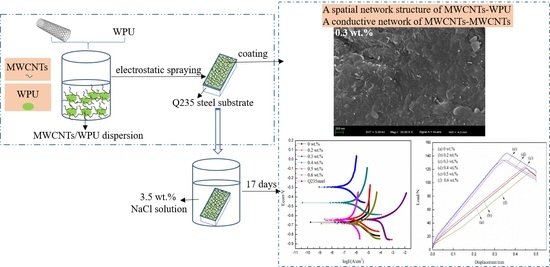
Properties of Waterborne Polyurethane Conductive Coating with Low MWCNTs Content by Electrostatic Spraying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Coatings
2.3. Measurements
2.3.1. Electrical Conductivity
2.3.2. Corrosion Resistance
2.3.3. Adhesive Strength
2.3.4. Fourier Transform Infrared Spectroscopy
2.3.5. Scanning Electron Microscope
3. Results and Discussion
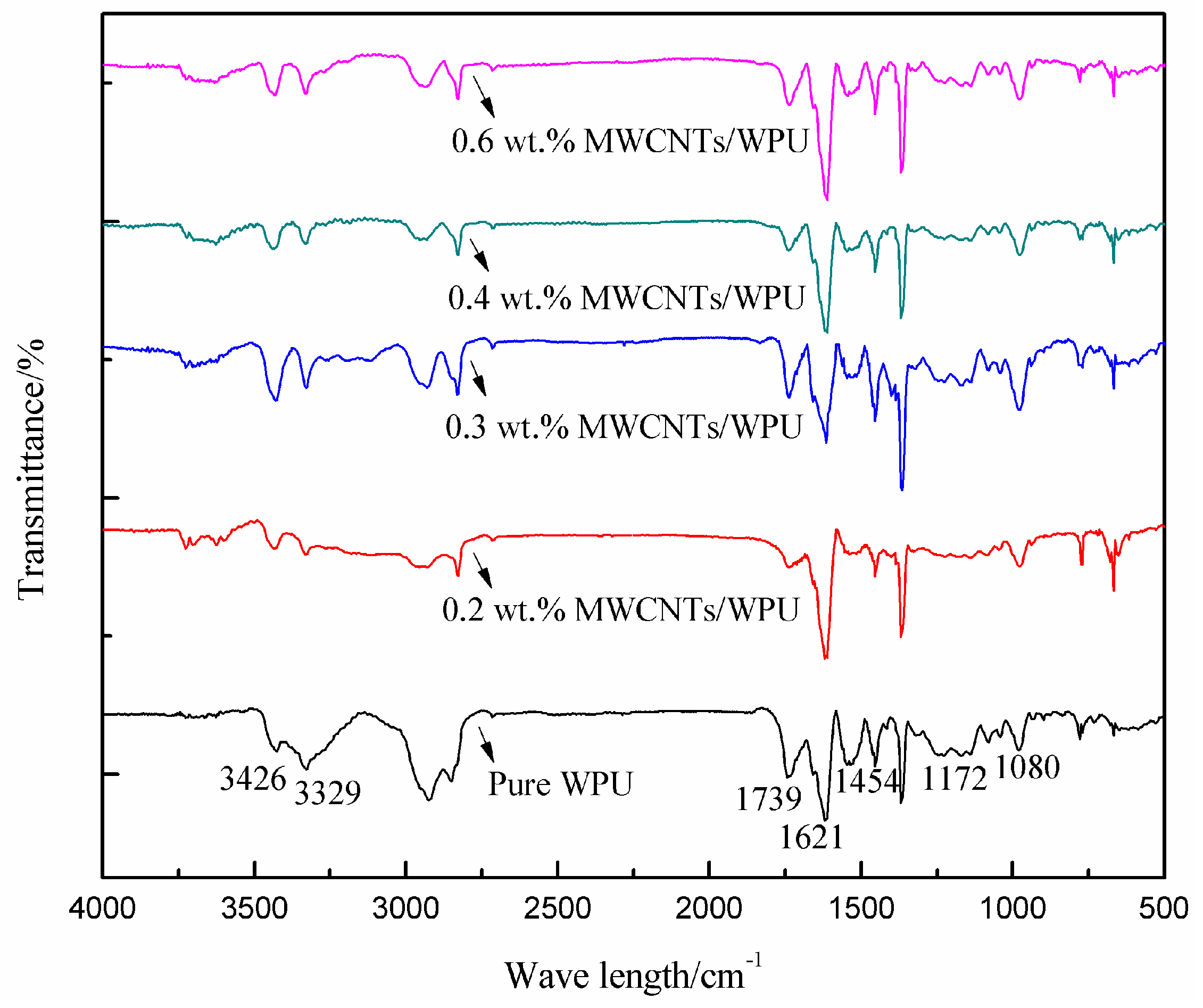
3.1. FTIR
3.2. SEM
3.3. Electrical Conductivity
3.4. Corrosion Resistance
3.4.1. Polarization Curve Analysis
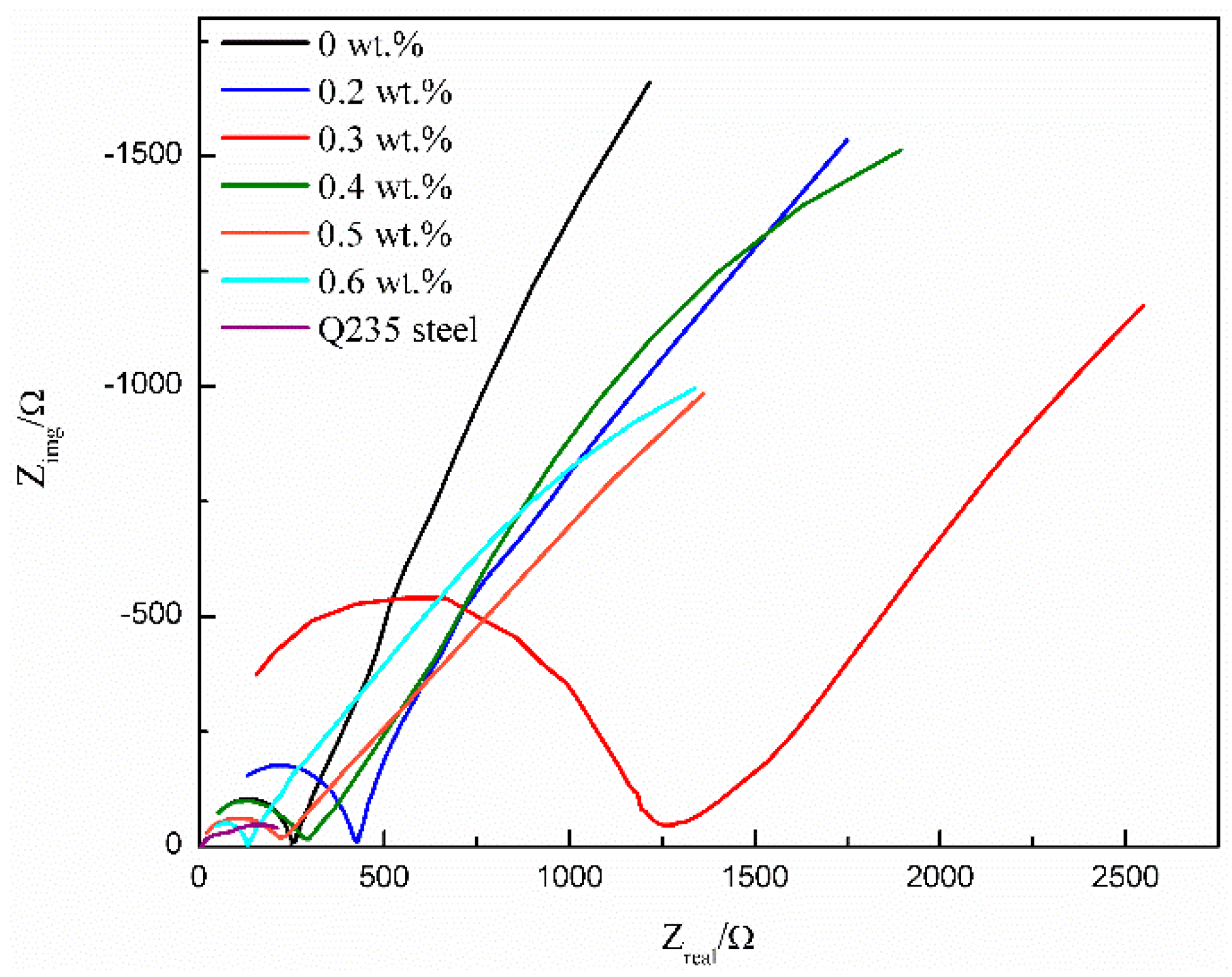
3.4.2. EIS Analysis
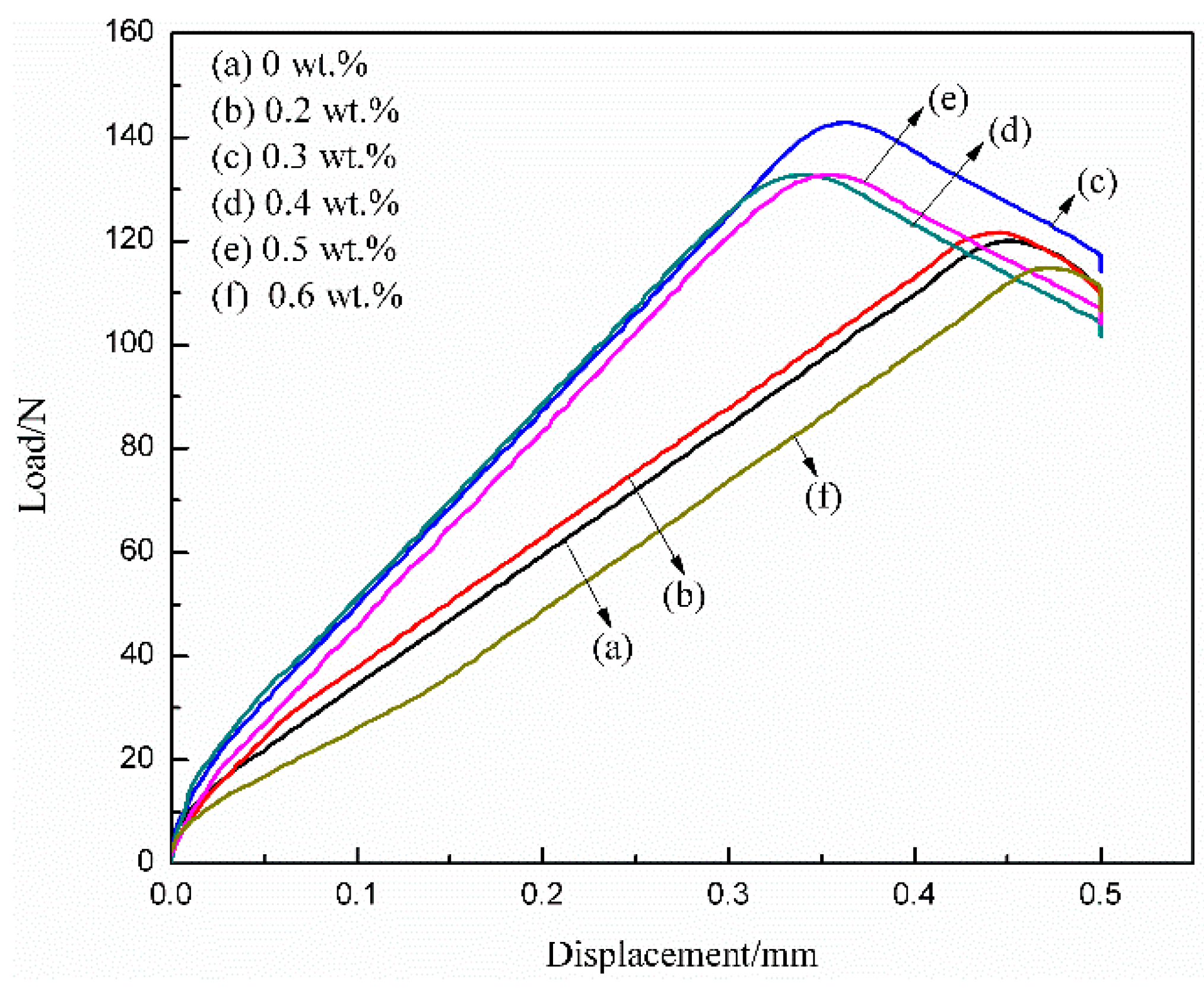
3.5. Adhesive Strength
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, R.; Dowden, A.; Baxendale, M.; Peijs, T. Conductive network formation in the melt of carbon nanotube/thermoplastic polyurethane composite. Compos. Sci. Technol. 2009, 69, 1499–1504. [Google Scholar] [CrossRef]
- Ashassisorkhabi, H.; Bagheri, R.; Rezaeimoghadam, B. Sonoelectrochemical Synthesis of PPy-MWCNTs-Chitosan Nanocomposite Coatings: Characterization and Corrosion Behavior. J. Mater. Eng. Perform. 2015, 24, 385–392. [Google Scholar] [CrossRef]
- Schnoor, T.I.W.; Smith, G.; Eder, D.; Koziol, K.K.; Burstein, G.T.; Windle, A.H.; Schulte, K. The production of aligned MWCNT/polypyrrole composite films. Carbon 2013, 60, 229–235. [Google Scholar] [CrossRef]
- Potschke, P.; Dudkin, S.M.; Alig, I. Dielectric spectroscopy on melt processed polycarbonate-multiwalled carbon nanotube composites. Polymer 2003, 44, 5023–5030. [Google Scholar] [CrossRef]
