Hydrothermal Synthesis of Cellulose Nanocrystal-Grafted-Acrylic Acid Aerogels with Superabsorbent Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
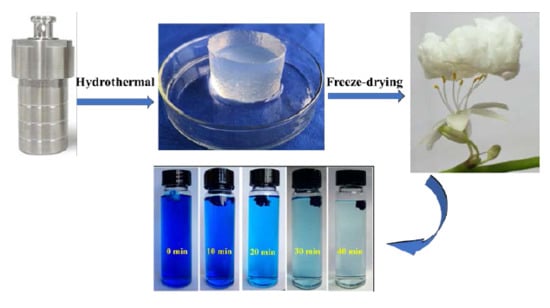
2.2. Preparation of CNC-g-AA Hydro/Aerogels
2.3. Characterization of CNC-g-AA Aerogels
2.4. Swelling Characterization of CNC-g-AA Aerogels in Distilled Water
2.5. Adsorption Studies
3. Results and Discussion
3.1. Morphology of CNC-g-AA Aerogels
3.2. FT-IR and NMR Characterization of CNC-g-AA Aerogels
3.3. Equilibrium Swelling Ratio in Distilled Water
3.4. Formation Mechanism of CNC-g-AA Aerogels
3.5. Adsorption Studies
3.5.1. Studies on Dye Adsorption
3.5.2. Adsorption Kinetics
3.5.3. Adsorption Isotherm and Thermodynamics
3.5.4. Adsorption and Regeneration Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.; Bai, Q.; Liang, T.; Bai, H.; Liu, X. Two-sided surface oxidized cellulose membranes modified with PEI: Preparation, characterization and application for dyes removal. Polymers 2017, 9, 455. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Vijwani, H.; Nadagouda, M.N.; Namboodiri, V.; Mukhopadhyay, S.M. Hierarchical hybrid carbon nano-structures as robust and reusable adsorbents: Kinetic studies with model dye compound. Chem. Eng. J. 2015, 268, 197–207. [Google Scholar] [CrossRef]
- Hu, E.; Shang, S.M.; Tao, X.M.; Jiang, S.X.; Chiu, K.L. Minimizing Freshwater Consumption in the Wash-Off Step in Textile Reactive Dyeing by Catalytic Ozonation with Carbon Aerogel Hosted Bimetallic Catalyst. Polymers 2018, 10, 193. [Google Scholar] [CrossRef]
- Lee, W.J.; Clancy, A.J.; Kontturi, E.; Bismarck, A.; Shaffer, M.S. Strong and Stiff: High-Performance Cellulose Nanocrystal/Poly(vinyl alcohol) Composite Fibers. ACS Appl. Mater. Interfaces 2016, 8, 31500–31504. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Buesch, C.; Smith, S.W.; Eschbach, P.; Conley, J.F., Jr.; Simonsen, J. The Microstructure of Cellulose Nanocrystal Aerogels as Revealed by Transmission Electron Microscope Tomography. Biomacromolecules 2016, 17, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Zhang, D.Z.; Lu, F.F.; Yao, J. New Approach for Single-Step Extraction of Carboxylated Cellulose Nanocrystals for Their Use as Adsorbents and Flocculants. ACS Sustain. Chem. Eng. 2016, 4, 2632–2643. [Google Scholar] [CrossRef]
- Liu, Q.; Jing, S.; Wang, S.; Zhuo, H.; Zhong, L.; Peng, X.; Sun, R. Flexible nanocomposites with ultrahigh specific areal capacitance and tunable properties based on a cellulose derived nanofiber-carbon sheet framework coated with polyaniline. J. Mater. Chem. A 2016, 4, 13352–13362. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically Cross-Linked Cellulose Nanocrystal Aerogels with Shape Recovery and Superabsorbent Properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. Chem. Mater. 2017, 5, 16105–16117. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Miettunen, K.; Vapaavuori, J.; Tiihonen, A.; Poskela, A.; Lahtinen, P.; Halme, J.; Lund, P. Nanocellulose aerogel membranes for optimal electrolyte filling in dye solar cells. Nano Energy 2014, 8, 95–102. [Google Scholar] [CrossRef] [Green Version]
