Electrosprayed Core–Shell Composite Microbeads Based on Pectin-Arabinoxylans for Insulin Carrying: Aggregation and Size Dispersion Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polysaccharides and Gelling Agents
2.2. Modification of Pectin Esterification Degree
2.3. Polysaccharides Dispersions
2.4. Insulin Solubilization
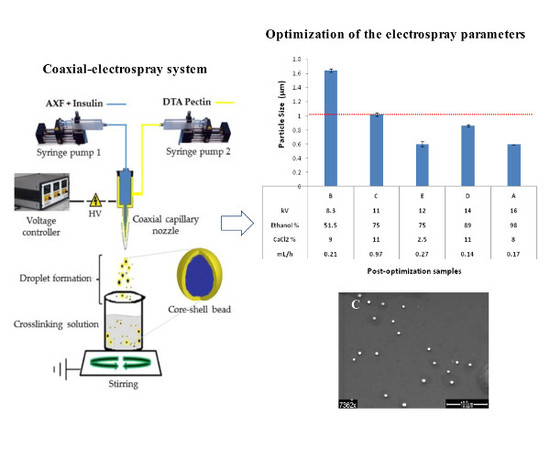
2.5. Electrospray Process
2.6. Measurement of Beads Size by DLS
2.7. Scanning Electron Microscopy Low Vacuum (SEM-LV)
2.8. Transmission Electron Microscopy
2.9. Confocal Laser Scanning Microscopy
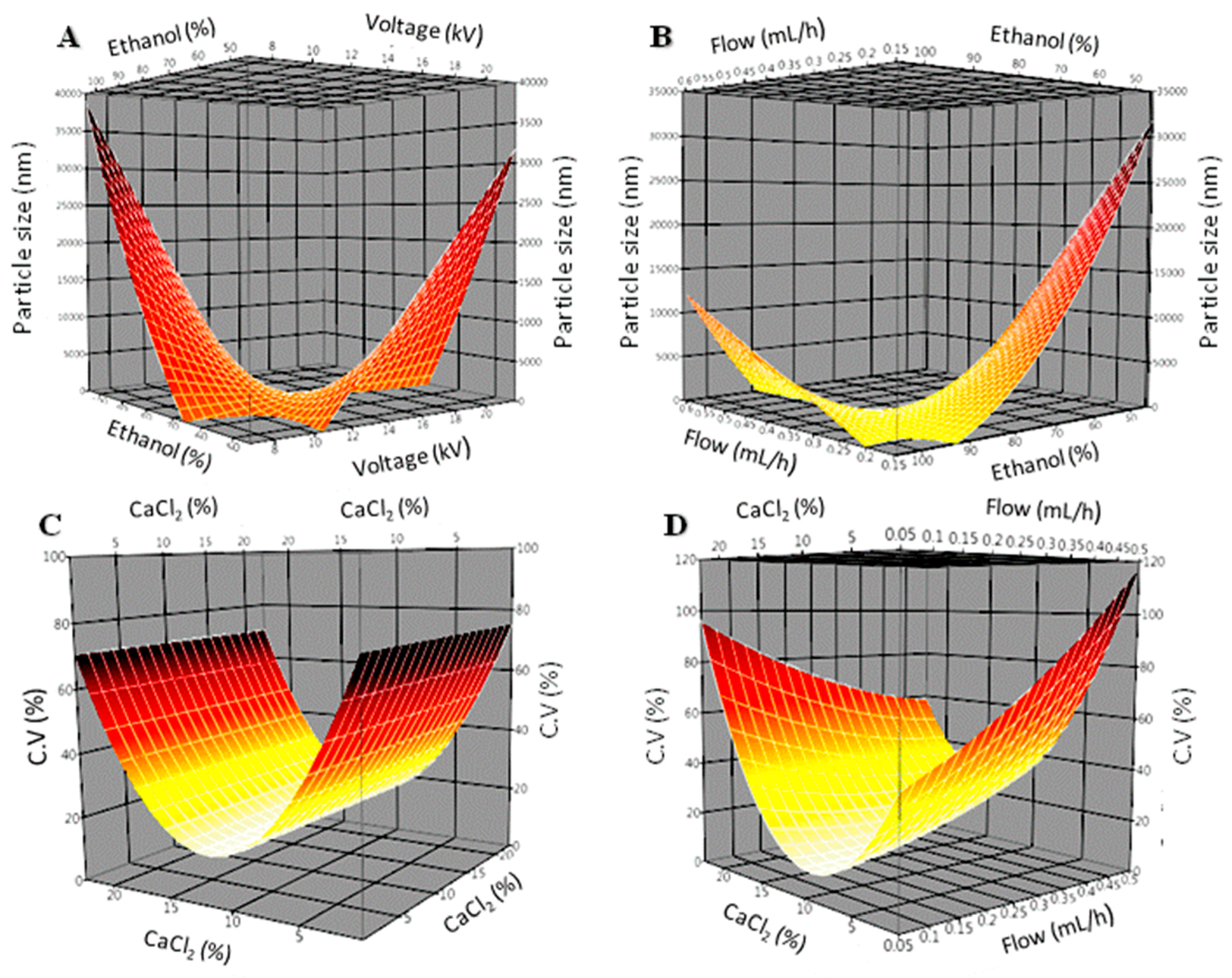
2.10. Experimental Design
2.11. Fabrication Post-Optimization
2.12. Scanning Electron Microscopy (SEM)
2.13. Sample Preparation for SEM Microscopy Analysis
3. Results and Discussion
3.1. Degree of Esterification
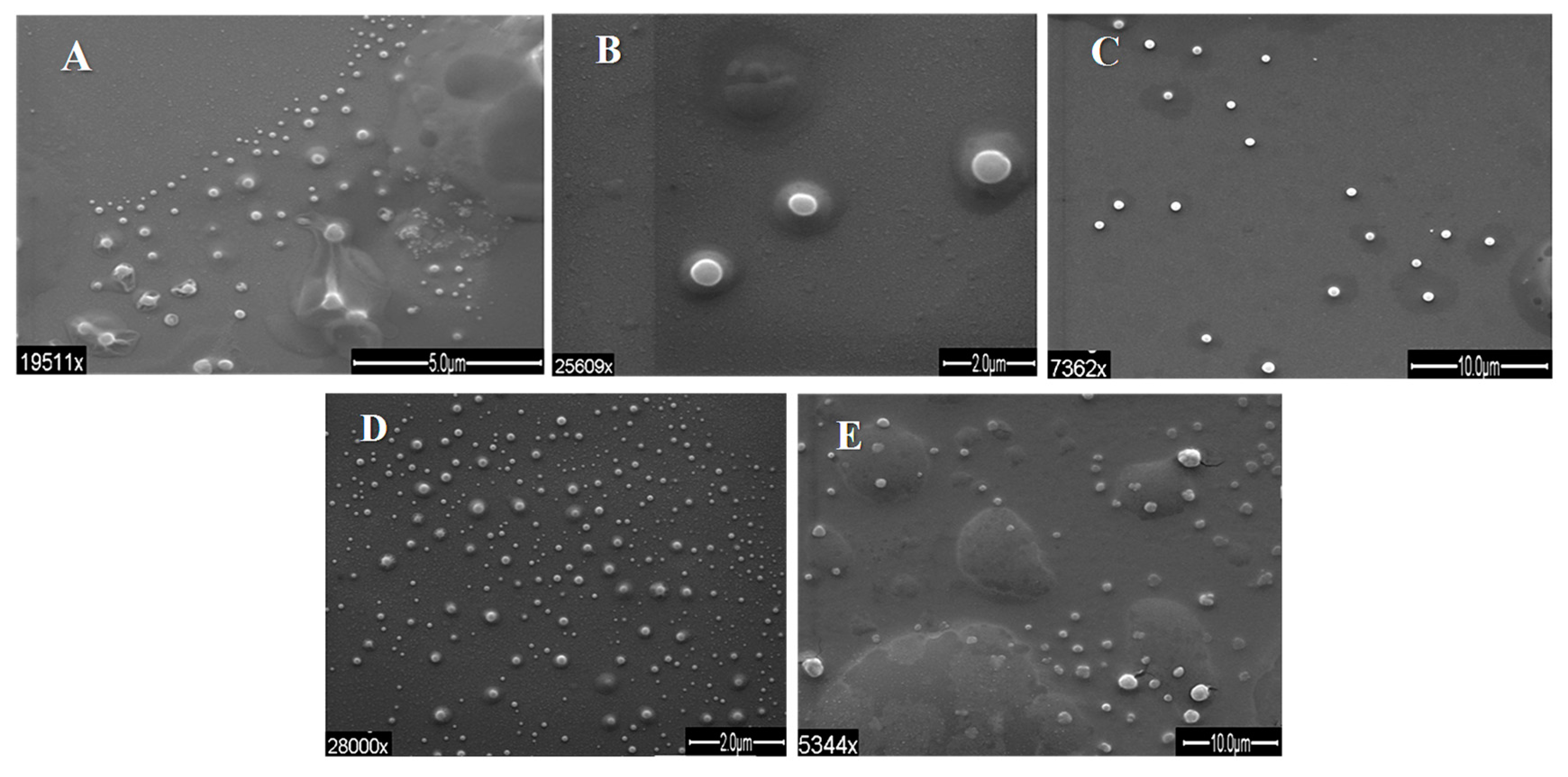
3.2. Shape and Size of Beads
3.3. Statistical Analysis
3.4. Post-Optimization Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berlanga-Reyes, C.M.; Carvajal-Millán, E.; Lizardi-Mendoza, J.; Rascón-Chu, A.; Marquez-Escalante, J.; Martínez-López, A.L. Maize arabinoxylan gels as protein delivery matrices. Molecules 2009, 14, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Arnold, R.D.; Wicker, L. Pectin and charge modified pectin hydrogel beads as a colon-targeted drug delivery carrier. Colloids Surf. B 2013, 104, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fishman, M.; Hicks, K. Pectin in controlled drug delivery—A review. Cellulose 2007, 14, 15–24. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.L.; Carvajal-Millan, E.; Miki-Yoshida, M.; Alvarez-Contreras, L.; Rascon-Chu, A.; Lizardi-Mendoza, J.; Lopez-Franco, Y. Arabinoxylan microspheres: Structural and textural characteristics. Molecules 2013, 18, 4640–4650. [Google Scholar] [CrossRef] [PubMed]
