Recent Achievements of Self-Healing Graphene/Polymer Composites
Abstract
:1. Introduction
2. Fabrication Methods
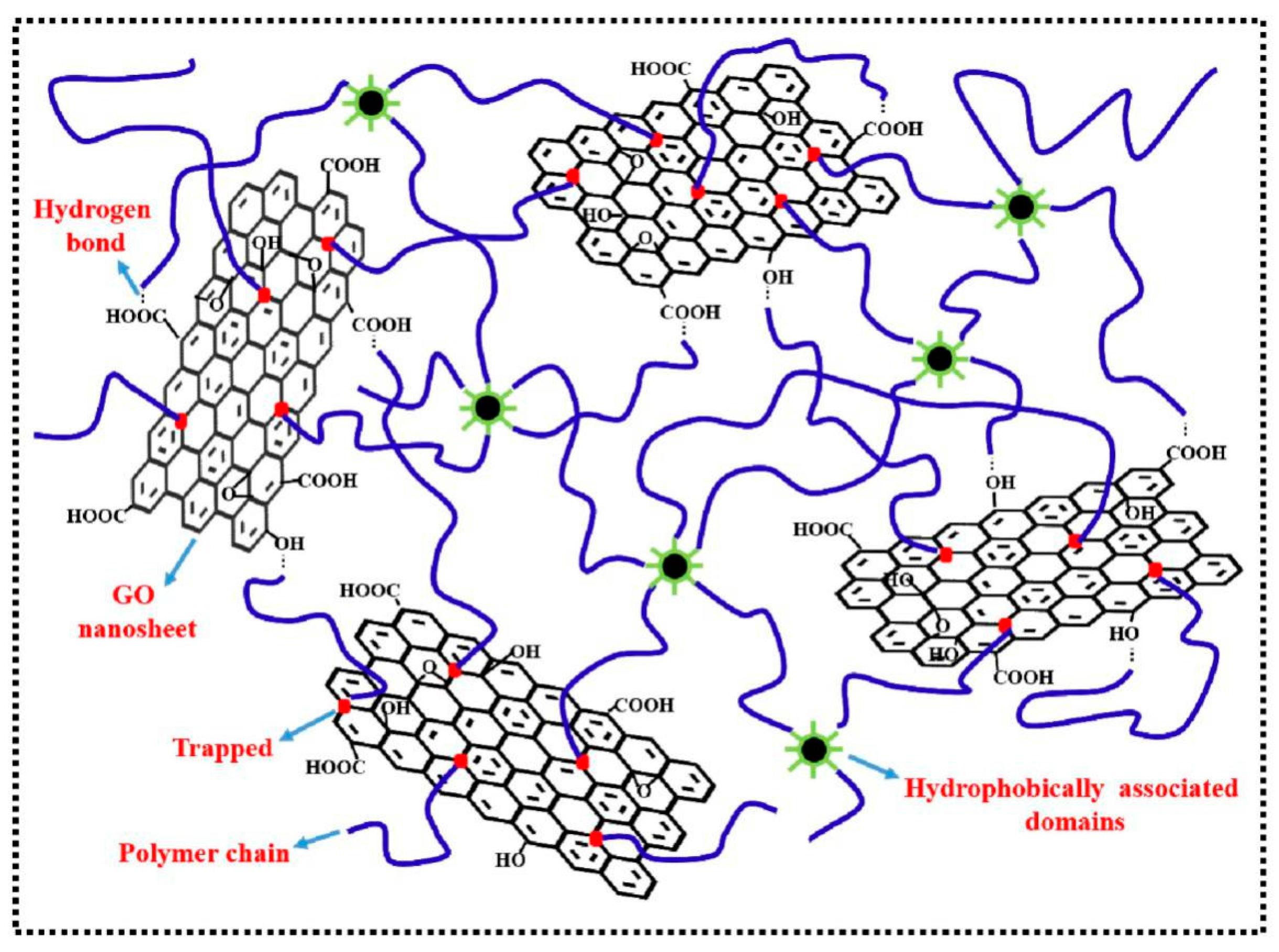
2.1. Simple Mixing
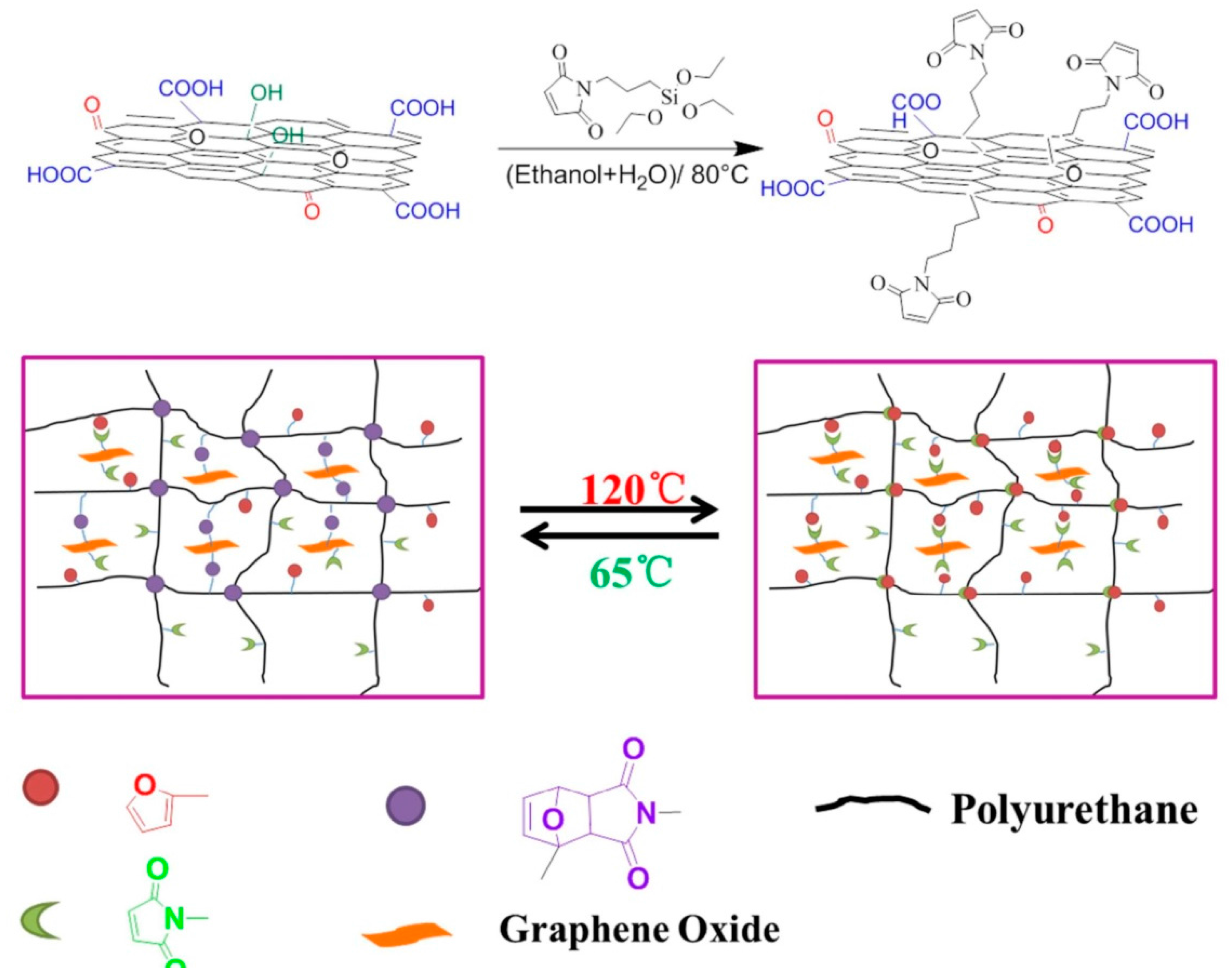
2.2. In Situ Polymerization
2.3. Diels-Alder (DA) Reactions
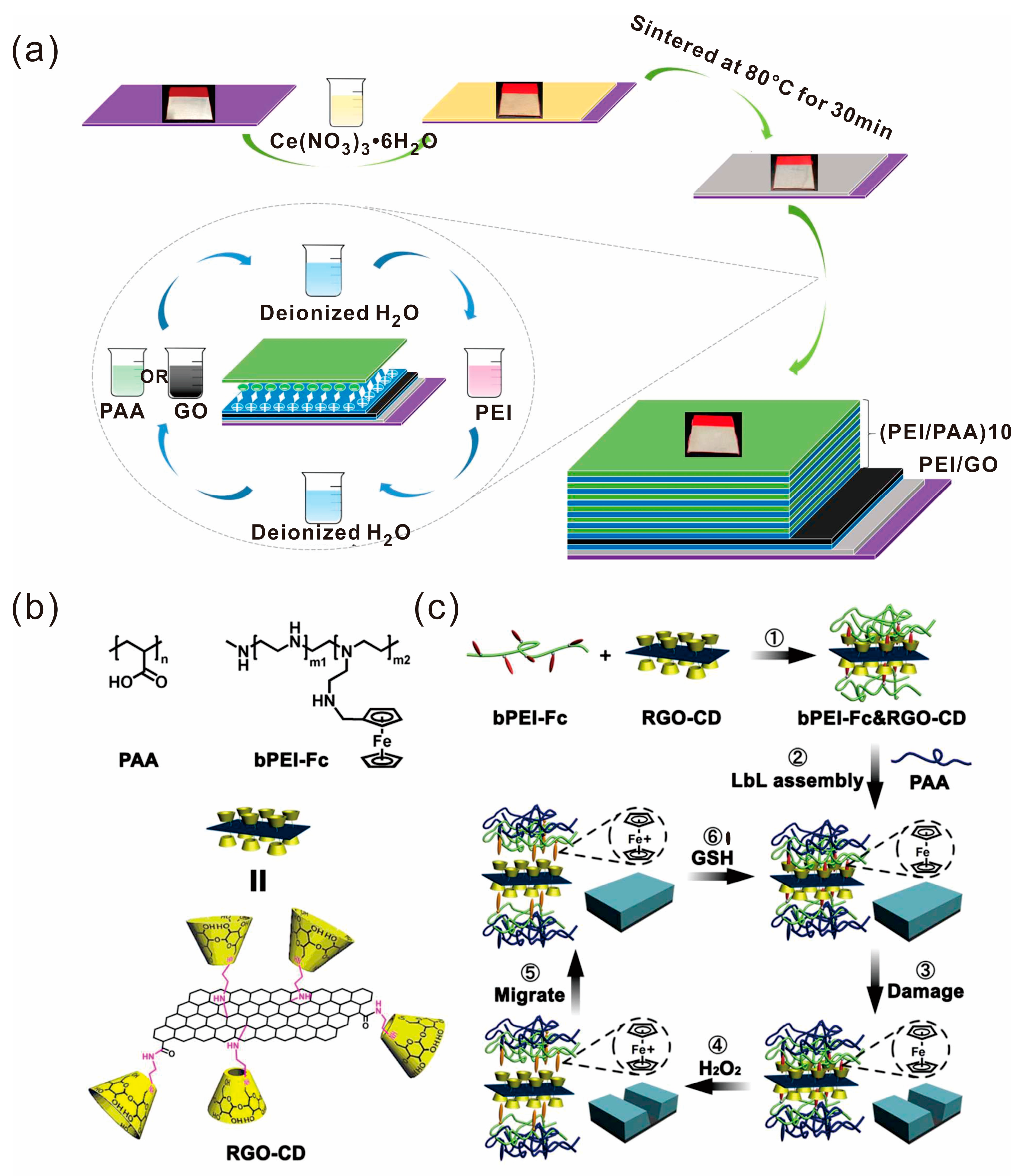
2.4. Layer-by-Layer Self-Assembly Technique
2.5. Hydrothermal Methods
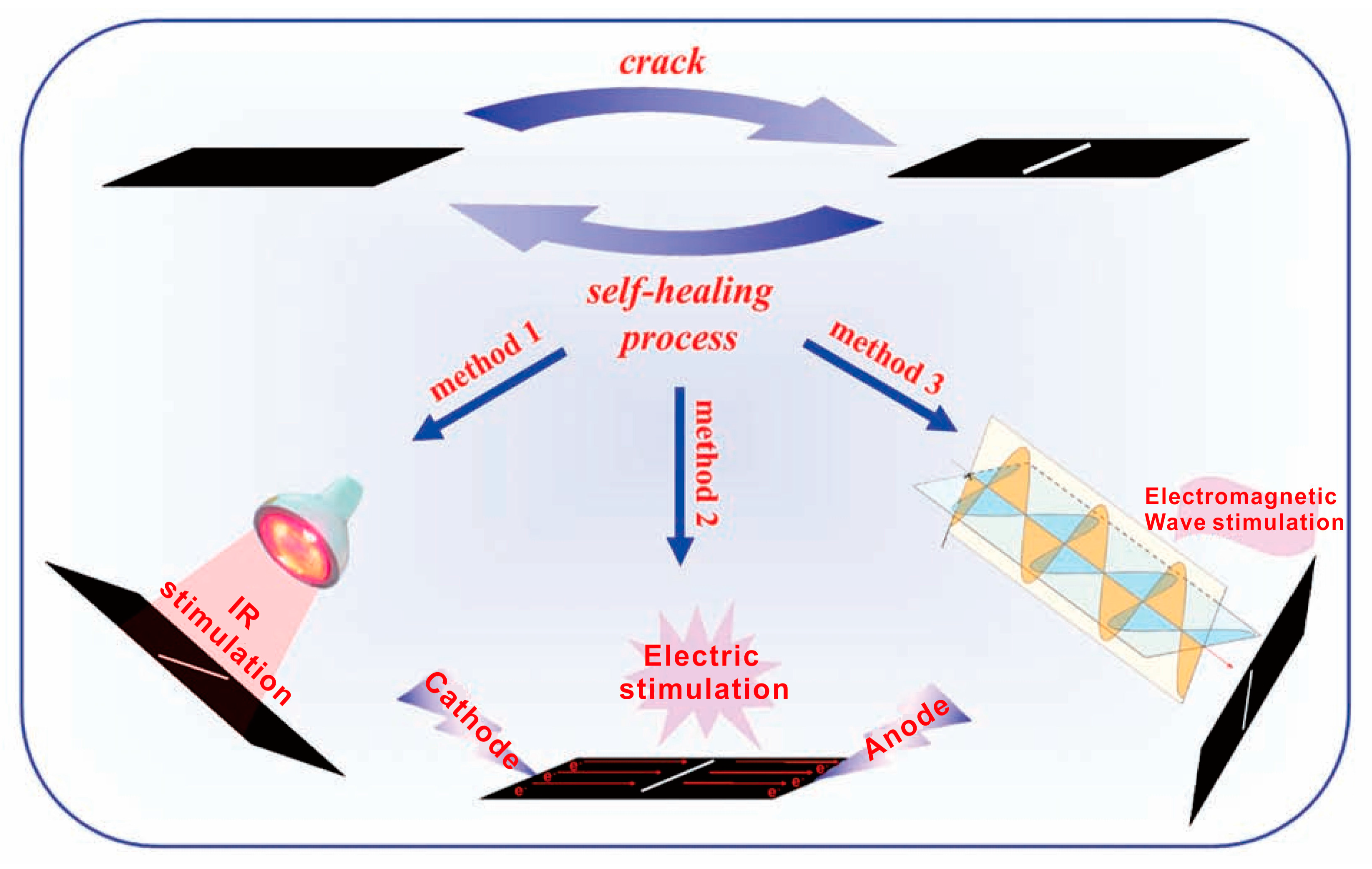
3. Self-Healing Condition
3.1. Heating
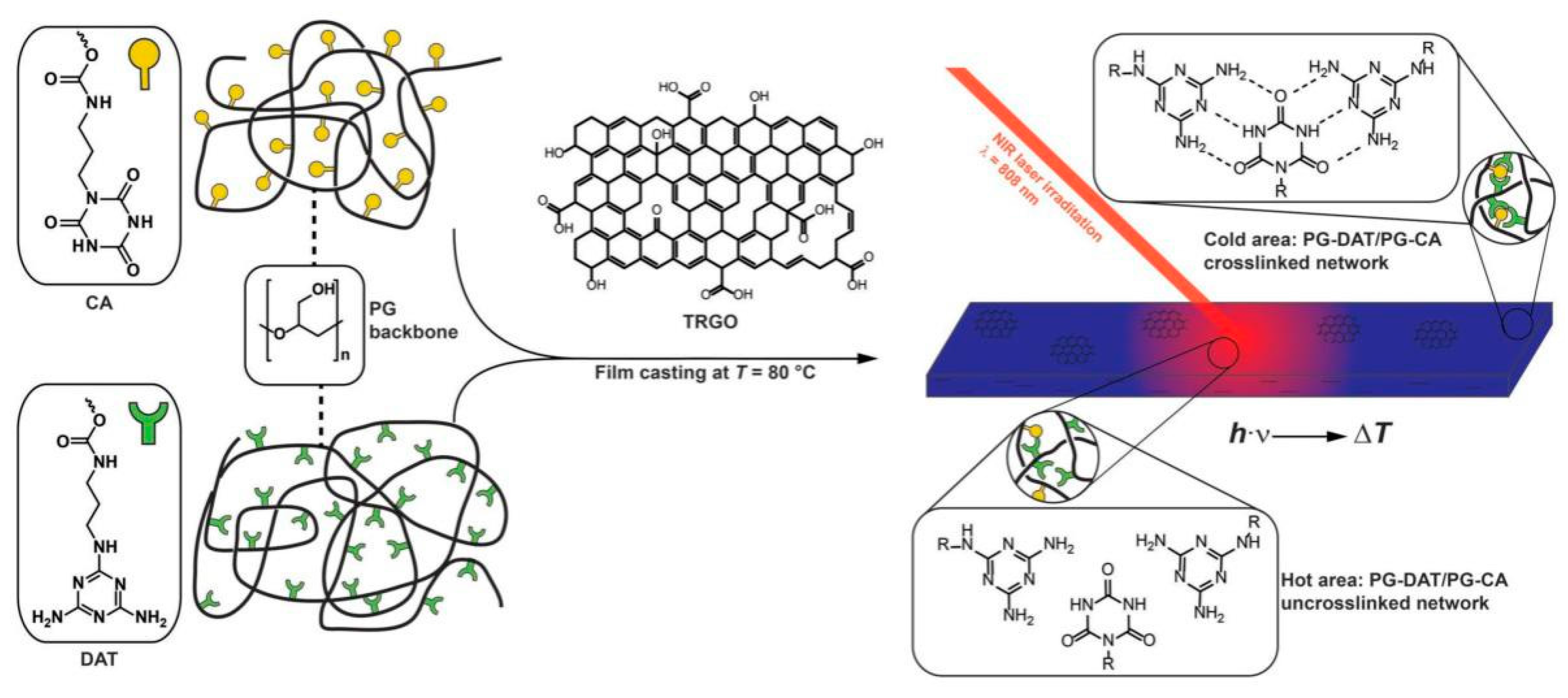
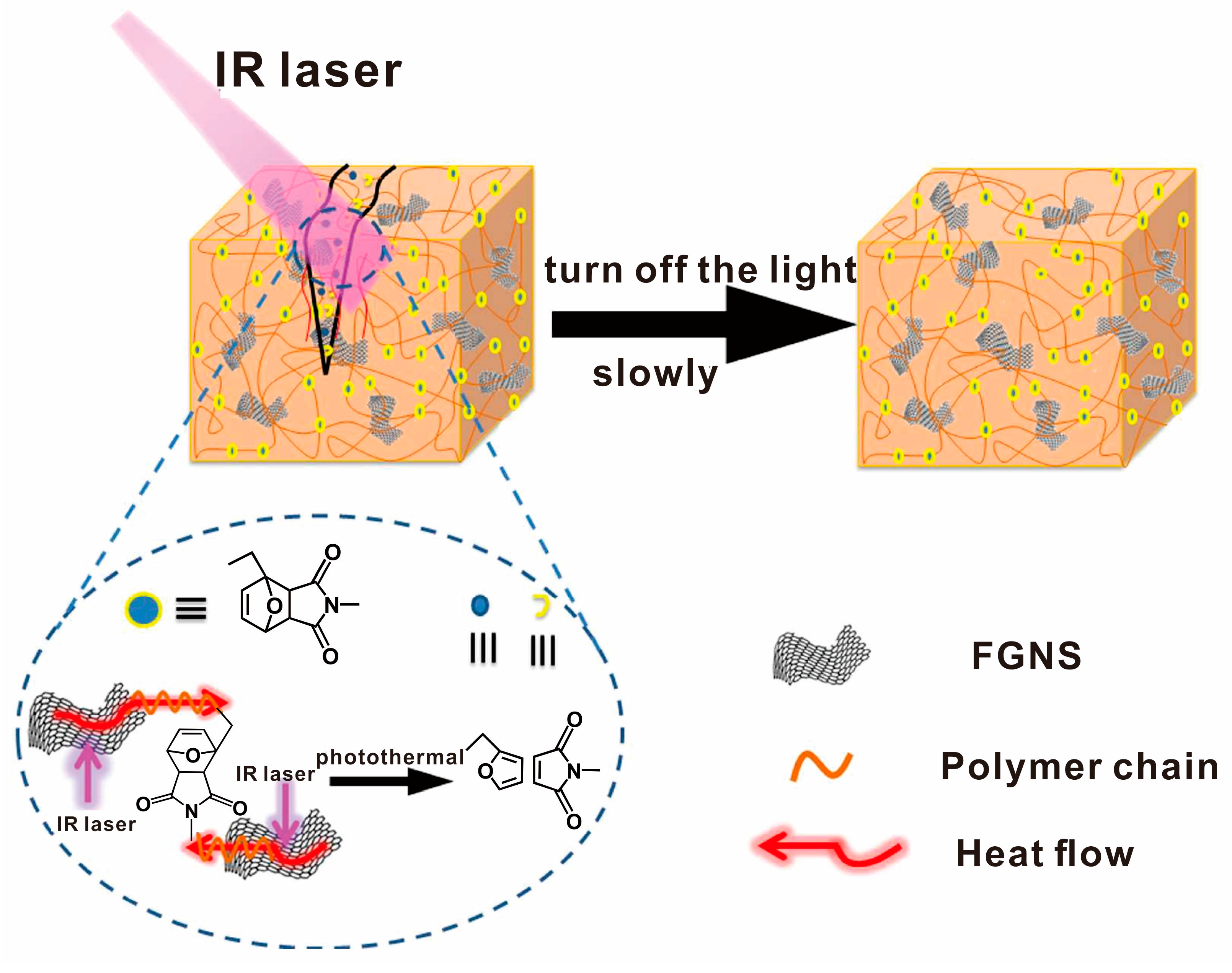
3.2. Light Radiation
3.3. Microwave
3.4. Solvent-Assistant Self-Healing
3.5. Simple Contacting without Any External Stimuli
3.6. Microcapsules and Others Healing Methods
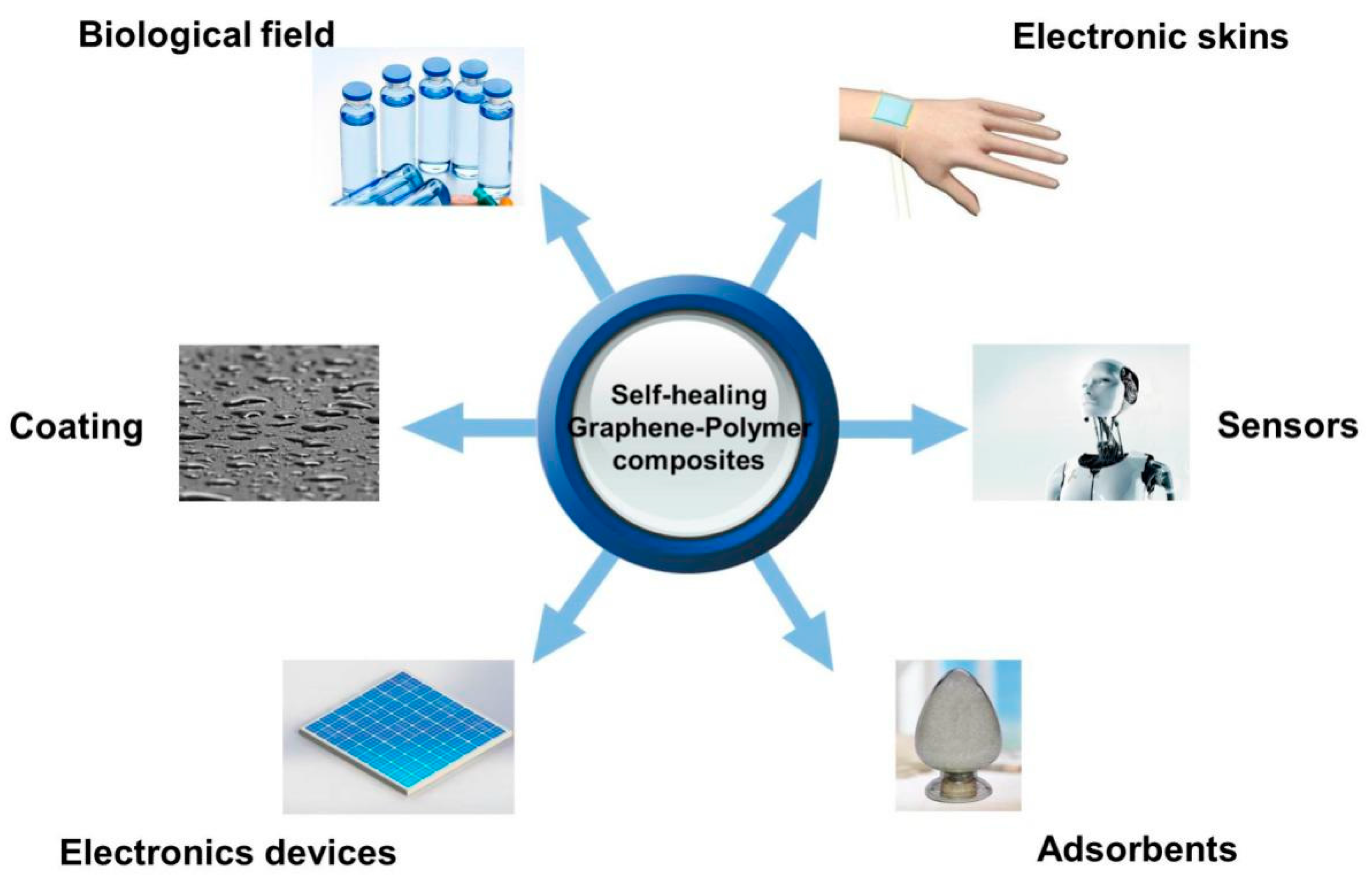
4. Properties and Applications
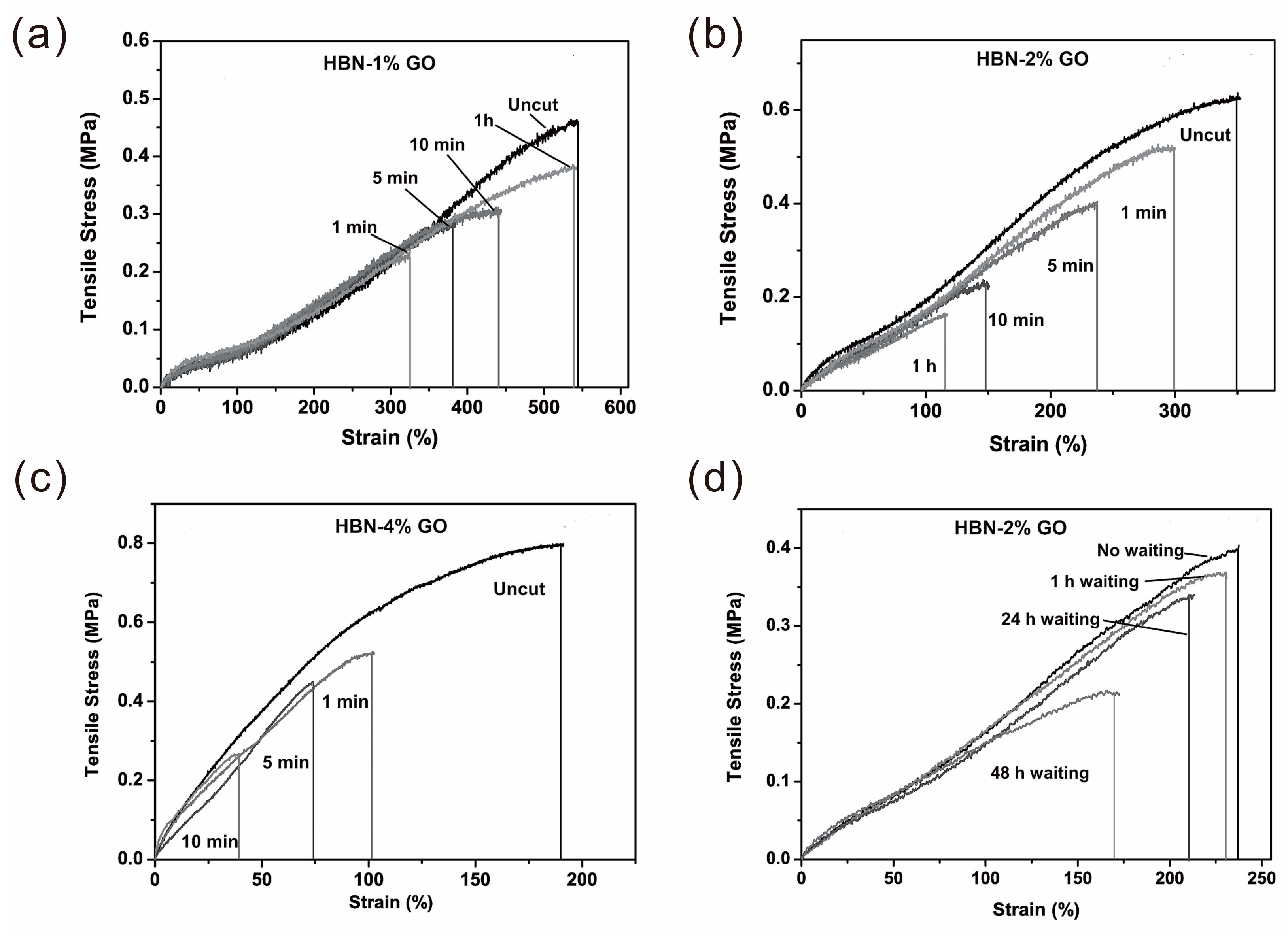
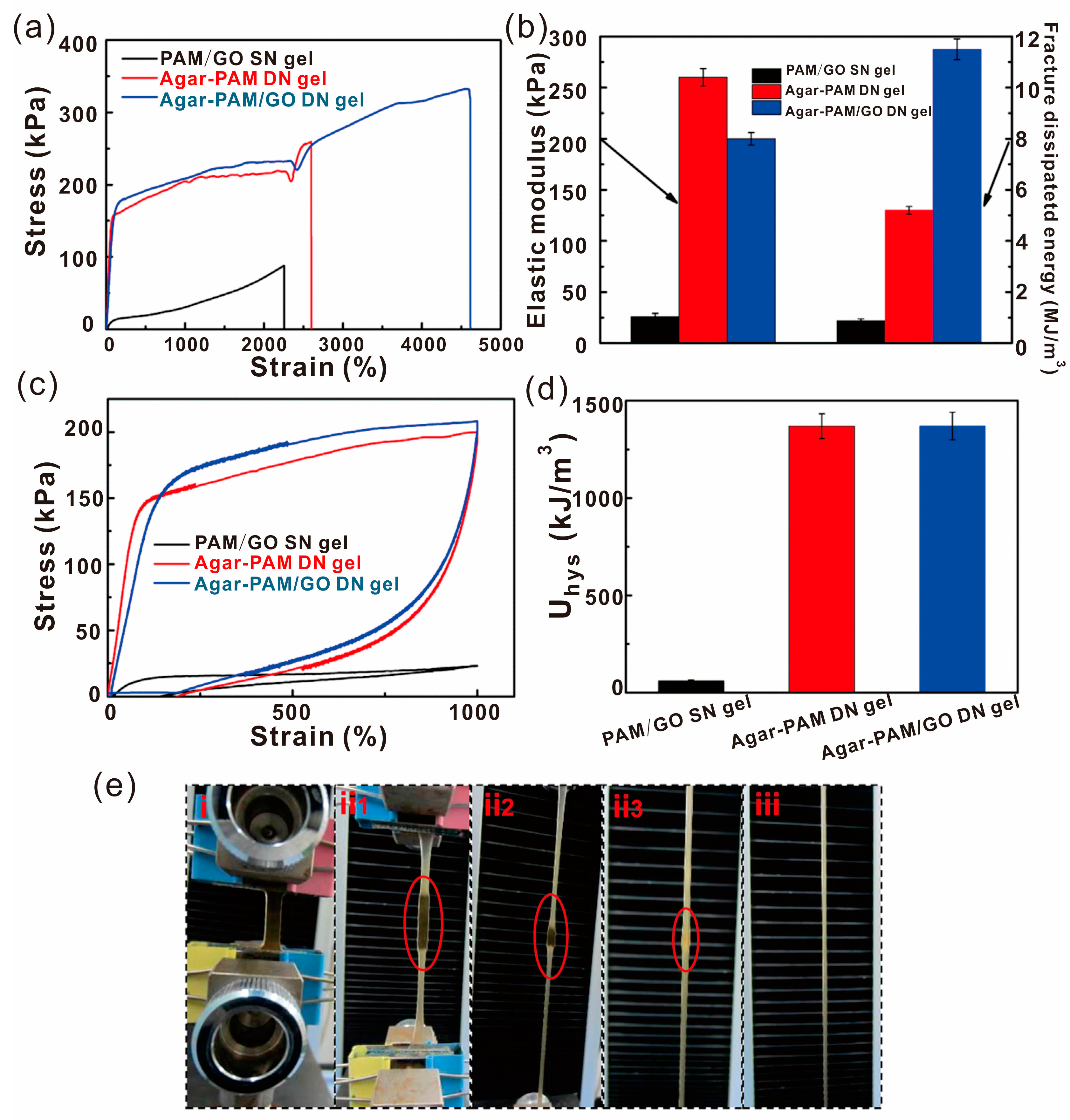
4.1. Mechanical Property
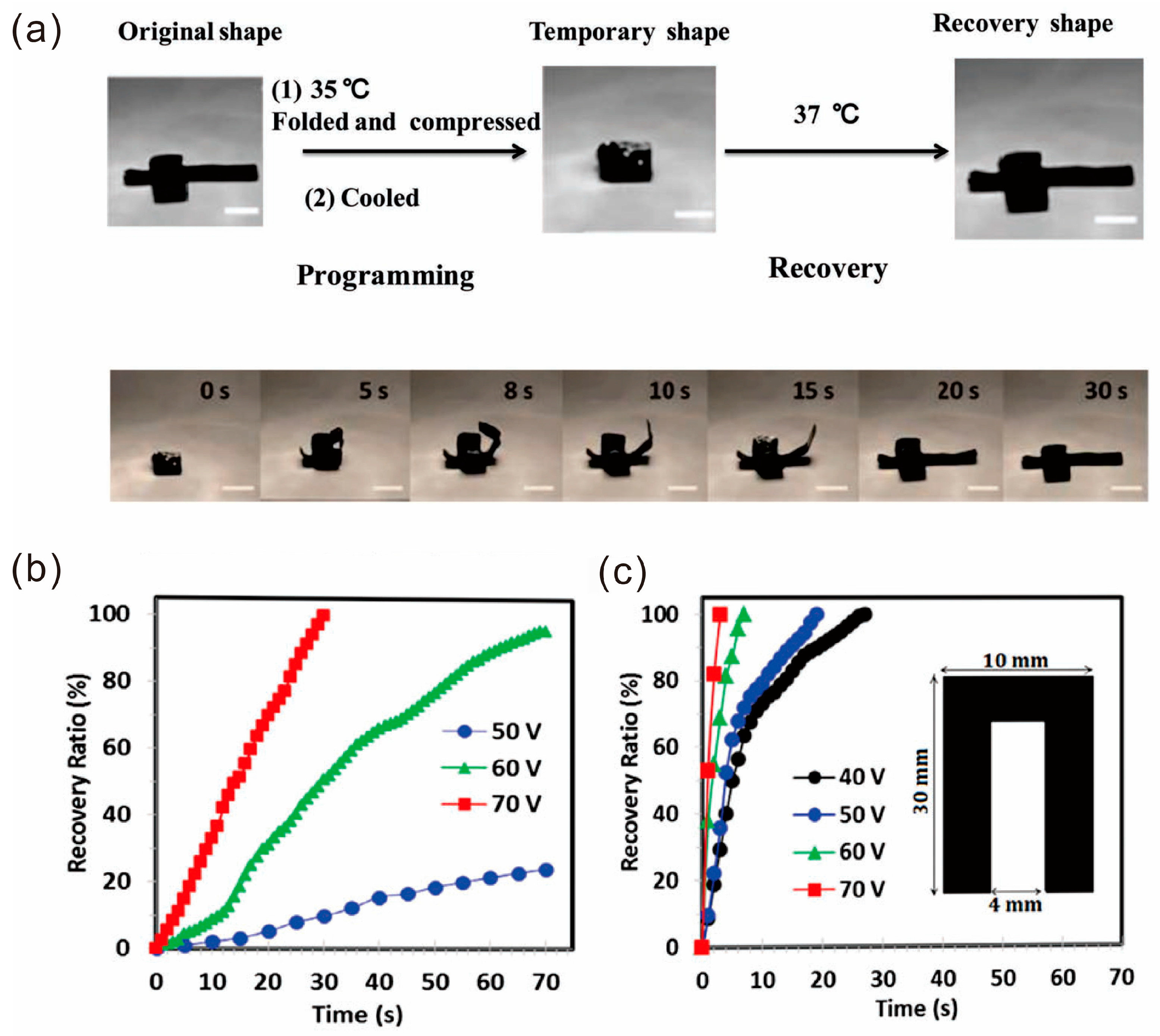
4.2. Shape Memory Property
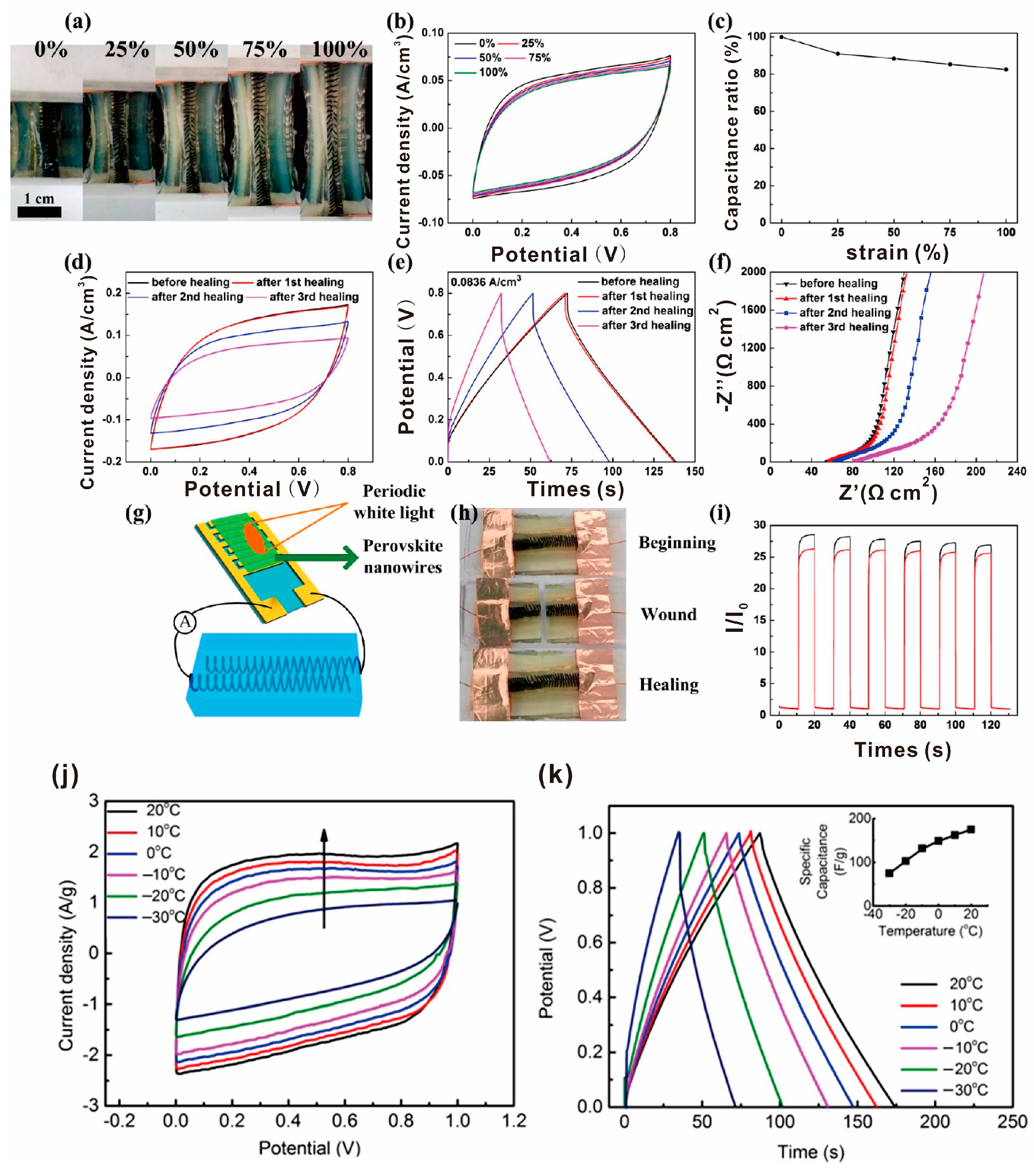
4.3. Conductivity and Electrical Devices
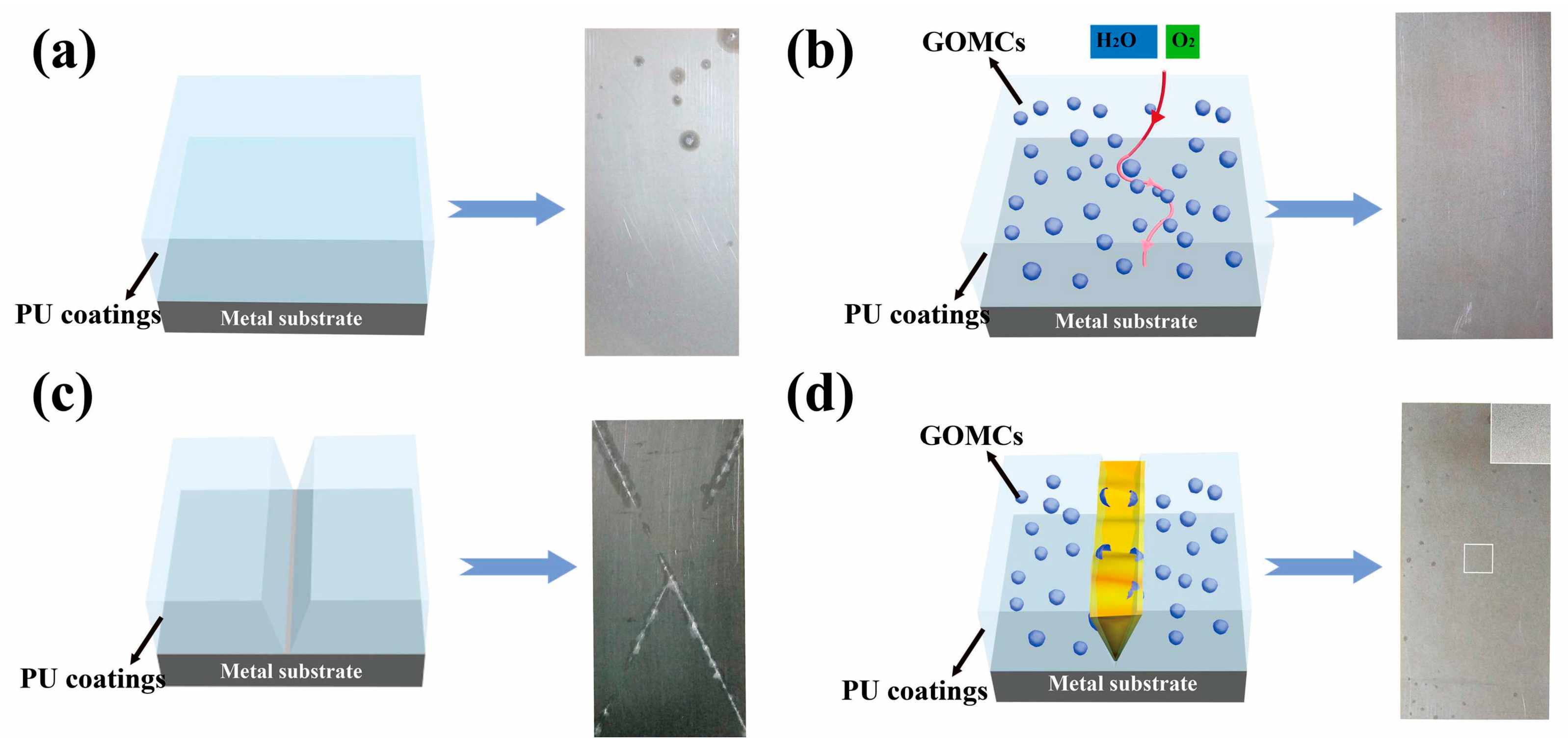
4.4. Anticorrosive Coating
4.5. Biological and Pharmaceutical Applications
5. Future Perspectives
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, P.; Zhang, H.; Yan, C.; Zheng, Z.; Wu, B.; Yu, Y. A Transparent, Highly stretchable, autonomous self-healing poly(dimethyl siloxane) elastomer. Macromol. Rapid Commun. 2017, 38. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Negulescu, I.; Zhang, D. Dynamic covalent polymer networks based on degenerative imine bond exchange: Tuning the malleability and self-healing properties by solvent. Macromolecules 2016, 49, 6277–6284. [Google Scholar] [CrossRef]
- Jian, X.; Hu, Y.; Zhou, W.; Xiao, L. Self-healing polyurethane based on disulfide bond and hydrogen bond. Polym. Adv. Technol. 2017, 29, 463–469. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, H.; He, Q.; Cai, S. Reprogrammable, reprocessible, and self-healable liquid crystal elastomer with exchangeable disulfide bonds. ACS Appl. Mater. Interfaces 2017, 9, 33119–33128. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, D. Self-healing Polyurethane/attapulgite nanocomposites based on disulfide bonds and shape memory effect. Mater. Chem. Phys. 2017, 195, 40–48. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, W.; Gu, H.; Feng, Y.; He, Z.; Chen, Q.; Mao, X.; Zhang, J.; Zheng, L. pH-Switchable and self-healable hydrogels based on ketone type acylhydrazone dynamic covalent bonds. Soft Matter 2017, 13, 7371–7380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Ruan, Y.B.; Zhang, B.Q.; Qiao, X.; Deng, G.; Chen, Y.; Liu, C.Y. A self-healing PDMS elastomer based on acylhydrazone groups and the role of hydrogen bonds. Polymer 2017, 120, 189–196. [Google Scholar] [CrossRef]
- Yu, F.; Cao, X.; Du, J.; Wang, G.; Chen, X. Multifunctional hydrogel with good structure integrity, self-healing, and tissue-adhesive property formed by combining diels-alder click reaction and acylhydrazone bond. ACS Appl. Mater. Interfaces 2015, 7, 24023–24031. [Google Scholar] [CrossRef] [PubMed]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kushner, A.M.; Williams, G.A.; Guan, Z. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat. Chem. 2012, 4, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Hart, L.R.; Nguyen, N.A.; Harries, J.L.; Mackay, M.E.; Colquhoun, H.M.; Hayes, W. Perylene as an electron-rich moiety in healable, complementary π–π stacked, supramolecular polymer systems. Polymer 2015, 69, 293–300. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Sari, M.; Oppermann, W.; Okay, O. Tough and self-healing hydrogels formed via hydrophobic interactions. Macromolecules 2011, 44, 4997–5005. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Argun, A.; Sahin, M.; Sari, M.; Okay, O. Structure optimization of self-healing hydrogels formed via hydrophobic interactions. Polymer 2012, 53, 5513–5522. [Google Scholar] [CrossRef]
- Yan, X.; Xu, D.; Chi, X.; Chen, J.; Dong, S.; Ding, X.; Yu, Y.; Huang, F. A multiresponsive, shape-persistent, and elastic supramolecular polymer network gel constructed by orthogonal self-assembly. Adv. Mater. 2012, 24, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Khamrai, M.; Banerjee, S.L.; Kundu, P.P. Modified bacterial cellulose based self-healable polyeloctrolyte film for wound dressing application. Carbohydr. Polym. 2017, 174, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Mozhdehi, D.; Ayala, S.; Cromwell, O.R.; Guan, Z. Self-healing multiphase polymers via dynamic metal-ligand interactions. J. Am. Chem. Soc. 2014, 136, 16128–16131. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.L.; Chortos, A.; Pfattner, R.; Lissel, F.; Chiu, Y.C.; Feig, V.; Xu, J.; Kurosawa, T.; Gu, X.; Wang, C. Stretchable self-healing polymeric dielectrics cross-linked through metal-ligand coordination. J. Am. Chem. Soc. 2016, 138, 6020–6027. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, M.; Ma, C.; Wang, Y.; Li, X.; Yu, G. A Conductive self-healing hybrid gel enabled by metal-ligand supramolecule and nanostructured conductive polymer. Nano Lett. 2015, 15, 6276–6281. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, D.; Yin, Z.; Yan, Q.; Zhang, H. Graphene and graphene-based materials for energy storage applications. Small 2014, 10, 3480–3498. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Geim, A.K.; Macdonald, A.H. Graphene: Exploring carbon flatland. Phys. Today 2007, 60, 35–41. [Google Scholar]
- Chen, G.; Wu, D.; Weng, W.; Wu, C. Exfoliation of graphite flake and its nanocomposites. Carbon 2003, 41, 619–621. [Google Scholar] [CrossRef]
- Silva, M.; Alves, N.M.; Paiva, M.C. Graphene-polymer nanocomposites for biomedical applications. Polym. Adv. Technol. 2017, 29, 687–700. [Google Scholar] [CrossRef]
- Tee, B.C.; Wang, C.; Allen, R.; Bao, Z. An electrically and mechanically self-healing composite with pressure-and flexion-sensitive properties for electronic skin applications. Nat. Nanotechnol. 2012, 7, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Nistor, R.A.; Newns, D.M.; Martyna, G.J. The role of chemistry in graphene doping for carbon-based electronics. ACS Nano 2011, 5, 3096–3103. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Chen, S.; Wang, L.; Jiang, K.; Shen, G. An ultra-sensitive and rapid response speed graphene pressure sensors for electronic skin and health monitoring. Nano Energy 2016, 23, 7–14. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L.; Pan, C.; Li, D. Review of recent achievements in self-healing conductive materials and their applications. J. Mater. Sci. 2017, 53, 27–46. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef]
