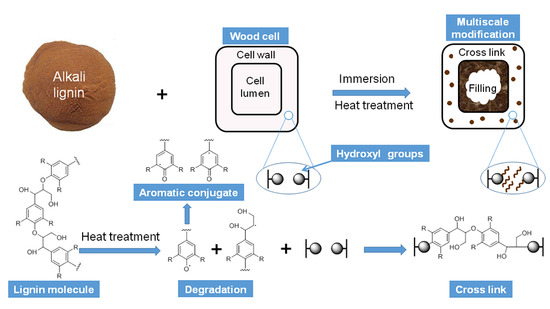
Multiscale Modification of Populus cathayana by Alkali Lignin Combined with Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Treatment Methods
2.3. SEM Analysis
2.4. CLSM Analysis
2.5. FTIR Spectroscopy Analysis
2.6. Leachability Test
2.7. Contact Angle
2.8. Water Resistance and Dimensional Stability
2.9. Compression Test
3. Results and Discussion
3.1. General Description of Treated Wood
3.2. SEM Analysis of Treated Wood
3.3. CLSM Analysis of Treated Wood
3.4. FTIR Spectroscopy Analysis
3.5. Contact Angle
3.6. Moisture Adsorption and Water Absorption
3.7. Dimensional Stability
3.8. Compressive Strength
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nandanwar, R. Utilization of industrial waste lignin in polymer systems. Int. J. Knowl. Eng. 2012, 31, 1323–1330. [Google Scholar]
- Strassberger, Z.; Tanase, S.; Rothenberg, G. The pros and cons of lignin valorisation in an integrated biorefinery. RSC Adv. 2014, 4, 25310–25318. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Liu, R.; Cao, J.; Chen, Y. Effects of UV weathering on surface properties of polypropylene composites reinforced with wood flour, lignin, and cellulose. Appl. Surf. Sci. 2014, 317, 385–392. [Google Scholar] [CrossRef]
- Kaewtatip, K.; Thongmee, J. Effect of kraft lignin and esterified lignin on the properties of thermoplastic starch. Mater. Des. 2013, 49, 701. [Google Scholar] [CrossRef]
- Behrooz, R.; Kordkheili, H.Y.; Najafi, S.K. Physical properties of lignin added wood flourpolypropylene composites: A comparison of direct and solvent mixing techniques. Asian. J. Chem. 2012, 24, 157. [Google Scholar]
- Braun, J.L.; Holtman, K.M.; Kadla, J.F. Ligninbased carbon fibers: Oxidative thermostabilization of kraft lignin. Carbon 2005, 43, 385–394. [Google Scholar] [CrossRef]
- Dallmeyer, I.; Chowdhury, S.; Kadla, J.F. Preparation and characterization of kraft lignin based moisture-responsive films with reversible shape-change capability. Biomacromolecules 2013, 14, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Jian, L. Preparation and characterization of softwood lignin-based carbon fibers. J. Tribol. 2013, 97, 656. [Google Scholar] [CrossRef]
- Wagenführ, A.; Scholz, F. Taschenbuch der Holztechnik; Hanser Fachbuch: München, Germany, 2007. [Google Scholar]
- Andersson, S.; Serimaa, R.; Väänänen, T.; Paakkari, T.; Jämsä, S.; Viitaniemi, P. X-ray scattering studies of thermally modified Scots pine (Pinus sylvestris L.). Holzforschung 2005, 35, 155–427. [Google Scholar] [CrossRef]
- Kavyashree, S.; Krishna, K.P. Effect of heat treatment on color changes, dimensional stability, and mechanical properties of wood. J. Wood. Chem. Technol. 2012, 32, 304–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, D.; Ma, L.; Wang, S.; Liu, X. Influence of Heat Treatment on the Water Uptake Behavior of Wood. Bioresources 2017, 12, 1697–1705. [Google Scholar] [CrossRef]
- Metsä-Kortelainen, S.; Antikainen, T.; Viitaniemi, P. The water absorption of sapwood and heartwood of Scots pine and Norway spruce heat-treated at 170 °C, 190°C, 210 °C and 230 °C. Holz Roh-Werkst 2006, 64, 192–197. [Google Scholar] [CrossRef]
- Macromolecule Academy. Physical Properties of Macromolecules; Kyoritsu Press: Tokyo, Japan, 1958. [Google Scholar]
- Hailwood, A.J.; Horrobin, S. Absorption of water by polymers: Analysis in terms of a simple model. J. Chem. Soc. Faraday Trans. 1946, 42, B084–B092. [Google Scholar] [CrossRef]
- Ma, J.F.; Yang, G.H.; Mao, J.Z.; Xu, F. Characterization of anatomy, ultrastructure and lignin microdistribution in Forsythia suspensa. Ind. Crop. Prod. 2011, 33, 358–363. [Google Scholar] [CrossRef]
- Sudo, K.; Shimizu, K.; Nakashima, N.; Yokoyama, A. A new modification method of exploded lignin for the preparation of a carbon fiber precursor. J. Appl. Polym. Sci. 1993, 48, 1485–1491. [Google Scholar] [CrossRef]