- Potschke, P.; Abdelgoad, M.; Alig, I.; Dudkin, S.; Lellinger, D. Rheological and dielectrical characterization of melt mixed polycarbonate-multiwalled carbon nanotube composites. Polymer 2004, 45, 8863–8870. [Google Scholar] [CrossRef]
- Caffrey, P.O.; Gupta, M.C. Electrically conducting superhydrophobic microtextured carbon nanotube nanocomposite. Appl. Surf. Sci. 2014, 314, 40–45. [Google Scholar] [CrossRef]
- Yun, S.; Im, H.; Kim, J. The effect of different hard segments in polyurethane on the electrical conductivity of polyurethane grafted multi-walled carbon nanotube/polyurethane nanocomposites. Synth. Met. 2011, 161, 1361–1367. [Google Scholar] [CrossRef]
- Bryning, M.B.; Islam, M.F.; Kikkawa, J.M.; Yodh, A.G. Very low conductivity threshold in bulk isotropic single-walled carbon nanotube-epoxy composites. Adv. Mater. 2010, 17, 1186–1191. [Google Scholar] [CrossRef]
- Chai, C.P.; Ma, Y.F.; Li, G.P.; Ge, Z.; Ma, S.Y.; Luo, Y.J. The preparation of high solid content waterborne polyurethane by special physical blending. Prog. Org. Coat. 2018, 115, 79–85. [Google Scholar] [CrossRef]
- Rana, D.; Lee, C.H.; Cho, K.; Lee, B.H.; Choe, S. Thermal and mechanical properties for binary blends of metallocene polyethylene with conventional polyolefins. J. Appl. Polym. Sci. 1998, 69, 2441–2450. [Google Scholar] [CrossRef]