- France, K.J.D.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Milani, A.H.; Fielding, L.A.; Greensmith, P.; Saunders, B.R.; Adlam, D.J.; Freemont, A.J.; Hoyland, J.A.; Hodson, N.W.; Elsawy, M.A.; Miller, A.F.; et al. Anisotropic pH-Responsive Hydrogels Containing Soft or Hard Rod-Like Particles Assembled Using Low Shear. Chem. Mater. 2017, 29, 3100–3110. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhao, J.; Xiao, M.; Zhang, W.; Lu, C. Mechanically Strong and Thermally Responsive Cellulose Nanofibers/Poly(N-isopropylacrylamide) Composite Aerogels. ACS Sustain. Chem. Eng. 2016, 4, 4321–4327. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, C.; He, X.; Zhang, X.; Zhang, W.; Zhang, X. Polyethylenimine-grafted cellulose nanofibril aerogels as versatile vehicles for drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Cranston, E.D.; Zhu, S. Flexible and Porous Nanocellulose Aerogels with High Loadings of Metal-Organic-Framework Particles for Separations Applications. Adv. Mater. 2016, 28, 7652–7657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javadi, A.; Zheng, Q.; Payen, F.; Javadi, A.; Altin, Y.; Cai, Z.; Sabo, R.; Gong, S. Polyvinyl alcohol-cellulose nanofibrils-graphene oxide hybrid organic aerogels. ACS Appl. Mater. Interfaces 2013, 5, 5969–5975. [Google Scholar] [CrossRef] [PubMed]
- Maatar, W.; Boufi, S. Poly(methacylic acid-co-maleic acid) grafted nanofibrillated cellulose as a reusable novel heavy metal ions adsorbent. Carbohydr. Polym. 2015, 126, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Napadensky, E.; Orlicki, J.A.; Snyder, J.F.; Chantawansri, T.L.; Kapllani, A. Cellulose Nanofibrils and Diblock Copolymer Complex: Micelle Formation and Enhanced Dispersibility. ACS Sustain. Chem. Eng. 2016, 5, 1264–1271. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, Y.; Fu, Y.; Fang, G.; Yan, X.; Guo, Z. Swelling behaviors of porous lignin based poly (acrylic acid). Chemosphere 2016, 163, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Noppakundilograt, S.; Choopromkaw, S.; Kiatkamjornwong, S. Hydrolyzed collagen-grafted-poly[(acrylicacid)-co-(methacrylic acid)] hydrogel for drug delivery. Appl. Polym. Sci. 2018, 135, 1–11. [Google Scholar] [CrossRef]
- Wan, C.; Li, J. Embedding ZnO nanorods into porous cellulose aerogels via a facile one-step low-temperature hydrothermal method. Mater. Des. 2015, 83, 620–625. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Li, Y.; Ma, C.; Huang, Z.; Wang, C.; Li, J.; Chen, Z.; Liu, S. One-step hydrothermal synthesis of fluorescent nanocrystalline cellulose/carbon dot hydrogels. Carbohydr. Polym. 2017, 175, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Chen, C.H. A one-step hydrothermal route to programmable stimuli-responsive hydrogels. Chem. Commun. 2015, 51, 6617–6620. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.; Derakhshandeh, M.; Hatzikiriakos, S.G.; Hamad, W.Y.; MacLachlan, M.J. Hydrothermal Gelation of Aqueous Cellulose Nanocrystal Suspensions. Biomacromolecules 2016, 17, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shi, Z.; Fu, S.; Chen, J.; Berry, R.M.; Tam, K.C. Strategy for Synthesizing Porous Cellulose Nanocrystal Supported Metal Nanocatalysts. ACS Sustain. Chem. Eng. 2016, 4, 5929–5935. [Google Scholar] [CrossRef]