- Palin, R.; Geitmann, A. The role of pectin in plant morphogenesis. BioSystems 2012, 109, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Ovodov, Y.S. Current views on pectin substances. Russ. J. Bioorg. Chem. 2009, 35, 269–284. [Google Scholar] [CrossRef]
- Voragen, A.J.; Coenen, G.-J.; Verhoef, R.; Schols, H. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Löfgren, C.; Hermansson, A.-M. Synergistic rheological behaviour of mixed HM/LM pectin gels. Food Hydrocoll. 2007, 21, 480–486. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular Basis of Ca2+-Induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Fraeye, I.; Doungla, E.; Duvetter, T.; Moldenaers, P.; Van Loey, A.; Hendrickx, M. Influence of intrinsic and extrinsic factors on rheology of pectin–calcium gels. Food Hydrocoll. 2009, 23, 2069–2077. [Google Scholar] [CrossRef]
- Mazumder, K.; York, W.S. Structural analysis of arabinoxylans isolated from ball-milled switchgrass biomass. Carbohydr. Res. 2010, 345, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Landillon, V.; Morel, M.H.; Rouau, X.; Doublier, J.L.; Micard, V. Arabinoxylan gels: Impact of the feruloylation degree on their structure and properties. Biomacromolecules 2005, 6, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Millan, E.; Guilbert, S.; Doublier, J.-L.; Micard, V. Arabinoxylan/protein gels: Structural, rheological and controlled release properties. Food Hydrocoll. 2006, 20, 53–61. [Google Scholar] [CrossRef]
- Lamprecht, A.; Schafer, U.; Lehr, C.M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001, 18, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Lacoeuille, F.; Garcion, E.; Benoit, J.P.; Lamprecht, A. Lipid nanocapsules for intracellular drug delivery of anticancer drugs. J. Nanosci. Nanotechnol. 2007, 7, 4612–4617. [Google Scholar] [PubMed]
