Influence of Ethylene Oxide Content in Nonionic Surfactant to the Hydrolysis of Reactive Dye in Silicone Non-Aqueous Dyeing System
Abstract
:1. Introduction
2. Experimental
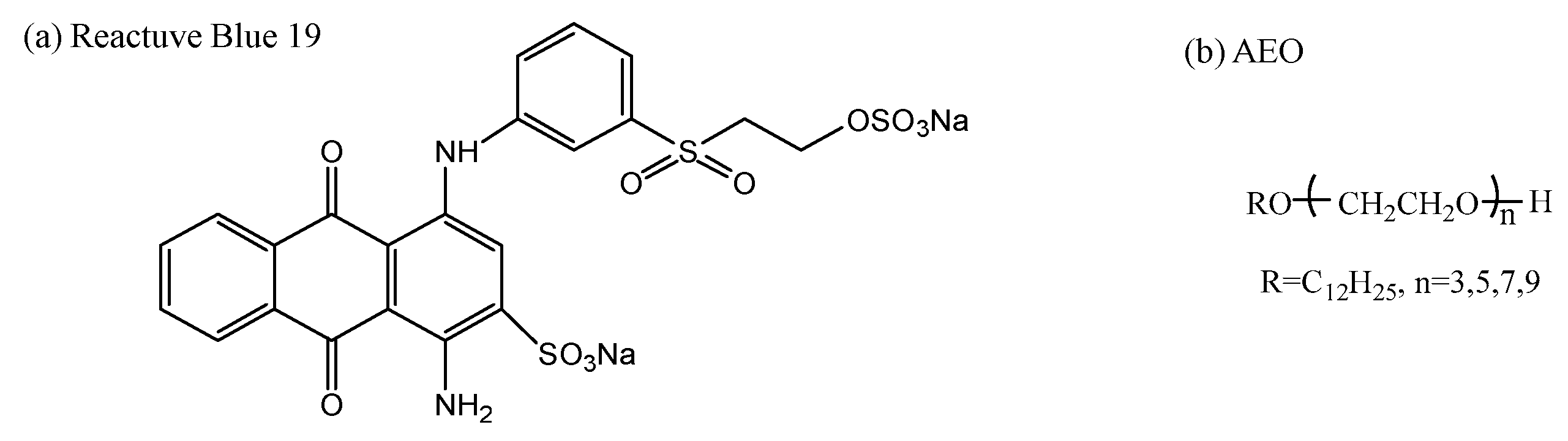
2.1. Materials
2.2. UV Analysis
2.3. Preparation of the Buffer Solution
2.4. HPLC Analysis
2.5. Hydrolysis of Reactive Blue 19
3. Results and Discussion
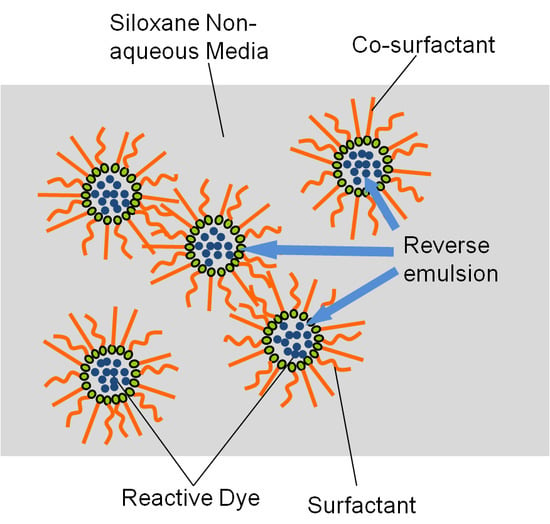
3.1. Hydrolytic Behavior of Reactive Blue 19 in the Siloxane Son-aqueous Dyeing System
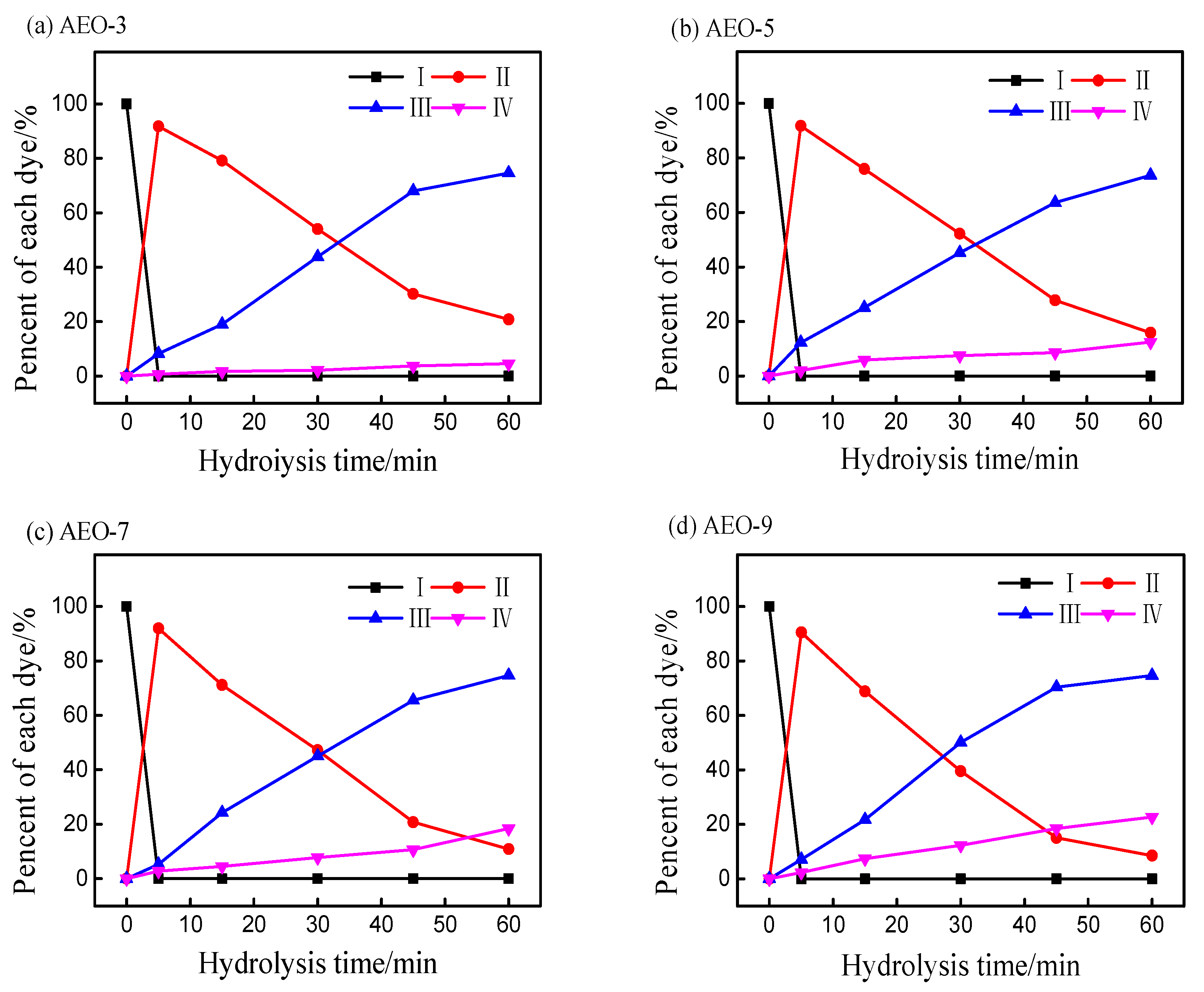
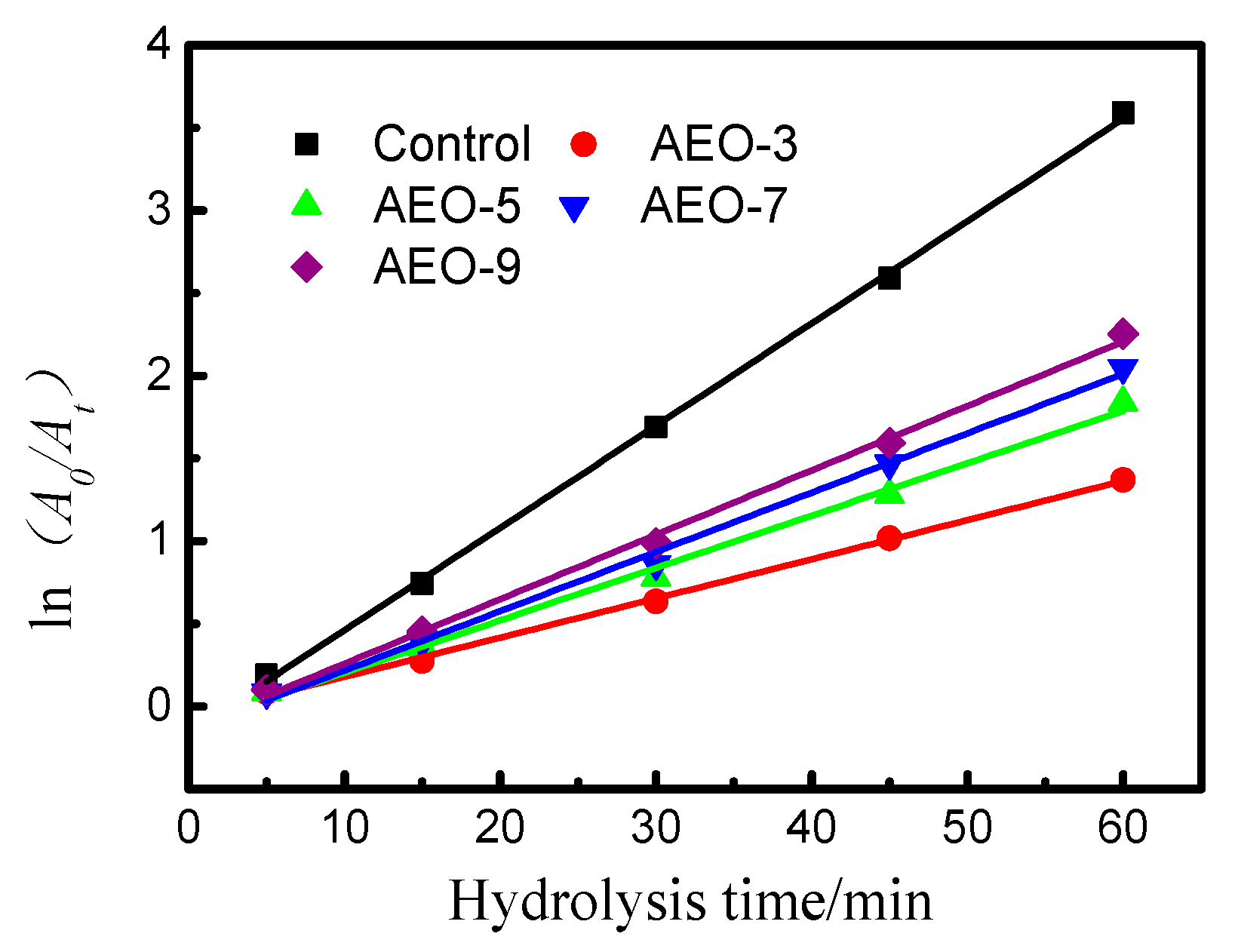
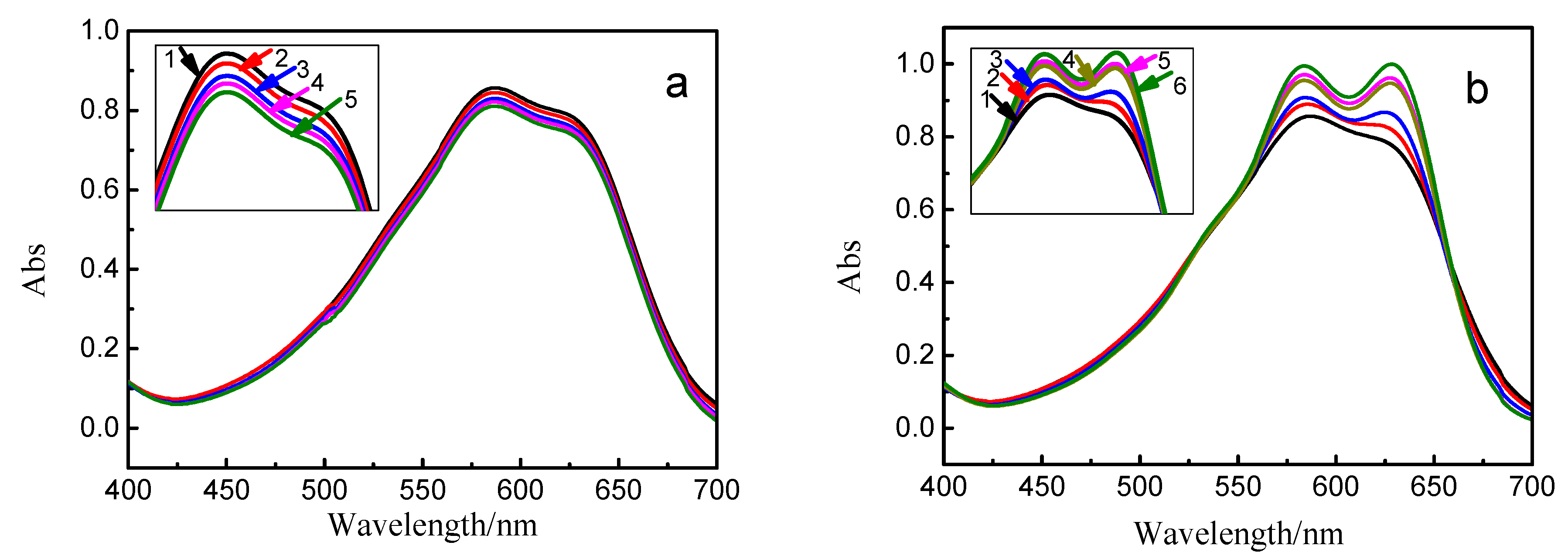
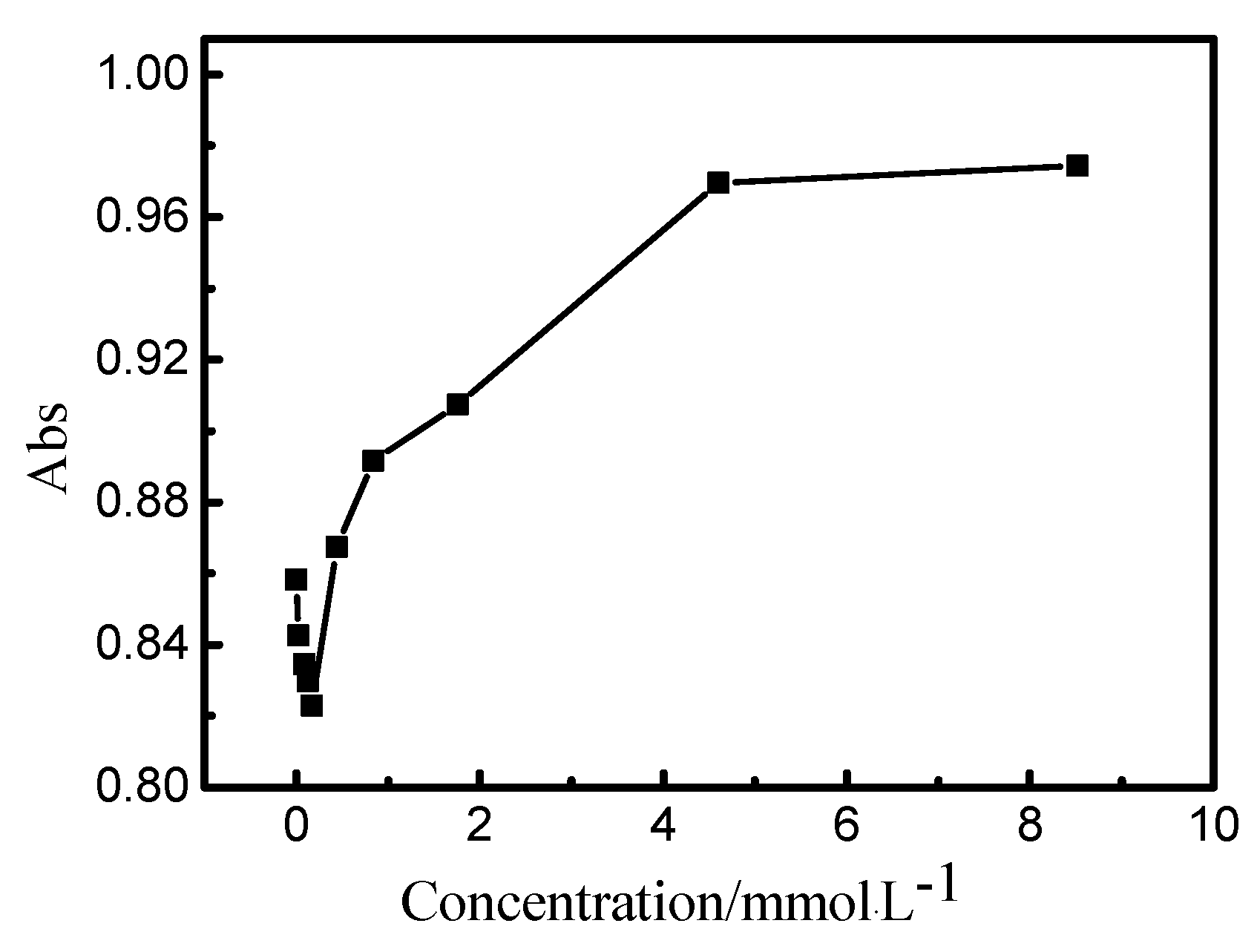
3.2. Effect of Ethylene Oxide Content on the Hydrolysis of Vinyl Sulfone Reactive Dye
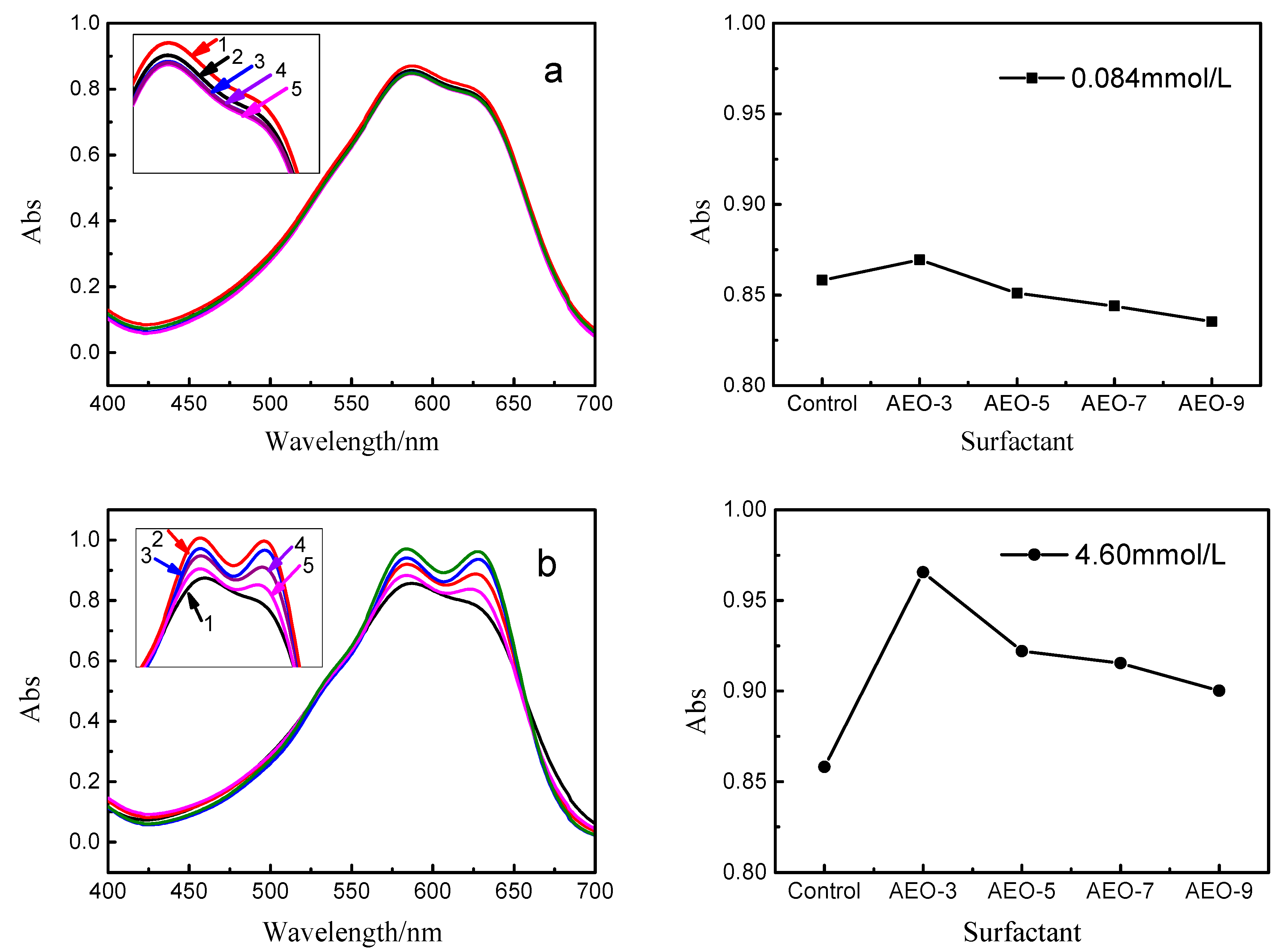
3.3. Effect of Nonionic Surfactants on UV Spectrum of Vinyl Sulfone Reactive Dye
3.4. Effect of the EO Chains on UV Spectrum of Vinyl Sulfone Reactive Dye
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, J. Interaction of surfactants and aminoindophenol dye. J. Colloid Interface Sci. 2004, 274, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Petcu, A.R.; Rogozea, E.A.; Lazar, C.A.; Olteanu, N.L.; Meghea, A.; Mihaly, M. Specific interactions within micelle microenvironment in different charged dye/surfactant systems. Arab. J. Chem. 