- Wang, M.; Duan, X.; Xu, Y.; Duan, X. Functional three-dimensional graphene/polymer composites. ACS Nano 2016, 10, 7231–7247. [Google Scholar] [CrossRef] [PubMed]
- Tjong, S.C. Polymer composites with graphene nanofillers: Electrical properties and applications. J. Nanosci. Nanotechnol. 2014, 14, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhang, Y.; Zou, X.; Zhang, R.; Wang, X.; Wu, Q.; Sun, P. Rapid self-healing and recycling of multiple-responsive mechanically enhanced epoxy resin/graphene nanocomposites. RSC Adv. 2017, 7, 46336–46343. [Google Scholar] [CrossRef]
- Li, J.; Feng, Q.; Cui, J.; Yuan, Q.; Qiu, H.; Gao, S.; Yang, J. Self-assembled graphene oxide microcapsules in Pickering emulsions for self-healing waterborne polyurethane coatings. Compos. Sci. Technol. 2017, 151, 282–290. [Google Scholar] [CrossRef]
- Noack, M.; Merindol, R.; Zhu, B.; Benitez, A.; Hackelbusch, S.; Beckert, F.; Seiffert, S.; Mülhaupt, R.; Walther, A. Light-fueled, spatiotemporal modulation of mechanical properties and rapid self-healing of graphene-doped supramolecular elastomers. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Shahabadi, S.I.S.; Kong, J.; Lu, X. Aqueous-only, green route to self-healable, UV-resistant, and electrically conductive polyurethane/graphene/lignin nanocomposite coatings. ACS Sustain. Chem. Eng. 2017, 5, 3148–3157. [Google Scholar] [CrossRef]
- Wang, S.; Liu, N.; Su, J.; Li, L.; Long, F.; Zou, Z.; Jiang, X.; Gao, Y. Highly stretchable and self-healable supercapacitor with reduced graphene oxide based fiber springs. ACS Nano 2017, 11, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Meng, Y.; Li, Y. Electric heating behavior of flexible graphene/natural rubber conductor with self-healing conductive network. Mater. Lett. 2016, 192, 115–118. [Google Scholar] [CrossRef]
- Sabzi, M.; Babaahmadi, M.; Samadi, N.; Mahdavinia, G.R.; Keramati, M.; Nikfarjam, N. Graphene network enabled high speed electrical actuation of shape memory nanocomposite based on poly(vinyl acetate). Polym. Int. 2016, 66, 665–671. [Google Scholar] [CrossRef]
- Dhar, P.; Katiyar, A.; Maganti, L.S. Smart viscoelastic and self-healing characteristics of graphene nano-gels. J. Appl. Phys. 2016, 120. [Google Scholar] [CrossRef]
- Valentini, L.; Bon, S.B.; Pugno, N.M. Severe graphene nanoplatelets aggregation as building block for the preparation of negative temperature coefficient and healable silicone rubber composites. Compos. Sci. Technol. 2016, 134, 125–131. [Google Scholar] [CrossRef]
- Samadi, N.; Sabzi, M.; Babaahmadi, M. Self-healing and tough hydrogels with physically cross-linked triple networks based on Agar/PVA/Graphene. Int. J. Biol. Macromol. 2017, 107, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yan, L. Supramolecular hydrogel of chitosan in the presence of graphene oxide nanosheets as 2D cross-linkers. ACS Sustain. Chem. Eng. 2013, 2, 296–300. [Google Scholar] [CrossRef]
- Pan, C.; Liu, L.; Chen, Q.; Zhang, Q.; Guo, G. Tough, stretchable, compressive novel polymer/graphene oxide nanocomposite hydrogels with excellent self-healing performance. ACS Appl. Mater. Interfaces 2017, 9, 38052–38061. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, J.; Wang, T.; Sun, W.; Tong, Z. Multiple shape memory, self-healable, and supertough PAA-GO-Fe3+ hydrogel. Macromol. Mater. Eng. 2016, 302. [Google Scholar] [CrossRef]