- Zhang, F.D.; Xu, C.H.; Li, M.Y.; Huang, A.; Sun, S. Rapid identification of Pterocarpus santalinus, and Dalbergia louvelii, by FTIR and 2D correlation IR spectroscopy. J. Mol. Struct. 2014, 1069, 89–95. [Google Scholar] [CrossRef]
- Huang, Y.X.; Ma, E.N.; Zhao, G.J. Thermal and structure analysis on reaction mechanisms during the preparation of activated carbon fibers by KOH activation from liquefied wood-based fibers. Ind. Crop. Prod. 2015, 69, 447–455. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ono, T. Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresour. Technol. 2013, 127, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.B. The Influence of Incipient Brown Rot on the Properties of Chinese Fir at Macroscopic and Tissue Level; Chinese Academy of Forestry: Beijing, China, 2011. [Google Scholar]
- Sui, X.J. Study on the Catalytic Liquefaction of Industrial Kraft Lignin for the Production of Phenols. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2011. [Google Scholar]
- Labbé, N.; Rials, T.G.; Kelley, S.S.; Cheng, Z.; Li, Y. FT-IR imaging and pyrolysis-molecular beam mass spectrometry: New tools to investigate wood tissues. Wood. Sci. Technol. 2005, 39, 61–77. [Google Scholar] [CrossRef]
- Müller, G.; Schöpper, C.; Vos, H.; Kharazipou, A.; Polle, A. FTIR-ATR spectroscopic analyses of changes in wood properties during particle-and fibreboard production of hard-and softwood trees. BioResources 2008, 4, 49–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.Q.; Li, H.; Yuan, T.Q.; Wang, Y.Y.; Sun, R. Heat treatment of industrial alkaline lignin and its potential application as adhesive for green wood-lignin composites. ACS Sustain. Chem. Eng. 2017, 5, 7269–7277. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, D.; Li, N.; Sun, X. Bio-Based Wood Adhesive from Camelina Protein (a Biodiesel Residue) and Depolymerized Lignin with Improved Water Resistance. ACS Omega 2017, 2, 7996–8004. [Google Scholar] [CrossRef]
- Crick, C.R.; Parkin, I.P. Preparation and characterisation of super-hydrophobic surfaces. Chemistry 2010, 16, 3568–3588. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Luo, J.J.; Shi, S.H.; Li, R.; Lu, P.; Guo, X. Effects of steam heat treatment on the surface contact angle of Chinese fir. Agric. Sci. Technol. 2014, 15, 127. [Google Scholar]
- Zhou, H.; Xu, R.; Ma, E. Effects of Removal of Chemical Components on Moisture Adsorption by Wood. Bioresources 2016, 11, 3110–3122. [Google Scholar] [CrossRef]
- Bakar, B.; Hiziroglu, S.; Tahir, P. Properties of some thermally modified wood species. Mater. Des. 2013, 43, 348–355. [Google Scholar] [CrossRef]
- Saidan, M. Improvement of linerboard compressive strength by hot-pressing and addition of recovered lignin from spent pulping liquor. Chem. Ind. Chem. Eng. Q. 2014, 21, 12. [Google Scholar] [CrossRef]









| Sample | WPG (%) | Mass Loss (%) | Leaching Rate (%) | 18/W (%) | σ (MPa) |
|---|---|---|---|---|---|
| Control | - | - | - | 5.4 (0.1) | 41.9 (1.3) |
| AL | 12.3 (0.3) | - | 3.3 (0.7) | 5.3 (0.1) | 42.5 (1.2) |
| AL 140 °C | 12.4 (1.0) | 0.8 (0.1) | 2.3 (0.2) | 5.2 (0.1) | 43.8 (1.4) |
| AL 160 °C | 11.2 (0.9) | 1.2 (0.1) | 1.9 (0.3) | 5.1 (0.1) | 45.4 (1.2) |
| AL 180 °C | 11.8 (0.7) | 2.2 (0.2) | 1.4 (0.1) | 4.9 (0.0) | 45.6 (1.1) |
| Sample | LI1263 | LI1427 | LI1507 | LI1632 |
|---|---|---|---|---|
| Control | 49.0% | 52.8% | 39.3% | 73.2% |
| AL | 78.6% | 73.2% | 72.1% | 86.0% |
| AL 180 °C | 55.2% | 60.6% | 48.8% | 139.4% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Li, J.; Ma, E. Multiscale Modification of Populus cathayana by Alkali Lignin Combined with Heat Treatment. Polymers 2018, 10, 1240. https://doi.org/10.3390/polym10111240
Zhou H, Li J, Ma E. Multiscale Modification of Populus cathayana by Alkali Lignin Combined with Heat Treatment. Polymers. 2018; 10(11):1240. https://doi.org/10.3390/polym10111240
Chicago/Turabian StyleZhou, Haizhen, Jingyu Li, and Erni Ma. 2018. "Multiscale Modification of Populus cathayana by Alkali Lignin Combined with Heat Treatment" Polymers 10, no. 11: 1240. https://doi.org/10.3390/polym10111240