2016, 9, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, L.; Fang, G.; Yan, N.; Ren, S.; Ma, Y. Hydrogenolysis and activation of soda lignin using [BMIM] Cl as a catalyst and solvent. Polymers 2017, 9, 279. [Google Scholar] [CrossRef]
- Oakes, J.; Gratton, P. Solubilisation of dyes by surfactant micelles. Part 1; Molecular interactions of azo dyes with nonionic and anionic surfactants. Color. Technol. 2003, 119, 91–99. [Google Scholar] [CrossRef]
- Oakes, J.; Gratton, P.; Dixon, S. Solubilisation of dyes by surfactant micelles. Part 3; A spectroscopic study of azo dyes in surfactant solutions. Color. Technol. 2003, 119, 301–306. [Google Scholar] [CrossRef]
- Fu, C.; Wang, J.; Shao, J.; Pu, D.; Chen, J.; Liu, J. A non-aqueous dyeing process of reactive dye on cotton. J. Text. Inst. 2015, 106, 152–161. [Google Scholar] [CrossRef]
- Pei, L.; Wu, P.; Liu, J.; Wang, J. Effect of nonionic surfactant on the micro-emulsifying water in silicone media. J. Surfactants Deterg. 2017, 20, 247–254. [Google Scholar] [CrossRef]
- Pei, L.; Liu, J.; Cai, G.; Wang, J. Study of hydrolytic kinetics of vinyl sulfone reactive dye in siloxane reverse micro-emulsion. Text. Res. J. 2017, 87, 2368–2378. [Google Scholar] [CrossRef]
- Pei, L.; Liu, J.; Wang, J. Study of Dichlorotriazine Reactive Dye Hydrolysis in Siloxane Reverse Micro-emulsion. J. Clean. Prod. 2017, 165, 994–1004. [Google Scholar] [CrossRef]