- Rana, D.; Cho, K.; Woo, T.; Lee, B.H.; Choe, S. Blends of ethylene 1-octene copolymer synthesized by Ziegler-Natta and metallocene catalysts. I. Thermal and mechanical properties. J. Appl. Polym. Sci. 1999, 74, 1169–1177. [Google Scholar] [CrossRef]
- Yang, Z.; Wicks, D.A.; Hoyle, C.E.; Pu, H.; Yuan, J.; Wan, D.; Liu, Y. Newly UV-curable polyurethane coatings prepared by multifunctional thiol-and ene-terminated polyurethane aqueous dispersions mixtures: Preparation and characterization. Polymer 2009, 50, 1717–1722. [Google Scholar] [CrossRef]
- Khun, N.W.; Frankel, G.S. Cathodic delamination of polyurethane/multiwalled carbon nanotube composite coatings from steel substrates. Prog. Org. Coat. 2016, 99, 55–60. [Google Scholar] [CrossRef]
- Jatin, S.; Essi, S.; Meysami, S.S.; Suihkonen, R.; Kumar, A.R.S.S.; Honkanen, M.; Keinänen, P.; Grobert, N.; Vuorinen, J. The effect of multi-wall carbon nanotube morphology on electrical and mechanical properties of polyurethane nanocomposites. Compos. Part A Appl. Sci. Manuf. 2017, 102, 305–313. [Google Scholar] [CrossRef]
- Li, G.; Feng, L.J.; Tong, P.; Zhai, Z. The properties of mwcnt/polyurethane conductive composite coating prepared by electrostatic spraying. Prog. Org. Coat. 2016, 90, 284–290. [Google Scholar] [CrossRef]
- Li, R.; Shan, Z. Research on structural features and thermal conductivity of waterborne polyurethane. Prog. Org. Coat. 2017, 104, 271–279. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Jung, Y.C.; So, H.H.; Cho, J.W. Polypyrrole coated carbon nanotubes: Synthesis, characterization, and enhanced electrical properties. Synth. Met. 2007, 157, 374–379. [Google Scholar] [CrossRef]
