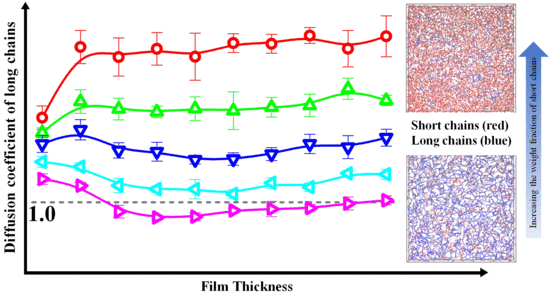
Effect of Bidispersity on Dynamics of Confined Polymer Films
Abstract
:1. Introduction
2. Model and Simulation Method
3. Results and Discussion
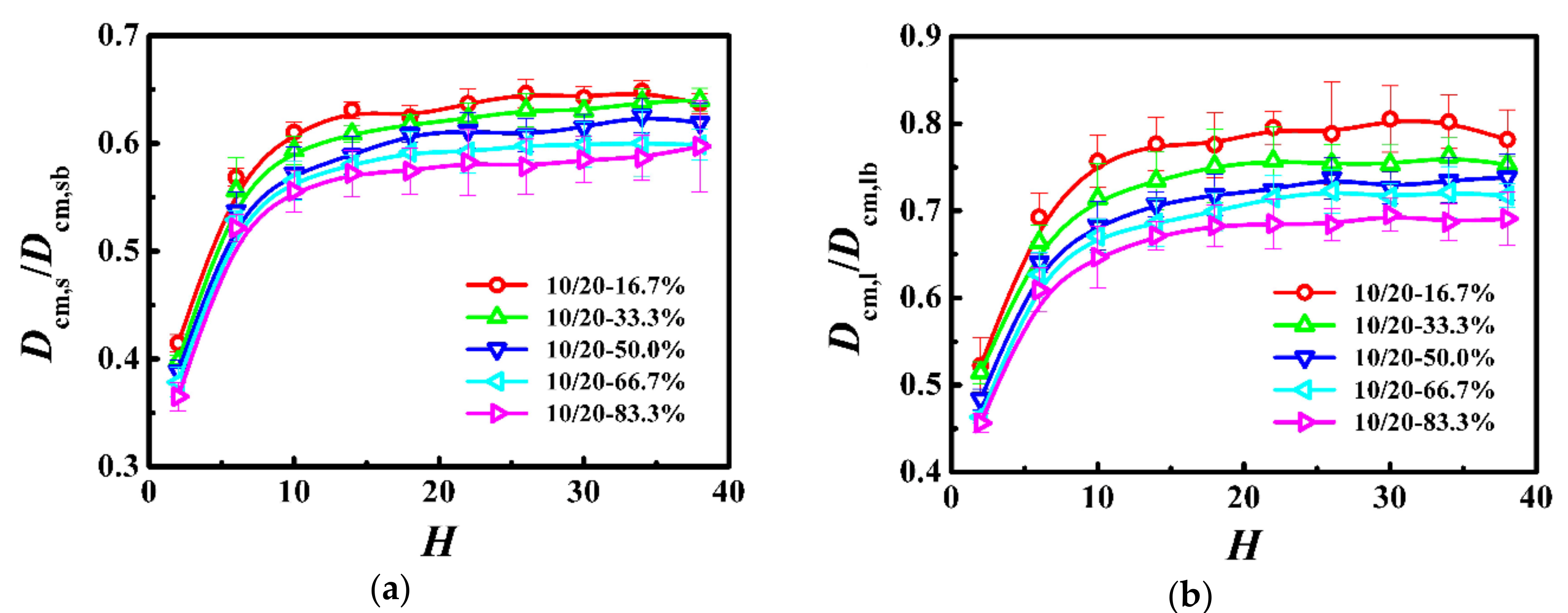
3.1. Model System of Ns = 10/Nl = 20
3.2. Model System of Ns = 20/Nl = 150
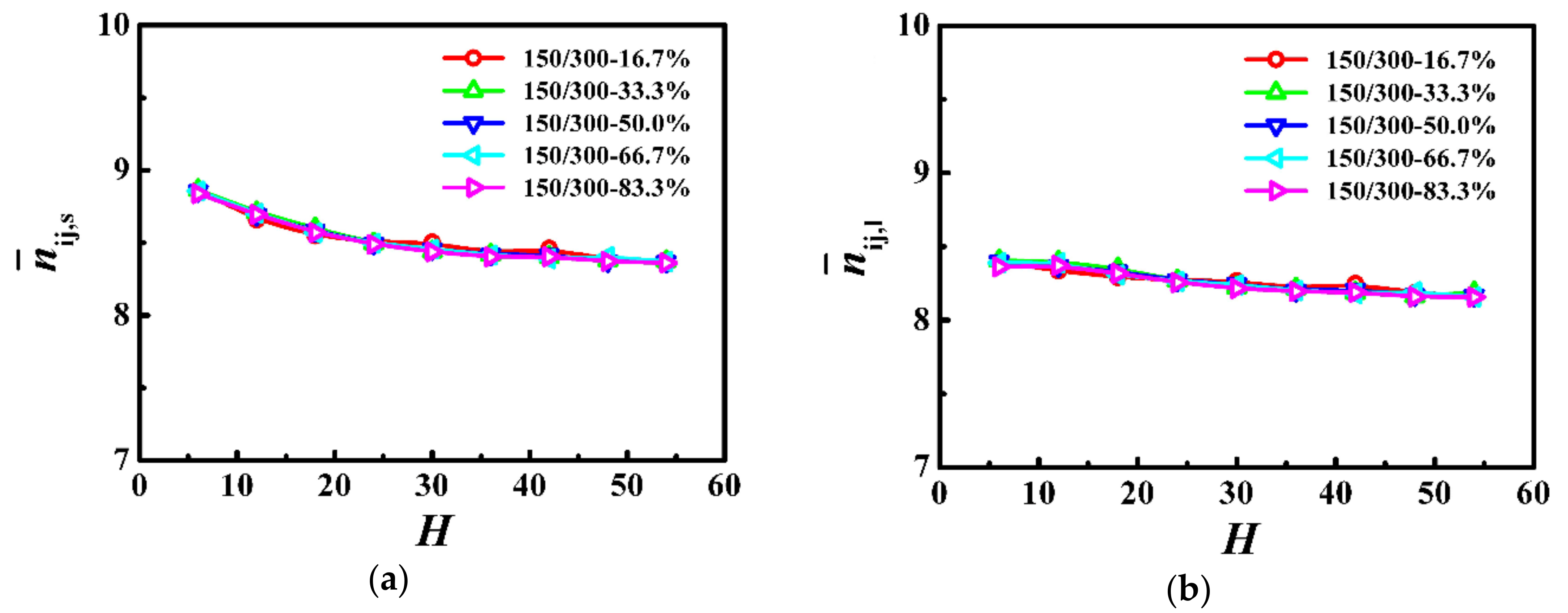
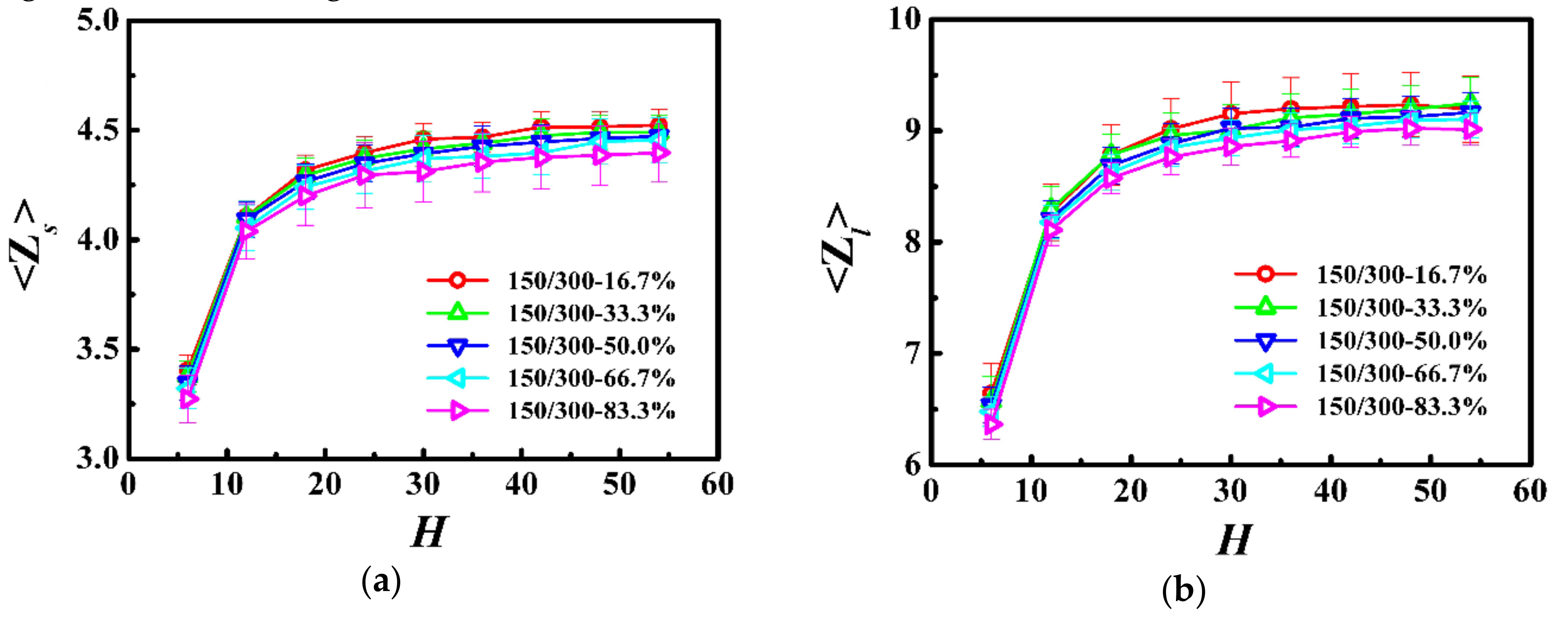
3.3. Model System of Ns = 150/Nl = 300
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, M.; Harrison, C.; Chaikin, P.M.; Register, R.A.; Adamson, D.H. Block copolymer lithography: Periodic arrays of ~1011 holes in 1 square centimeter. Science 1997, 276, 1401–1404. [Google Scholar] [CrossRef]
- Feller, L.M.; Cerritelli, S.; Textor, M.; Hubbell, J.A.; Tosatti, S.G. Influence of poly (propylene sulfide-block-ethylene glycol) di-and triblock copolymer architecture on the formation of molecular adlayers on gold surfaces and their effect on protein resistance: A candidate for surface modification in biosensor research. Macromolecules 2005, 38, 10503–10510. [Google Scholar] [CrossRef]
- Olson, D.C.; Piris, J.; Collins, R.T.; Shaheen, S.E.; Ginley, D.S. Hybrid photovoltaic devices of polymer and ZnO nanofiber composites. Thin Solid Films 2006, 496, 26–29. [Google Scholar] [CrossRef]
- Stockelhuber, K.W.; Svistkov, A.S.; Pelevin, A.G.; Heinrich, G. Impact of filler surface modification on large scale mechanics of styrene butadiene/silica rubber composites. Macromolecules 2011, 44, 4366–4381. [Google Scholar] [CrossRef]
- Saeed, F.; Ansarifar, A.; Ellis, R.J.; Haile-Meskel, Y.; Irfan, M.S. Two advanced styrene-butadiene/polybutadiene rubber blends filled with a silanized silica nanofiller for potential use in passenger car tire tread compound. J. Appl. Polym. Sci. 2012, 123, 1518–1529. [Google Scholar] [CrossRef]
- Wu, S. Polymer Interface and Adhesion; Marcel Dekker: New York, NY, USA, 1982. [Google Scholar]
- Batistakis, C.; Michels, M.A.J.; Lyulin, A.V. Confinement-induced stiffening of thin elastomer films: Linear and nonlinear mechanics vs. local dynamics. Macromolecules 2014, 47, 4690–4703. [Google Scholar] [CrossRef]