- Miao, H.; Liu, J.; Li, Y.; Fu, C.; Zhang, Y. Study on hydrolysis kinetics of reactive dyes in dye/D5 suspending system. J. Text. Res. 2013, 34, 77–82. [Google Scholar]
- Cai, G.; Sun, L.; Wu, J.; Wang, J. Influence of Nonionic Surfactant on Hydrolysis of Vinyl Sulfone Reactive Dye. J. Surfactants Deterg. 2015, 18, 1127–1135. [Google Scholar] [CrossRef]
- Tunc, S.; Duman, O.; Kanci, B. Spectrophotometric investigation of the interactions between cationic dye (C.I. Basic Yellow 2) and anionic surfactant (sodium dioctylsulfosuccinate) in the premicellar and micellar region. Dyes Pigment. 2012, 94, 233–238. [Google Scholar] [CrossRef]
- Fazeli, S.; Sohrabi, B.; Tehrani-Bagha, A.R. The study of Sunset Yellow anionic dye interaction with gemini and conventional cationic surfactants in aqueous solution. Dyes Pigment. 2012, 95, 768–775. [Google Scholar] [CrossRef]
- Simoncic, B.; Span, J. A study of dye–surfactant interactions. Part 3. Thermodynamics of the association of C.I. Acid Orange 7 and cetylpyridinium chloride in aqueous solutions. Dyes Pigment. 2000, 46, 1–8. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Kumari, N.; Pathania, S.; Sharma, K. A conductometric study of interactions between gelatin and sodium dodecyl sulfate (SDS) in aqueous rich mixtures of dimethylsulfoxide. Colloids Surf. A 2007, 293, 157–161. [Google Scholar] [CrossRef]