- Dong, X.M.; Revol, J.F.; Gray, D.G. Effect of microcrystallite preparationconditions on the formation of colloid crystals of cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, Y.; Wang, S.Q.; Jiang, H.; Liu, S.; Yao, Y.; Zhang, T.M.; Li, Q. Synthesis and characterization of amine-modified spherical nanocellulose aerogels. J. Mater. Sci. 2018, 53, 13304–13315. [Google Scholar] [CrossRef]
- Hu, K.; Sun, J.; Guo, Z.; Wang, P.; Chen, Q.; Ma, M.; Gu, N. A novel magnetic hydrogel with aligned magnetic colloidal assemblies showing controllable enhancement of magnetothermal effect in the presence of alternating magnetic field. Adv. Mater. 2015, 27, 2507–2514. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, X.; Ruan, W.; Zhao, B.; Xu, W.; Lombardi, J.R. Adsorption study of 4-MBA on TiO2 nanoparticles by surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 2004–2008. [Google Scholar] [CrossRef]
- Marrocchelli, D.; Madden, P.A.; Norberg, S.T.; Hull, S. Structural disorder in doped zirconias, part ii: Vacancy ordering effects and the conductivity maximum. Chem. Mater. 2011, 23, 1365–1373. [Google Scholar] [CrossRef]
- Chau, M.; De France, K.J.; Kopera, B.; Machado, V.R.; Rosenfeldt, S.; Reyes, L.; Chan, K.J.W.; Förster, S.; Cranston, E.D.; Hoare, T.; et al. Composite Hydrogels with Tunable Anisotropic Morphologies and Mechanical Properties. Chem. Mater. 2016, 28, 3406–3415. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan-present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Rao, K.M.; Han, S.S. Synthesis of mechanically stiff and bioactive hybrid hydrogels for bone tissue engineering applications. Chem. Eng. J. 2017, 317, 119–131. [Google Scholar] [CrossRef]
- Fu, J.; Wang, S.; He, C.; Lu, Z.; Huang, J.; Chen, Z. Facilitated fabrication of high strength silica aerogels using cellulose nanofibrils as scaffold. Carbohydr. Polym. 2016, 147, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Han, C.R.; Zhang, X.M.; Xu, F.; Sun, R.C. Cellulose Nanocrystals Mechanical Reinforcement in Composite Hydrogels with Multiple Cross-Links: Correlations between Dissipation Properties and Deformation Mechanisms. Macromolecules 2014, 47, 4077–4086. [Google Scholar] [CrossRef]
- Tang, L.R.; Huang, B.; Yang, N.T.; Li, T.; Lu, Q.L.; Lin, W.Y.; Che, X.R. Organicsolvent-free and efficient manufacture of functionalized cellulose nanocrystalsvia one-pot tandem reactions. Green. Chem. 2013, 15, 2369–2373. [Google Scholar] [CrossRef]