- Laroui, H.; Wilson, D.S.; Dalmasso, G.; Salaita, K.; Murthy, N.; Sitaraman, S.V.; Merlin, D. Nanomedicine in GI. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G371–G383. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Goto, T.; Peppas, N.A.; Joseph, J.I.; Torjman, M.C.; Munsick, C.; Nakamura, K.; Yamagata, T.; Takayama, K.; Lowman, A.M. Mucosal insulin delivery systems based on complexation polymer hydrogels: Effect of particle size on insulin enteral absorption. J. Control. Release 2004, 97, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; MacKay, J.A.; McDaniel, J.R.; Chilkoti, A.; Clark, R.L. Fabrication of elastin-like polypeptide nanoparticles for drug delivery by electrospraying. Biomacromolecules 2009, 10, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Zheng, Z.-B.; Yu, D.-G.; Liu, X.-K.; Qu, Y.-L.; Li, H.-L. Electrosprayed sperical ethylcellulose nanoparticles for an improved sustained-release profile of anticancer drug. Cellulose 2017, 24, 5551–5564. [Google Scholar] [CrossRef]
- Almería, B.; Deng, W.; Fahmy, T.M.; Gomez, A. Controlling the morphology of electrospray-generated PLGA microparticles for drug delivery. J. Colloid Interface Sci. 2010, 343, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-P.; Zhang, Y.-Y.; Yu, D.-G.; Wu, D.; Li, H.-L. Fabrication of sustained-release zein nanoparticles via modified coaxial electrospraying. Chem. Eng. J. 2018, 334, 807–816. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Zhang, M.; Liu, Z.-P.; Wang, K.; Yu, D.-G. Meletin sustained-release gliadin nanoparticles prepared via solvent surface modification on blending electrospraying. Appl. Surf. Sci. 2018, 434, 1040–1047. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, J.-J.; Zhang, M.; Williams, G.R. High-quality Janus nanofibers prepared using three-fluid electrospinning. Chem. Commun. 2017, 53, 4542–4545. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Bingham, D.; de Juan, L.; Tang, K. Generation of monodisperse protein nanoparticles by electrospray drying. MRS Online Proc. Libr. 1998, 550. [Google Scholar] [CrossRef]