- Navarro, A.; Sanz, F. Chemical interaction between nonionic surfactants and an acid dye. J. Colloid Interface Sci. 2001, 237, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tunc, S.; Duman, O. Investigation of interactions between some anionic dyes and cationic surfactans by conductometric method. Fluid Phase Equilibria 2007, 251, 1–7. [Google Scholar] [CrossRef]
- Tunc, S.; Duman, O. The effect of different molecular weight of poly (ethylene glycol) on the electrokinetic and rheological properties of Na-bentonite suspensions. Colloids Surf. A 2008, 317, 93–99. [Google Scholar] [CrossRef]
- Lins, L.C.; Bugatti, V.; Livi, S.; Gorrasi, G. Ionic liquid as surfactant agent of hydrotalcite: Influence on the final properties of polycaprolactone matrix. Polymers 2018, 10, 44. [Google Scholar] [CrossRef]
- Chen, K.M.; Lin, L.H.; Wang, C.F.; Hwang, M.C. Interactions between new multi-anionic surfactants and direct dyes and their effects on the dyeing of cotton fabrics. Colloids Surf. A 2010, 356, 46–50. [Google Scholar] [CrossRef]
- Heyna, J. Reactive dyes containing vinylsulfonyl groups. Angew. Chem. Int. Ed. 1963, 2, 20–23. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, M.; Shao, J. Study on the hydrolysis kinetics of vinyl sulfone reactive dyes with HPLC analysis. J. Text. Res. 2006, 27, 9. [Google Scholar]