- Das, D.; Ghosh, P.; Dhara, S.; Panda, A.B.; Pal, S. Dextrin and Poly(acrylic acid)-Based Biodegradable, Non-Cytotoxic, Chemically Cross-Linked Hydrogel for Sustained Release of Ornidazole and Ciprofloxacin. ACS Appl. Mater. Interfaces 2015, 7, 4791–4803. [Google Scholar] [CrossRef] [PubMed]
- Spagnol, C.; Rodrigues, F.H.A.; Pereira, A.G.B.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogel nanocomposites based on starch-g-poly(sodium acrylate) matrix filled with cellulose nanowhiskers. Cellulose 2012, 19, 1225–1237. [Google Scholar] [CrossRef]
- Chau, M.; Sriskandha, S.E.; Pichugin, D.; Therien-Aubin, H.; Nykypanchuk, D.; Chauve, G.; Methot, M.; Bouchard, J.; Gang, O.; Kumacheva, E. Ion-Mediated Gelation of Aqueous Suspensions of Cellulose Nanocrystals. Biomacromolecules 2015, 16, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Fatona, A.; Berry, R.M.; Brook, M.A.; Moran-Mirabal, J.M. Versatile Surface Modification of Cellulose Fibers and Cellulose Nanocrystals through Modular Triazinyl Chemistry. Chem. Mater. 2018, 30, 2424–2435. [Google Scholar] [CrossRef]
- Mukerabigwi, J.F.; Lei, S.; Fan, L.; Wang, H.; Luo, S.; Ma, X.; Qin, J.; Huang, X.; Cao, Y. Eco-friendly nano-hybrid superabsorbent composite from hydroxyethyl cellulose and diatomite. RSC Adv. 2016, 6, 31607–31618. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.R.; Duan, J.F.; Ma, M.G.; Zhang, X.M.; Xu, F.; Sun, R.C.; Xie, X.M. Studies on the properties and formation mechanism of flexible nanocomposite hydrogels from cellulose nanocrystals and poly(acrylic acid). J. Mater. Chem. 2012, 22, 22467–22480. [Google Scholar] [CrossRef]
- Chen, W.; Li, Q.; Wang, Y.; Yi, X.; Zeng, J.; Yu, H.; Liu, Y.; Li, J. Comparative study of aerogels obtained from differently prepared nanocellulose fibers. ChemSusChem 2014, 7, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Ouhab, D.; Boisseau, S.M.; Heux, L. Versatile gas-phase reactions for surface to bulk esterification of cellulose microfibrils aerogels. Biomacromolecules 2013, 14, 3246–3255. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Echols, I.; Wang, P.; Lei, S.; Luo, J.; Peng, B.; He, L.; Zhang, L.; Huang, D.; Mejia, C.; et al. Highly Biocompatible, Underwater Superhydrophilic and Multifunctional Biopolymer Membrane for Efficient Oil-Water Separation and Aqueous Pollutant Removal. ACS Sustain. Chem. Eng. 2018, 6, 3879–3887. [Google Scholar] [CrossRef]
- Štefelová, J.; Slovák, V.; Siqueira, G.; Olsson, R.T.; Tingaut, P.; Zimmermann, T.; Sehaqui, H. Drying and Pyrolysis of Cellulose Nanofibers from Wood, Bacteria, and Algae for Char Application in Oil Absorption and Dye Adsorption. ACS Sustain. Chem. Eng. 2017, 5, 2679–2692. [Google Scholar] [CrossRef]
- He, X.; Male, K.B.; Nesterenko, P.N.; Brabazon, D.; Paull, B.; Luong, J.H. Adsorption and desorption of methylene blue on porous carbon monoliths and nanocrystalline cellulose. ACS Appl. Mater. Interfaces 2013, 5, 8796–8804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Sheikhi, A.; van de Ven, T.G. Reusable Green Aerogels from Cross-Linked Hairy NanocrystallineCellulose and Modified Chitosan for Dye Removal. Langmuir 2016, 32, 11771–11779. [Google Scholar] [CrossRef] [PubMed]