- Díaz-Baca, J.; Martinez-Lopez, A.L.; Carvajal-Millan, E.; Pérez-López, E.; González-Ríos, H.; Balandrán-Quintana, R.; Rascón-Chu, A. Fabrication and characterization of core–shell microspheres composed of pectin and arabinoxylans as controlled release systems for insulin. In Nanotechnology 2014: MEMS, Fluidics, Bio Systems, Medical, Computational & Photonics Materials for Drug & Gene Delivery & Cancer Nanotech; NSTI: Washington, DC, USA, 2014; pp. 327–330. ISBN 978-1-4822-5827-1. [Google Scholar]
- Carvajal-Millan, E.; Rascón-Chu, A.; Márquez-Escalante, J.A.; Micard, V.; Ponce de León, N.P.; Gardea, A. Maize bran gum: Extraction, characterization and functional properties. Carbohydr. Polym. 2007, 69, 280–285. [Google Scholar] [CrossRef]
- Urias-Orona, V.; Rascon-Chu, A.; Lizardi-Mendoza, J.; Carvajal-Millan, E.; Gardea, A.A.; Ramirez-Wong, B. A novel pectin material: Extraction, characterization and gelling properties. Int. J. Mol. Sci. 2010, 11, 3686–3695. [Google Scholar] [CrossRef] [PubMed]
- Martínez López, A.L.; Carvajal Millan, E.; Sotelo Cruz, N.; Micard, V.; Rascón Chu, A.; Prakash, S.; Lizardi Mendoza, J.; López Franco, Y.L.; Cannet Romero, R.; Toledo Guillén, A.R. Biodegradable Covalent Matrices for the Oral Delivery of Insulin to the Colon, Activated by Micriobiota and Production Method Thereof. International Patent Application WO2017/105212A2, 22 June 2017. [Google Scholar]
- Ghayempour, S.; Mortazavi, S.M. Fabrication of micro-nanocapsules by a new electrospraying method using coaxial jets and examination of effective parameters on their production. J. Electrost. 2013, 71, 717–727. [Google Scholar] [CrossRef]