- Klančnik, M. The influence of temperature on the kinetics of concurrent hydrolysis and methanolysis reactions of a monochlorotriazine reactive dye. Dyes Pigment. 2000, 46, 9–15. [Google Scholar] [CrossRef]
- Klančnik, M.; Gorenšek, M. Kinetics of hydrolysis of monofunctional and bifunctional monochloro-s-triazine reactive dyes. Dyes Pigment. 1997, 33, 337–350. [Google Scholar] [CrossRef]
- Weber, E.J.; Stickney, V.C. Hydrolysis kinetics of reactive blue 19-vinyl sulfone. Water Res. 1993, 27, 63–67. [Google Scholar] [CrossRef]
- Patel, U.; Parekh, P.; Sastry, N.V.; Aswal, V.K.; Bahadur, P. Surface activity, micellization and solubilization of cationic gemini surfactant-conventional surfactants mixed systems. J. Mol. Liq. 2017, 225, 888–896. [Google Scholar] [CrossRef]
- Rashidi-Alavijeh, M.; Javadian, S.; Gharibi, H.; Moradi, M.; Tehrani-Bagha, A.R.; Shahir, A.A. Intermolecular interactions between a dye and cationic surfactants: effects of alkyl chain, head group, and counterion. Colloids Surf. A 2011, 380, 119–127. [Google Scholar] [CrossRef]