- Zhu, P.; Hu, M.; Deng, Y.; Wang, C. One-Pot Fabrication of a Novel agar-polyacrylamide/graphene oxide nanocomposite double network hydrogel with high mechanical properties. Adv. Eng. Mater. 2016, 18, 1799–1807. [Google Scholar] [CrossRef]
- Cui, W.; Ji, J.; Cai, F.Y.; Li, H.; Rong, R. Robust, anti-fatigue, and self-healing graphene oxide/hydrophobic association composite hydrogels and their use as recyclable adsorbents for dye wastewater treatment. J. Mater. Chem. A 2015, 3, 17445–17458. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Tuning of sunlight-induced self-cleaning and self-healing attributes of an elastomeric nanocomposite by judicious compositional variation of the TiO2–reduced graphene oxide nanohybrid. J. Mater. Chem. A 2015, 3, 12334–12342. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhong, M.; Xie, X.M. Self-healable, super tough graphene oxide/poly(acrylic acid) nanocomposite hydrogels facilitated by dual cross-linking effects through dynamic ionic interactions. J. Mater. Chem. B 2015, 3, 4001–4008. [Google Scholar]
- Zhang, E.; Wang, T.; Zhao, L.; Sun, W.; Liu, X.; Tong, Z. Fast self-healing of graphene oxide-hectorite clay-poly(N,N-dimethylacrylamide) hybrid hydrogels realized by near-infrared irradiation. ACS Appl. Mater. Interfaces 2014, 6, 22855–22861. [Google Scholar] [CrossRef] [PubMed]
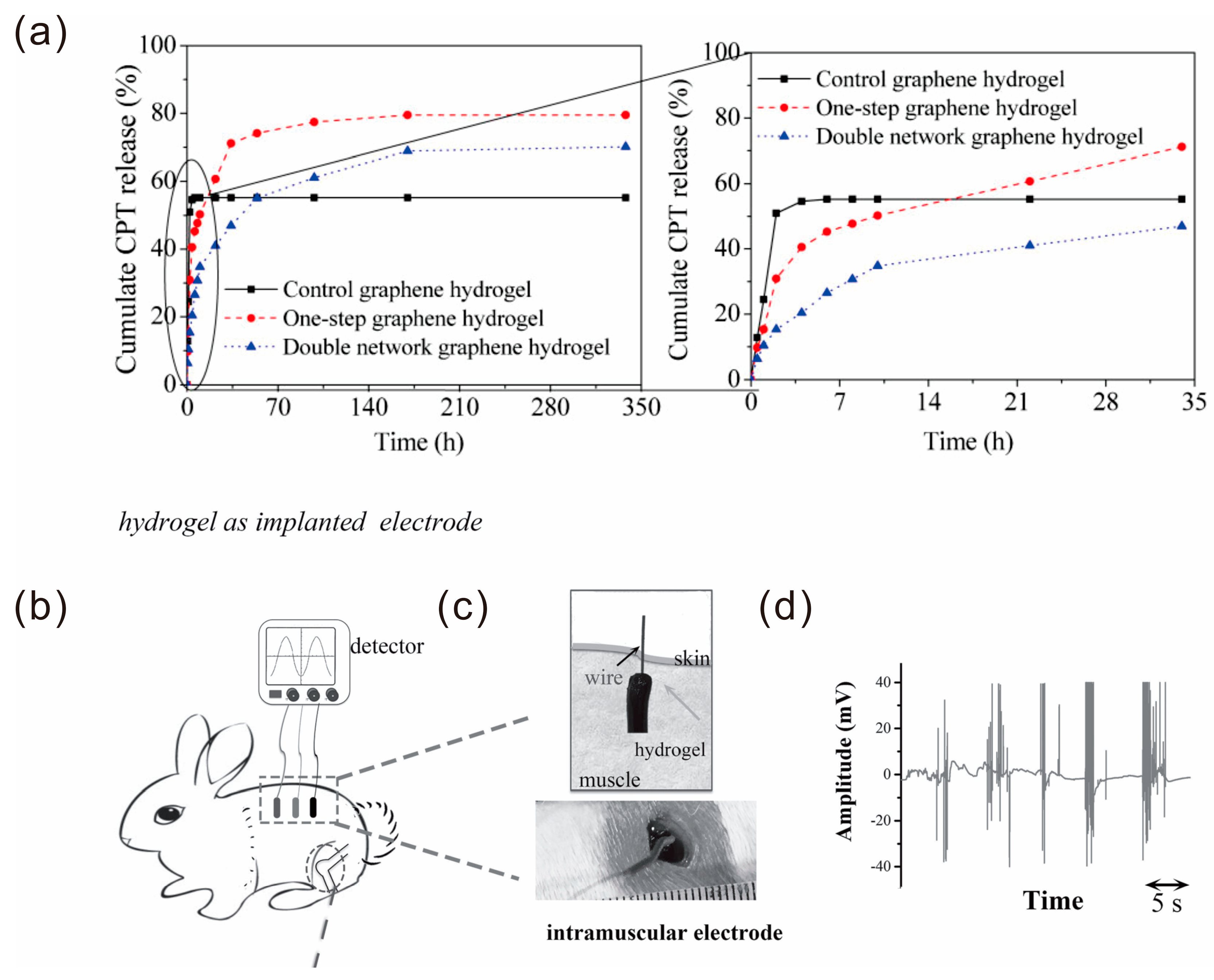
- Chen, P.; Wang, X.; Wang, G.Y.; Duo, Y.R.; Zhang, X.Y.; Hu, X.H.; Zhang, X.J. Double network self-healing graphene hydrogel by two step method for anticancer drug delivery. Mater. Technol. 2014, 29, 210–213. [Google Scholar] [CrossRef]
- Das, S.; Irin, F.; Ma, L.; Bhattacharia, S.K.; Hedden, R.C.; Green, M.J. Rheology and morphology of pristine graphene/polyacrylamide gels. ACS Appl. Mater. Interfaces 2013, 5, 8633–8640. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.P.; Wang, P.; Yu, S.H. Stretchable and self-healing graphene oxide–polymer composite hydrogels: A dual-network design. Chem. Mater. 2013, 25, 3357–3362. [Google Scholar] [CrossRef]
- Liu, J.; Song, G.; He, C.; Wang, H. Self-healing in tough graphene oxide composite hydrogels. Macromol. Rapid Commun. 2013, 34, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Duan, Y.; Zhang, Q.; Wang, H.; Li, Y. Bio-applicable and electroactive near-infrared laser-triggered self-healing hydrogels based on graphene networks. J. Mater. Chem. 2012, 22, 14991–14996. [Google Scholar] [CrossRef]
- Hou, C.; Huang, T.; Wang, H.; Yu, H.; Zhang, Q.; Li, Y. A strong and stretchable self-healing film with self-activated pressure sensitivity for potential artificial skin applications. Sci. Rep. 2013, 3, 3138. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Karak, N. A tough smart elastomeric bio-based hyperbranched polyurethane nanocomposite. New J. Chem. 2015, 39, 2146–2154. [Google Scholar] [CrossRef]
- Thakur, S.; Das, G.; Raul, P.K.; Karak, N. Green one-step approach to prepare sulfur/reduced graphene oxide nanohybrid for effective mercury ions removal. J. Phys. Chem. C 2013, 117, 7636–7642. [Google Scholar] [CrossRef]
- Thakur, S.; Barua, S.; Karak, N. Self-healable castor oil based tough smart hyperbranched polyurethane nanocomposite with antimicrobial attributes. RSC Adv. 2014, 5, 2167–2176. [Google Scholar] [CrossRef]