- Cao, L.; Luo, J.; Tu, K.; Wang, L.Q.; Jiang, H. Generation of nano-sized core–shell particles using a coaxial tri-capillary electrospray-template removal method. Colloids Surf. B 2014, 115, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Ferreira, D.; Neufeld, R. Oral bioavailability of insulin contained in polysaccharide nanoparticles. Biomacromolecules 2007, 8, 3054–3060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, W.; Lv, P.; Wang, L.; Ma, G. Preparation and evaluation of alginate-chitosan microspheres for oral delivery of insulin. Eur. J. Pharm. Biopharm. 2011, 77, 11–19. [Google Scholar] [CrossRef] [PubMed]





| Run | Voltage (KV) | Flow (mL/h) | Ethanol Conc. (%) | CaCl2 Conc. (%) |
|---|---|---|---|---|
| A | 16 | 0.175 | 98 | 8 |
| B | 8.3 | 0.208 | 51.5 | 9 |
| C | 11 | 0.972 | 75 | 11 |
| D | 14.5 | 0.142 | 89.5 | 11 |
| E | 12 | 0.275 | 75 | 2.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rascón-Chu, A.; Díaz-Baca, J.A.; Carvajal-Millan, E.; Pérez-López, E.; Hotchkiss, A.T.; González-Ríos, H.; Balandrán-Quintana, R.; Campa-Mada, A.C. Electrosprayed Core–Shell Composite Microbeads Based on Pectin-Arabinoxylans for Insulin Carrying: Aggregation and Size Dispersion Control. Polymers 2018, 10, 108. https://doi.org/10.3390/polym10020108
Rascón-Chu A, Díaz-Baca JA, Carvajal-Millan E, Pérez-López E, Hotchkiss AT, González-Ríos H, Balandrán-Quintana R, Campa-Mada AC. Electrosprayed Core–Shell Composite Microbeads Based on Pectin-Arabinoxylans for Insulin Carrying: Aggregation and Size Dispersion Control. Polymers. 2018; 10(2):108. https://doi.org/10.3390/polym10020108
Chicago/Turabian StyleRascón-Chu, Agustín, Jonathan A. Díaz-Baca, Elizabeth Carvajal-Millan, Elías Pérez-López, Arland T. Hotchkiss, Humberto González-Ríos, Rene Balandrán-Quintana, and Alma C. Campa-Mada. 2018. "Electrosprayed Core–Shell Composite Microbeads Based on Pectin-Arabinoxylans for Insulin Carrying: Aggregation and Size Dispersion Control" Polymers 10, no. 2: 108. https://doi.org/10.3390/polym10020108