- Ghosh, S.; Mondal, S.; Das, S.; Biswas, R. Spectroscopic investigation of interaction between crystal violet and various surfactants (cationic, anionic, nonionic and gemini) in aqueous solution. Fluid Phase Equilibria 2012, 332, 1–6. [Google Scholar] [CrossRef]
- Borodin, O.; Smith, G.D.; Bandyopadhyaya, R.; Byutner, O. Molecular dynamics study of the influence of solid interfaces on poly (ethylene oxide) structure and dynamics. Macromolecules 2003, 36, 7873–7883. [Google Scholar] [CrossRef]
- Chakraborty, T.; Chakraborty, I.; Ghosh, S. The methods of determination of critical micellar concentrations of the amphiphilic systems in aqueous medium. Arab. J. Chem. 2011, 4, 265–270. [Google Scholar] [CrossRef]
- Ghosh, S.; Das Burman, A.; De, G.C.; Das, A.R. Interfacial and self-aggregation of binary mixtures of anionic and nonionic amphiphiles in aqueous medium. J. Phys. Chem. B 2011, 115, 11098–11112. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Glenn, K.M.; Palepu, R.M. Spectroscopic investigations on the interaction of crystal violet with nonionic micelles of brij and igepal surfactants in aqueous media. J. Solut. Chem. 2007, 36, 563–571. [Google Scholar] [CrossRef]
- Tang, A.Y.; Lee, C.; Wang, Y.; Kan, C.W. Octane-Assisted Reverse Micellar Dyeing of Cotton with Reactive Dyes. Polymers 2017, 9, 678. [Google Scholar] [CrossRef]
