:G | ITO/ PEDOT:PSS/P3HT:G/LiF/Al | 4.0 | 0.72 | 0.38 | 1.1 | 61 |
| P3OT:PCBM–G | ITO/PEDOT:PSS/P3OT:PCBM–G/LiF/Al | 4.6 | 0.67 | 0.37 | 1.14 | 62 |
| P3HT-f-MWCNTs-SPFG | ITO/PEDOT:PSS/P3HT-f-MWCNTs-SPFG/LiF/Al | 4.7 | 0.67 | 0.32 | 1.05 | 63 |
| P3HT-grafted GO | ITO/PEDOT:PSS/G-P3HT:C60/Al | 3.5 | 0.43 | 0.41 | 0.61 | 64 |
| C60-rGO:P3HT | ITO/PEDOT:PSS/C60-rGO:P3HT/Al | 4.45 | 0.56 | 0.49 | 1.22 | 65 |
| P3HT:GQDs | ITO/PEDOT:PSS/P3HT:GQDs/Al | 6.33 | 0.67 | 0.30 | 1.28 | 66 |
| P3HT/ANI-GQDs | ITO/PEDOT:PSS/P3HT/ANI-GQDs/Al | 3.51 | 0.61 | 0.53 | 1.14 | 67 |
| P3HT:PCBM:GQDs | ITO/PEDOT:PSS/P3HT:PCBM:GQDs/LiF/Al | 26.46 | 0.60 | 0.33 | 5.24 | 68 |
| GOQDs:PTB7: PC71BM | ITO/PEDOT:PSS/GOQDs:PTB7: PC71BM/Al | 15.2 | 0.74 | 0.68 | 7.6 | 69 |
| N-dopedG:P3HT: PCBM | ITO/PEDOT:PSS/N-doped G:P3HT:PCBM/Al | 14.95 | 0.59 | 0.51 | 4.48 | 70 |
| PTM21-GO | ITO/PEDOT:PSS/P3HT:PTM21-GO: PCBM/LiF/Al | 8.11 | 0.59 | 0.30 | 1.43 | 71 |
| GO/PEDOT:PSS | ITO/GO/PEDOT:PSS/PCDTBT: PC71BM/Al | 10.44 | 0.82 | 0.50 | 4.28 | 72 |
| Au@PEG–GO | ITO/PEDOT:PSS(Au@PEG–GO) /PBDTTT-CT | 16.1 | 0.75 | 0.60 | 7.26 | 73 |
| PRGO-PTB7-th | NA | 14.78 | 0.76 | 0.64 | 7.24 | 74 |
| ZnO@G:EC | ITO/ZnO@G:EC/P3HT:PC61BM/MoO3/Ag | 7.73 | 0.63 | 0.73 | 3.9 | 75 |
| GO/P3HT:PCBM | ITO/GO/P3HT:PCBM/Al | 11.4 | 0.57 | 0.54 | 3.5 | 76 |
| G:PEDOT:PSS | ITO/FLGs-PEDOT:PSS/PCBM/P3HT/Ca/Al | 9.44 | 0.58 | 0.55 | 3.7 | 77 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez-Pascual, A.M.; Luceño Sánchez, J.A.; Peña Capilla, R.; García Díaz, P. Recent Developments in Graphene/Polymer Nanocomposites for Application in Polymer Solar Cells. Polymers 2018, 10, 217. https://doi.org/10.3390/polym10020217
Díez-Pascual AM, Luceño Sánchez JA, Peña Capilla R, García Díaz P. Recent Developments in Graphene/Polymer Nanocomposites for Application in Polymer Solar Cells. Polymers. 2018; 10(2):217. https://doi.org/10.3390/polym10020217
Chicago/Turabian StyleDíez-Pascual, Ana Maria, José Antonio Luceño Sánchez, Rafael Peña Capilla, and Pilar García Díaz. 2018. "Recent Developments in Graphene/Polymer Nanocomposites for Application in Polymer Solar Cells" Polymers 10, no. 2: 217. https://doi.org/10.3390/polym10020217