- Jin, T.K.; Kim, B.K.; Kim, E.Y.; Sun, H.K.; Han, M.J. Synthesis and properties of near IR induced self-healable polyurethane/graphene nanocomposites. Eur. Polym. J. 2013, 49, 3889–3896. [Google Scholar]
- Li, J.; Zhang, G.; Sun, R.; Wong, C.P. A covalently cross-linked reduced functionalized graphene oxide/polyurethane composite based on Diels–Alder chemistry and its potential application in healable flexible electronics. J. Mater. Chem. C 2016, 5, 220–228. [Google Scholar] [CrossRef]
- Wool, R.P. Self-healing materials: A review. Soft Matter 2008, 4, 400–418. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.A.; Picchioni, F. Thermally self-healing polymeric materials: The next step to recycling thermoset polymers? Macromolecules 2009, 42, 1906–1912. [Google Scholar] [CrossRef] [Green Version]
- Kuang, X.; Liu, G.; Dong, X.; Liu, X.; Xu, J.; Wang, D. Facile fabrication of fast recyclable and multiple self-healing epoxy materials through diels-alder adduct cross-linker. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2094–2103. [Google Scholar] [CrossRef]
- Lin, C.; Sheng, D.; Liu, X.; Xu, S.; Ji, F.; Dong, L.; Zhou, Y.; Yang, Y. A self-healable nanocomposite based on dual-crosslinked Graphene oxide/polyurethane. Polymer 2017, 127, 241–250. [Google Scholar] [CrossRef]
- Wu, S.; Li, J.; Zhang, G.; Yao, Y.; Li, G.; Sun, R.; Wong, C.P. Ultrafastly self-healing nanocomposites via infrared laser and its application in flexible electronics. ACS Appl. Mater. Interfaces 2017, 9, 3040–3049. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Tugba, C.B.; Oytun, F.; Hasan, A.M.; Jeong, S.H.; Choi, H.; Basarir, F. Fabrication of a transparent conducting electrode based on graphene/silver nanowires via layer-by-layer method for organic photovoltaic devices. J. Colloid Interface Sci. 2017, 505, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.; Wu, M.; Sun, J. Polyelectrolyte multilayers impart healability to highly electrically conductive films. Adv. Mater. 2012, 24, 4578–4582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, T.; Zhang, L.; Tong, Y.; Zhang, Z.; Shi, X.; Guo, L.; Huang, H.; Yang, Q.; Huang, W. Tracking deep crust by zircon xenocrysts within igneous rocks from the northern Alxa, China: Constraints on the southern boundary of the Central Asian Orogenic Belt. J. Asian Earth Sci. 2015, 108, 150–169. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Kirkland, J.J. Porous Thin-layer modified glass bead supports for gas liquid chromatography. Anal. Chem. 1965, 37, 1458–1461. [Google Scholar] [CrossRef]
- Fan, F.; Zhou, C.; Wang, X.; Szpunar, J. Layer-by-layer assembly of a self-healing anticorrosion coating on magnesium alloys. ACS Appl. Mater. Interfaces 2015, 7, 27271–27278. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, C.; Ren, J.; Liu, C.; Ge, L. Graphene improved electrochemical property in self-healing multilayer polyelectrolyte film. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 26–31. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhang, L.; Li, Y.; Yuan, T.; Zhang, W.; Sun, J. Reduced graphene oxide-reinforced polymeric films with excellent mechanical robustness and rapid and highly efficient healing properties. ACS Nano 2017, 11, 7134–7141. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Yu, Y.; Chen, S.; Yang, Y.; Tang, Y. Conductive nanocomposite hydrogels with self-healing property. RSC Adv. 2014, 4, 35149–35155. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Deng, L.; Zhao, S.; Gao, Y.; Jiang, K.; Sun, R.; Wong, C. In situ polymerization of mechanically reinforced, thermally healable graphene oxide/polyurethane composites based on Diels-Alder chemistry. J. Mater. Chem. A 2014, 2, 20642–20649. [Google Scholar] [CrossRef]