- Muhulet, A.; Miculescu, F.; Voicu, S.I.; Schütt, F.; Thakur, V.K.; Mishra, Y.K. Fundamentals and scopes of doped carbon nanotubes towards energy and biosensing applications. Mater. Today Energy 2018, 9, 154–186. [Google Scholar] [CrossRef]
- Deng, H.; Skipa, T.; Zhang, R.; Lellinger, D.; Bilotti, E.; Alig, I.; Peijs, T. Effect of melting and crystallization on the conductive network in conductive polymer composites. Polymer 2009, 50, 3747–3754. [Google Scholar] [CrossRef]
- Rana, D.; Kim, H.L.; Kwag, H.; Choe, S. Hybrid blends of similar ethylene 1-octene copolymers. Polymer 2000, 41, 7067–7082. [Google Scholar] [CrossRef]
- Rana, D.; Kim, H.L.; Kwag, H.; Rhee, J.; Cho, K.; Woo, T.; Lee, B.H.; Choe, S. Blends of ethylene 1-octene copolymer synthesized by Ziegler–Natta and metallocene catalysts. II. Rheology and morphological behaviors. J. Appl. Polym. Sci. 2000, 76, 1950–1964. [Google Scholar] [CrossRef]
- Zhang, W.D.; Song, Z.N.; Song, J.; Shi, Y.; Qu, J.; Qin, J.; Zhang, T.; Li, Y.; Zhang, H.; Zhang, R. A systematic laboratory study on an anticorrosive cool coating of oil storage tanks for evaporation loss control and energy conservation. Energy 2013, 58, 617–627. [Google Scholar] [CrossRef]
- Nezhad, H.Y.; Thakur, V.K. Effect of Morphological Changes due to Increasing Carbon Nanoparticles Content on the Quasi-Static Mechanical Response of Epoxy Resin. Polymers 2018, 10, 1106. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C. White/light white polyethylene joints obtained by diode laser welding process. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]