- Batistakis, C.; Michels, M.A.J.; Lyulin, A.V. Glassy boundary layers vs enhanced mobility in capped polymer films. J. Chem. Phys. 2013, 139, 024906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batistakis, C.; Lyulin, A.V.; Michels, M.A.J. Slowing down versus acceleration in the dynamics of confined polymer films. Macromolecules 2012, 45, 7282–7292. [Google Scholar] [CrossRef]
- Bäumchen, O.; Fetzer, R.; Jacobs, K. Reduced interfacial entanglement density affects the boundary conditions of polymer flow. Phys. Rev. Lett. 2009, 103, 247801. [Google Scholar] [CrossRef] [PubMed]
- Bodiguel, H.; Fretigny, C. Reduced viscosity in thin polymer films. Phys. Rev. Lett. 2006, 97, 266105. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Massa, M.V.; Dalnoki-Veress, K.; Brown, H.R.; Jones, R.A. Chain entanglement in thin freestanding polymer films. Phys. Rev. Lett. 2005, 94, 127801. [Google Scholar] [CrossRef] [PubMed]
- Sussman, D.M. Spatial distribution of entanglements in thin free-standing films. Phys. Rev. E 2016, 94, 012503. [Google Scholar] [CrossRef] [PubMed]
- Sussman, D.M.; Tung, W.-S.; Winey, K.I.; Schweizer, K.S.; Riggleman, R.A. Entanglement reduction and anisotropic chain and primitive path conformations in polymer melts under thin film and cylindrical confinement. Macromolecules 2014, 47, 6462–6472. [Google Scholar] [CrossRef]
- Sarabadani, J.; Milchev, A.; Vilgis, T.A. Structure and dynamics of polymer melt confined between two solid surfaces: A molecular dynamics study. J. Chem. Phys. 2014, 141, 044907. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, D.; Han, C.C.; Liao, Q. Dynamics of polymer melts confined by smooth walls: Crossover from nonentangled region to entangled region. J. Chem. Phys. 2007, 126, 204907. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Ding, M.; Shi, T. Effects of polymer–wall interactions on entanglements and dynamics of confined polymer films. J. Phys. Chem. B 2017, 121, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Baschnagel, J.; Paul, W.; Tries, V.; Binder, K. Statics and dynamics of bidisperse polymer melts: A Monte Carlo study of the bond-fluctuation model. Macromolecules 1998, 31, 3856–3867. [Google Scholar] [CrossRef]