- Safavi, A.; Abdollahi, H.; Maleki, N.; Zeinali, S. Interaction of anionic dyes and cationic surfactants with ionic liquid character. J. Colloid Interface Sci. 2008, 322, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Purkait, M.K.; Dasgupta, S.; De, S. Removal of dye from wastewater using micellar-enhanced ultrafiltration and recovery of surfactant. Sep. Purif. Technol. 2004, 37, 81–92. [Google Scholar] [CrossRef]
- Kartal, Ç.; Akbaş, H. Study on the interaction of anionic dye–nonionic surfactants in a mixture of anionic and nonionic surfactants by absorption spectroscopy. Dyes Pigment. 2005, 65, 191–195. [Google Scholar] [CrossRef]
- Maza, A.D.L.; Parra, J.L. Interaction of Equimolecular Mixtures of Nonionic/Anionic Surfactants with Liposomes. Langmuir 1996, 12, 3393–3398. [Google Scholar] [CrossRef]
- Roscigno, P.; Paduano, L.; D’Erric, G.; Vitagliano, V. On the Presumed Specific Interaction of Anionic Surfactants with Nonionic Polymers. Aqueous Solution of Sodium Alkylsulfonate in the Presence of Poly(vinylpyrrolidone): An “Excluded Volume” Effect. Langmuir 2001, 17, 4510–4518. [Google Scholar] [CrossRef]
- Zhu, P.; Zhu, Y.; Xu, Z.C.; Zhang, L.; Zhang, L.; Zhao, S. Effect of polymer on dynamic interfacial tensions of anionic–nonionic surfactant solutions. J. Disper. Sci. Technol. 2016, 37, 820–829. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, B.; Cao, X.; Zhang, J.; Song, X.; Ding, M.; Chen, W. Interaction between polymer and anionic/nonionic surfactants and its mechanism of enhanced oil recovery. J. Disper. Sci. Technol. 2017, 39, 1178–1184. [Google Scholar] [CrossRef]