- Dong, J.; Ding, J.; Weng, J.; Dai, L. Graphene enhances the shape memory of poly (acrylamide-co-acrylic acid) grafted on graphene. Macromol. Rapid Commun. 2013, 34, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Q.; Sun, Y.; Bai, H.; Shi, G. Three-dimensional self-assembly of graphene oxide and DNA into multifunctional hydrogels. ACS Nano 2010, 4, 7358–7362. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xie, T.; Cheng, Y.T. Self-healable graphene polymer composites. J. Mater. Chem. 2010, 20, 3508–3514. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Wu, M.; Sun, J. Rapid and efficient multiple healing of flexible conductive films by near-infrared light irradiation. ACS Appl. Mater. Interfaces 2014, 6, 16409–16415. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Casalongue, H.S.; Vinh, D.; Dai, H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hou, W.; Liang, Y.; Zhang, Z.; Ren, L.; Zhao, Q.; Hou, W.; Liang, Y.; Zhang, Z.; Ren, L. Design and fabrication of bilayer hydrogel system with self-healing and detachment properties achieved by near-infrared irradiation. Polymers 2017, 9, 237. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepić, D.P.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramićanin, M.D.; Trajkovic, V.S. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Si, L.; Wu, F.; Chan, S.Y.; Yu, P.; Fei, B. Electrical and mechanical self-healing membrane using gold nanoparticles as localized “nano-heaters”. J. Mater. Chem. C 2016, 4, 10018–10025. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, Z.; Wan, W.; Gogotsi, Y.; Qiu, J. Ultralight and highly compressible graphene aerogels. Adv. Mater. 2013, 25, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Velamakanni, A.; Piner, R.D.; Ruoff, R.S. Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 2010, 48, 2118–2122. [Google Scholar] [CrossRef]
- Huang, L.; Yi, N.; Wu, Y.; Zhang, Y.; Zhang, Q.; Huang, Y.; Ma, Y.; Chen, Y. Multichannel and repeatable self-healing of mechanical enhanced graphene-thermoplastic polyurethane composites. Adv. Mater. 2013, 25, 2224–2228. [Google Scholar] [CrossRef] [PubMed]
- South, A.B.; Lyon, L.A. Autonomic self-healing of hydrogel thin films. Angew. Chem. 2010, 49, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, F.; Zheng, X.; Sun, J. Water-enabled self-healing of polyelectrolyte multilayer coatings. Angew. Chem. 2011, 123, 11580–11583. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-repairable polyurethane networks by atmospheric carbon dioxide and water. Angew. Chem. 2014, 53, 12142–12147. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Mi, H.Y.; Napiwocki, B.N.; Peng, X.F.; Turng, L.S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Konwar, A.; Kalita, S.; Kotoky, J.; Chowdhury, D. Chitosan-iron oxide coated graphene oxide nanocomposite hydrogel: A robust and soft antimicrobial bio-film. ACS Appl. Mater. Interfaces 2016, 8, 20625–20634. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jeon, Y.S.; Park, H.S.; Kim, J.H. Self-healable mussel-mimetic nanocomposite hydrogel based on catechol-containing polyaspartamide and graphene oxide. Mater. Sci. Eng. C 2016, 69, 160–170. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, E.; Barg, S.; Ni, N.; Rocha, V.G.; Saiz, E. Self-healing graphene-based composites with sensing capabilities. Adv. Mater. 2015, 27, 4788–4794. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, N.; Allen, R.; Tok, J.B.; Wu, Y.; Zhang, F.; Chen, Y.; Bao, Z. A rapid and efficient self-healing thermo-reversible elastomer crosslinked with graphene oxide. Adv. Mater. 2013, 25, 5785–5790. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; He, J.; Wang, H.; Zhou, Y.; You, X.; Nan, N.; Shao, W.; Wang, L.; Ding, B.; Cui, S. A highly stretchable nanofiber-based electronic skin with pressure-, strain-, and flexion-sensitive properties for health and motion monitoring. ACS Appl. Mater. Interfaces 2017, 9, 42951–42960. [Google Scholar] [CrossRef] [PubMed]
- Palleau, E.; Reece, S.; Desai, S.C.; Smith, M.E.; Dickey, M.D. Self-healing stretchable wires for reconfigurable circuit wiring and 3D microfluidics. Adv. Mater. 2013, 25, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y. A Mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, B. A mechanically and electrically self-healing graphite composite dough for stencil-printable stretchable conductors. J. Mater. Chem. C 2016, 4, 4150–4154. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Wang, X.; Ok, Y.S.; Jaw, E.; Chang, S.X.; Chung, H.J. Flexible and self-healing aqueous supercapacitors for low temperature applications: Polyampholyte gel electrolytes with biochar electrodes. Sci. Rep. 2017, 7, 1685. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Deka, J.; Raidongia, K.; Manna, U. Robust and self-healable bulk-superhydrophobic polymeric coating. Chem. Mater. 2017, 29, 8720–8728. [Google Scholar] [CrossRef]
- Qiu, S.; Li, W.; Zheng, W.; Zhao, H.; Wang, L. Synergistic effect of polypyrrole-intercalated graphene for enhanced corrosion protection of aqueous coating in 3.5% NaCl solution. ACS Appl. Mater. Interfaces 2017, 9, 34294–34304. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, Y.; Cheng, Q.; Shi, G.; Jiang, L.; Qu, L. Self-healing graphene oxide based functional architectures triggered by moisture. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Sazou, D.; Deshpande, P.P. Conducting polyaniline nanocomposite-based paints for corrosion protection of steel. Chem. Pap. 2017, 71, 459–487. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Zhang, X.; Lv, C.; Yuan, R.; Zhu, Y.; Mu, L.; Zhu, J. Durable Self-healing superhydrophobic coating with biomimic “Chloroplast” analogous structure. Adv. Mater. Interfaces 2016, 3. [Google Scholar] [CrossRef]
- Niratiwongkorn, T.; Luckachan, G.E.; Mittal, V. Self-healing protective coatings of polyvinyl butyral/polypyrrole-carbon black composite on carbon steel. RSC Adv. 2016, 6, 43237–43249. [Google Scholar] [CrossRef]


| Materials | Self-Healing Mechanisms | Self-Healing Condition | Self-Healing Efficiency | Original Mechanical Property | Applications | Reference |
|---|---|---|---|---|---|---|
| FG/TPU material | PU chains diffuse | IR light, Electricity, Microwave (2.45 GHz) | 98% of electrical conductivity | Tensile strength 40 MPa | Transport industries, construction industries, electronics | [91] |