- Thompson, P.A.; Grest, G.S.; Robbins, M.O. Phase transitions and universal dynamics in confined films. Phys. Rev. Lett. 1992, 68, 3448–3451. [Google Scholar] [CrossRef] [PubMed]
- Minoshima, W.; White, J.L.; Spruiell, J.E. Experimental investigation of the influence of molecular weight distribution on the rheological properties of polypropylene melts. Polym. Eng. Sci. 1980, 20, 1166–1176. [Google Scholar] [CrossRef]
- Minoshima, W.; White, J.L.; Spruiell, J.E. Experimental investigations of the influence of molecular weight distribution on melt spinning and extrudate swell characteristics of polypropylene. J. Appl. Polym. Sci. 1980, 25, 287–306. [Google Scholar] [CrossRef]
- Doi, M.; Edwards, S.F. The Theory of Polymer Dynamics; Oxford University Press: Oxford, UK, 1988; Volume 73. [Google Scholar]
- Doi, M.; Graessley, W.W.; Helfand, E.; Pearson, D.S. Dynamics of polymers in polydisperse melts. Macromolecules 1987, 20, 1900–1906. [Google Scholar] [CrossRef]
- Marrucci, G. Relaxation by reptation and tube enlargement: A model for polydisperse polymers. J. Polym. Sci. Part B Polym. Phys. 1985, 23, 159–177. [Google Scholar] [CrossRef]
- Sabzevari, S.M.; Cohen, I.; Wood-Adams, P.M. Wall slip of bidisperse linear polymer melts. Macromolecules 2014, 47, 3154–3160. [Google Scholar] [CrossRef]
- Sabzevari, S.M.; Cohen, I.; Wood-Adams, P.M. Wall slip of tridisperse polymer melts and the effect of unentangled versus weakly entangled chains. Macromolecules 2014, 47, 8033–8040. [Google Scholar] [CrossRef]
- Sabzevari, S.M.; McGraw, J.D.; Wood-Adams, P.M. Short chains enhance slip of highly entangled polystyrenes during thin film dewetting. RSC Adv. 2016, 6, 91163–91170. [Google Scholar] [CrossRef] [Green Version]
- Eslami, H.; Muüller-Plathe, F. Viscosity of nanoconfined polyamide-6,6 oligomers: Atomistic reverse nonequilibrium molecular dynamics simulation. J. Phys. Chem. B 2010, 114, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Rorrer, N.A.; Dorgan, J.R. Effects of polydispersity on confined homopolymer melts: A Monte Carlo study. J. Chem. Phys. 2014, 141, 214905. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, M.; Shi, T. Effect of bidispersity on structure and entanglement of confined polymer films. J. Phys. Chem. B 2017, 121, 7502–7507. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, J.S. Effects of chain topology on polymer dynamics: Bulk melts. J. Chem. Phys. 1994, 101, 4205–4213. [Google Scholar] [CrossRef]
- Shaffer, J.S. Effects of chain topology on polymer dynamics: Configurational relaxation in polymer melts. J. Chem. Phys. 1995, 103, 761–772. [Google Scholar] [CrossRef]
- Termonia, Y. Two-dimensional nanometric confinement of entangled polymer melts. Polymer 2011, 52, 5193–5196. [Google Scholar] [CrossRef]
- Kröger, M. Shortest multiple disconnected path for the analysis of entanglements in two-and three-dimensional polymeric systems. Comput. Phys. Commun. 2005, 168, 209–232. [Google Scholar] [CrossRef]
- Shanbhag, S.; Kröger, M. Primitive path networks generated by annealing and geometrical methods: Insights into differences. Macromolecules 2007, 40, 2897–2903. [Google Scholar] [CrossRef]
- Karayiannis, N.C.; Kröger, M. Combined molecular algorithms for the generation, equilibration and topological analysis of entangled polymers: Methodology and performance. Int. J. Mol. Sci. 2009, 10, 5054–5089. [Google Scholar] [CrossRef] [PubMed]
- Hoy, R.S.; Foteinopoulou, K.; Kröger, M. Topological analysis of polymeric melts: Chain-length effects and fast-converging estimators for entanglement length. Phys. Rev. E 2009, 80, 031803. [Google Scholar] [CrossRef] [PubMed]
- Baig, C.; Mavrantzas, V.G.; Kroöger, M. Flow effects on melt structure and entanglement network of linear polymers: Results from a nonequilibrium molecular dynamics simulation study of a polyethylene melt in steady shear. Macromolecules 2010, 43, 6886–6902. [Google Scholar] [CrossRef]
- Paul, W.; Binder, K.; Heermann, D.W.; Kremer, K. Dynamics of polymer solutions and melts. Reptation predictions and scaling of relaxation times. J. Chem. Phys. 1991, 95, 7726–7740. [Google Scholar] [CrossRef]
- Rouse, P.E. A theory of the linear viscoelastic properties of dilute solutions of coiling polymers. J. Chem. Phys. 1953, 21, 1272–1280. [Google Scholar] [CrossRef]