| Sample | 0.2 wt % | 0.3 wt % | 0.4 wt % | 0.5 wt % | 0.6 wt % |
|---|---|---|---|---|---|
| Square resistance (MΩ/□) | 195.0 ± 3 | 156.2 ± 5 | 25.8 ± 3 | 4.7 ± 0.3 | 2.6 ± 0.2 |
| Thickness (μm) | 65.0 ± 4 | 62.2 ± 3 | 65.0 ± 4 | 63.5 ± 2 | 63.0 ± 3 |
| Resistivity (Ω·m) | 12,675.0 | 9715.6 | 1677.0 | 298.5 | 163.8 |
| Sample | 0 wt % | 0.2 wt % | 0.3 wt % | 0.4 wt % | 0.5 wt % | 0.6 wt % | Q235 Steel |
|---|---|---|---|---|---|---|---|
| I0 (A/cm2) | 1.8706 × 10−6 | 1.7713 × 10−6 | 4.8596 × 10−6 | 7.6046 × 10−6 | 1.2015 × 10−6 | 4.3907 × 10−6 | 1.9749 × 10−6 |
| Corrosion rate (mm/a) | 0.022058 | 0.020888 | 0.005731 | 0.008968 | 0.014169 | 0.051777 | 2.328900 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Feng, L.; Li, G. Properties of Waterborne Polyurethane Conductive Coating with Low MWCNTs Content by Electrostatic Spraying. Polymers 2018, 10, 1406. https://doi.org/10.3390/polym10121406
Wang F, Feng L, Li G. Properties of Waterborne Polyurethane Conductive Coating with Low MWCNTs Content by Electrostatic Spraying. Polymers. 2018; 10(12):1406. https://doi.org/10.3390/polym10121406
Chicago/Turabian StyleWang, Fangfang, Lajun Feng, and Guangzhao Li. 2018. "Properties of Waterborne Polyurethane Conductive Coating with Low MWCNTs Content by Electrostatic Spraying" Polymers 10, no. 12: 1406. https://doi.org/10.3390/polym10121406