| P(AM-co-DAC)/GO Hydrogels | Hydrogen bonds, electrostatic interaction | Drop water | >92% of tensile strength, >99% of tensile strain and >93% of toughness | Young’s modulus 1 MPa Tensile strength 2 MPa | - | [46] |
| Chitosan/GO Hydrogel | π-π stacking, hydrogen bonds | Contact (room temperature) | - | Adhesive strength 1 MPa compressive stress 14 KPa | Electroactive tissue engineering applications | [95] |
| PU-DA-mGO | Dynamic covalent bonds | Heating (120 °C after 10 min) | 90% of tensile strength | Stress 38 MPa | Aerospace, automobile, coating, electronics, energy, etc. | [67] |
| Graphene/PU | Diels-Alder chemistry | IR | 96% of tensile strength | Breaking strength 36 MPa Young’s modulus 127 MPa | Flexible electronics | [68] |
| PVAc/graphene nanocomposites | Diffusion of the polymer chains | 60 °C for 1 h | 89% (mechanical properties) | - | Sensors and fast deployable and actuating devices | [41] |
| RFGO/PU composites | Diels–Alder chemistry | Microwaves | 93% (mechanical properties) | Stress 24 MPa Young’s module 52 MPa | Flexible conductors, strain sensors | [63] |
| PAA-GO-Fe3+ Hydrogel | Ionic binding | Contact and immersed in FeCl3/HCl | Nearly 100% tensile | Tensile strength 2.5 MPa Elongation 700% | Soft actuators, robots | [47] |
| PDA-pGO-PAM hydrogel | Non-covalent bonds | Contact | 60% of tensile strength 95% of electrical conductivity | Tensile strength 75 kPa | Bioelectronics | [102] |
| PAM/GO DN gel | Hydrogen bond | Heating 80 °C for 3 h | 48% of tensile strength | Elongation 4600% Fracture strength 332 kPa | Engineering fields | [48] |
| Au@PCLx/rGO/Ag | Fibers soften and flow | Light irradiation (532 nm) | 90% of tensile strength 91% of conductivity | Tensile strength 4.85 MPa | Optoelectronic devices | [88] |
| SR/GNP composite | Reversible bonds | Thermal annealing | 87% of tensile strength | Stress 1.3 MPa | Seals, hoses and automotive sector | [43] |
| HPU-IO-RGO | Diffusion of the polymer chains | Microwave sunlight | 99% | Tensile strength 24.15 MPa Tensile modulus 28.55 MPa Toughness 110.8 MJ m3 | Transport, construction, electronics | [59] |
| GO-Clay-PDMAA Hybrid Hydrogels | Diffusion of the polymer chains hydrogen bonds | NIR | 96% (mechanical strength) | Strength 184 kPa Elongation 1890% | Surgical dressing | [52] |
| GO/PU composites | Covalent bonding | Heating | 78% of tensile stress | Tensile modulus 21.95 MPa Fracture stress 8 MPa Young’s modulus 22 MPa | Smart materials and structural material | [79] |
| SHPU/grapheme composites | Interchain diffusion | NIR | 39% | Stress 4 MPa | Functional polymer | [62] |
| GO/PAA composite hydrogels | Diffusion of polymer chains hydrogen bond | Contact at different temperatures | 88% (mechanical properties) | Tensile strength 0.35 MPa Elongation 4900% (healed) | Biomedical and engineering fields | [56] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Li, D.; Liu, L.; Gai, G. Recent Achievements of Self-Healing Graphene/Polymer Composites. Polymers 2018, 10, 114. https://doi.org/10.3390/polym10020114
Du Y, Li D, Liu L, Gai G. Recent Achievements of Self-Healing Graphene/Polymer Composites. Polymers. 2018; 10(2):114. https://doi.org/10.3390/polym10020114
Chicago/Turabian StyleDu, Yongxu, Dong Li, Libin Liu, and Guangjie Gai. 2018. "Recent Achievements of Self-Healing Graphene/Polymer Composites" Polymers 10, no. 2: 114. https://doi.org/10.3390/polym10020114