| Time (min) | Solvent A (%) | Solvent B (%) |
|---|---|---|
| 0 | 61 | 39 |
| 20 | 60 | 40 |
| Surfactant | Concentration (mmol/L) | k/min−1 | R2 |
|---|---|---|---|
| Control | / | 5.47 × 10−2 | 0.997 |
| AEO-3 | 4.60 | 2.24 × 10−2 | 0.994 |
| AEO-5 | 4.60 | 2.91 × 10−2 | 0.986 |
| AEO-7 | 4.60 | 3.26 × 10−2 | 0.985 |
| AEO-9 | 4.60 | 3.60 × 10−2 | 0.989 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, Y.; Dou, H.; Pei, L. Influence of Ethylene Oxide Content in Nonionic Surfactant to the Hydrolysis of Reactive Dye in Silicone Non-Aqueous Dyeing System. Polymers 2018, 10, 1158. https://doi.org/10.3390/polym10101158
Wang J, Zhang Y, Dou H, Pei L. Influence of Ethylene Oxide Content in Nonionic Surfactant to the Hydrolysis of Reactive Dye in Silicone Non-Aqueous Dyeing System. Polymers. 2018; 10(10):1158. https://doi.org/10.3390/polym10101158
Chicago/Turabian StyleWang, Jiping, Yongbo Zhang, Huashu Dou, and Liujun Pei. 2018. "Influence of Ethylene Oxide Content in Nonionic Surfactant to the Hydrolysis of Reactive Dye in Silicone Non-Aqueous Dyeing System" Polymers 10, no. 10: 1158. https://doi.org/10.3390/polym10101158