- Kremer, K.; Grest, G.S. Dynamics of entangled linear polymer melts: A molecular-dynamics simulation. J. Chem. Phys. 1990, 92, 5057–5086. [Google Scholar] [CrossRef]
- Harmandaris, V.A.; Mavrantzas, V.G.; Theodorou, D.N. Atomistic molecular dynamics simulation of polydisperse linear polyethylene melts. Macromolecules 1998, 31, 7934–7943. [Google Scholar] [CrossRef]
- Smith, S.W.; Hall, C.K.; Freeman, B.D. Molecular dynamics study of transport coefficients for hard-chain fluids. J. Chem. Phys. 1995, 102, 1057–1073. [Google Scholar] [CrossRef]
- Barsky, S. Molecular dynamics study of diffusion in bidisperse polymer melts. J. Chem. Phys. 2000, 112, 3450–3456. [Google Scholar] [CrossRef]
- Vladkov, M.; Barrat, J.-L. Local dynamics and primitive path analysis for a model polymer melt near a surface. Macromolecules 2007, 40, 3797–3804. [Google Scholar] [CrossRef]
- Eslami, H.; Muüller-Plathe, F. How thick is the interphase in an ultrathin polymer film? Coarse-grained molecular dynamics simulations of polyamide-6,6 on graphene. J. Phys. Chem. C 2013, 117, 5249–5257. [Google Scholar] [CrossRef]
- Viovy, J.L.; Rubinstein, M.; Colby, R.H. Constraint release in polymer melts: Tube reorganization versus tube dilation. Macromolecules 1991, 24, 3587–3596. [Google Scholar] [CrossRef]
- Baig, C.; Stephanou, P.S.; Tsolou, G.; Mavrantzas, V.G.; Kröger, M. Understanding dynamics in binary mixtures of entangled cis-1,4-polybutadiene melts at the level of primitive path segments by mapping atomistic simulation data onto the tube model. Macromolecules 2010, 43, 8239–8250. [Google Scholar] [CrossRef]
- Stephanou, P.S.; Mavrantzas, V.G. Accurate prediction of the linear viscoelastic properties of highly entangled mono and bidisperse polymer melts. J. Chem. Phys. 2014, 140, 214903. [Google Scholar] [CrossRef] [PubMed]
- Langeloth, M.; Masubuchi, Y.; Böhm, M.C.; Müller-Plathe, F. Reptation and constraint release dynamics in bidisperse polymer melts. J. Chem. Phys. 2014, 141, 194904. [Google Scholar] [CrossRef] [PubMed]




| System (Ns/Nl-φl) | ns | nl |
|---|---|---|
| 10/20-16.7% | 1120 | 112 |
| 10/20-33.3% | 900 | 225 |
| 10/20-50.0% | 674 | 337 |