| C0 (mg/L) | qe,exp (mg/g) | Lagergren-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Mode | ||||
|---|---|---|---|---|---|---|---|
| K1 × 10−3 (1/min) | R2 | K2 × 10−4 (g/mg min) | R2 | ||||
| 20 | 97.6388 | 96.9829 | 21.95 | 0.9240 | 98.1354 | 19.00 | 0.9999 |
| 40 | 194.8410 | 194.1255 | 8.31 | 0.9489 | 196.8505 | 5.50 | 0.9992 |
| 60 | 291.0470 | 290.5800 | 9.00 | 0.9591 | 302.1148 | 1.50 | 0.9972 |
| 80 | 383.0140 | 327.9173 | 7.43 | 0.9910 | 341.2970 | 1.00 | 0.9957 |
| 100 | 473.2217 | 416.6342 | 12.42 | 0.8629 | 452.4887 | 0.35 | 0.9926 |
| Langmuir | Frendlich | |||||
|---|---|---|---|---|---|---|
| T (°C) | KL (L/mg) | qmax (mg/g) | R2 | KF | n | R2 |
| 15 | 0.3467 | 724.6377 | 0.9903 | 169.8320 | 1.5524 | 0.9731 |
| 25 | 0.3200 | 735.2941 | 0.9914 | 176.3358 | 1.5596 | 0.9635 |
| 35 | 0.2773 | 757.5758 | 0.9925 | 148.2559 | 1.5818 | 0.9731 |
| 45 | 0.2628 | 751.8797 | 0.9912 | 153.2657 | 1.5139 | 0.9771 |
| 55 | 0.2681 | 729.9270 | 0.9980 | 150.1148 | 1.4996 | 0.9823 |
| Concentration (mg/L) | Temperature (℃) | Kd | ∆G (KJ/mol) | ∆H (KJ/mol) | ∆S (J/mol K) |
|---|---|---|---|---|---|
| 20 | 15 | 216.7150 | −12.8853 | ||
| 25 | 206.7594 | −13.2160 | |||
| 35 | 166.6885 | −13.1073 | −12.4781 | 26.62 | |
| 45 | 166.6885 | −13.5727 | |||
| 55 | 166.6885 | −13.9580 | |||
| 40 | 15 | 232.8024 | −13.0569 | ||
| 25 | 197.1423 | −13.0979 | |||
| 35 | 162.6617 | −13.0447 | −12.3480 | 22.73 | |
| 45 | 155.8044 | −13.3540 | |||
| 55 | 146.9974 | −13.6150 | |||
| 60 | 15 | 149.0488 | −11.8986 | ||
| 25 | 144.8095 | −12.3332 | |||
| 35 | 136.4267 | −12.5940 | −11.6424 | 25.72 | |
| 45 | 122.1927 | −12.7113 | |||
| 55 | 121.0599 | −13.0854 | |||
| 80 | 15 | 109.4590 | −11.2490 | ||
| 25 | 104.1624 | −11.5165 | |||
| 35 | 97.8559 | −11.7427 | −10.8564 | 25.72 | |
| 45 | 92.1381 | −11.9645 | |||
| 55 | 91.1484 | −12.3111 | |||
| 100 | 15 | 92.2062 | −10.8381 | ||
| 25 | 88.3592 | −11.1086 | |||
| 35 | 79.5277 | −11.2114 | −10.5610 | 19.87 | |
| 45 | 76.6037 | −11.4761 | |||
| 55 | 71.4717 | −11.6477 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yang, R.; Xu, M.; Ma, C.; Li, W.; Yin, Y.; Huang, Q.; Wu, Y.; Li, J.; Liu, S. Hydrothermal Synthesis of Cellulose Nanocrystal-Grafted-Acrylic Acid Aerogels with Superabsorbent Properties. Polymers 2018, 10, 1168. https://doi.org/10.3390/polym10101168
Liu X, Yang R, Xu M, Ma C, Li W, Yin Y, Huang Q, Wu Y, Li J, Liu S. Hydrothermal Synthesis of Cellulose Nanocrystal-Grafted-Acrylic Acid Aerogels with Superabsorbent Properties. Polymers. 2018; 10(10):1168. https://doi.org/10.3390/polym10101168
Chicago/Turabian StyleLiu, Xuehua, Rue Yang, Mingcong Xu, Chunhui Ma, Wei Li, Yu Yin, Qiongtao Huang, Yiqiang Wu, Jian Li, and Shouxin Liu. 2018. "Hydrothermal Synthesis of Cellulose Nanocrystal-Grafted-Acrylic Acid Aerogels with Superabsorbent Properties" Polymers 10, no. 10: 1168. https://doi.org/10.3390/polym10101168