| 10/20-66.7% | 450 | 450 |
| 10/20-83.3% | 224 | 562 |
| 20/150-16.7% | 4500 | 120 |
| 20/150-33.3% | 3600 | 240 |
| 20/150-50.0% | 2700 | 360 |
| 20/150-66.7% | 1800 | 480 |
| 20/150-83.3% | 900 | 600 |
| 150/300-16.7% | 960 | 96 |
| 150/300-33.3% | 768 | 192 |
| 150/300-50.0% | 576 | 288 |
| 150/300-66.7% | 386 | 386 |
| 150/300-83.3% | 192 | 480 |
| Ns = 10/Nl = 20 | |||
|---|---|---|---|
| Lx = Ly | H = Lz | H/Rgs,b | H/Rgl,b |
| 116 | 2 | 1.04 | 0.71 |
| 68 | 6 | 3.11 | 2.14 |
| 52 | 10 | 5.18 | 3.57 |
| 44 | 14 | 7.26 | 4.99 |
| 38 | 18 | 9.33 | 6.42 |
| 36 | 22 | 11.40 | 7.85 |
| 32 | 26 | 13.47 | 9.27 |
| 30 | 30 | 15.54 | 10.68 |
| 28 | 34 | 17.62 | 12.10 |
| 26 | 38 | 19.69 | 13.52 |
| Ns = 20/Nl = 150 | |||
| Lx = Ly | H = Lz | H/Rgs,b | H/Rgl,b |
| 330 | 2 | 0.71 | 0.25 |
| 190 | 6 | 2.14 | 0.74 |
| 146 | 10 | 3.57 | 1.24 |
| 124 | 14 | 4.99 | 1.74 |
| 110 | 18 | 6.42 | 2.23 |
| 100 | 22 | 7.85 | 2.73 |
| 90 | 26 | 9.27 | 3.23 |
| 84 | 30 | 10.68 | 3.72 |
| 80 | 34 | 12.10 | 4.22 |
| 76 | 38 | 13.52 | 4.72 |
| Ns = 150/Nl = 300 | |||
| Lx = Ly | H = Lz | H/Rgs,b | H/Rgl,b |
| 240 | 6 | 0.74 | 0.52 |
| 170 | 12 | 1.49 | 1.04 |
| 138 | 18 | 2.23 | 1.56 |
| 120 | 24 | 2.98 | 2.08 |
| 106 | 30 | 3.72 | 2.60 |
| 98 | 36 | 4.47 | 3.12 |
| 90 | 42 | 5.21 | 3.64 |
| 84 | 48 | 5.96 | 4.16 |
| 80 | 54 | 6.70 | 4.68 |
| M | N | Lx = Ly = Lz | Rg,b | Dcm,b × 106 | 〈Z〉b |
|---|---|---|---|---|---|
| 1350 | 10 | 30 | 1.93 | 1220.50 | |
| 675 | 20 | 30 | 2.81 | 444.43 | |
| 213 | 150 | 40 | 8.06 | 12.05 | 4.7162 |
| 208 | 300 | 50 | 11.53 | 2.24 | 9.2341 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Chen, Q.; Ding, M.; Shi, T. Effect of Bidispersity on Dynamics of Confined Polymer Films. Polymers 2018, 10, 1327. https://doi.org/10.3390/polym10121327
Li S, Chen Q, Ding M, Shi T. Effect of Bidispersity on Dynamics of Confined Polymer Films. Polymers. 2018; 10(12):1327. https://doi.org/10.3390/polym10121327
Chicago/Turabian StyleLi, Sijia, Qiaoyue Chen, Mingming Ding, and Tongfei Shi. 2018. "Effect of Bidispersity on Dynamics of Confined Polymer Films" Polymers 10, no. 12: 1327. https://doi.org/10.3390/polym10121327