Flexible Biocomposites with Enhanced Interfacial Compatibility Based on Keratin Fibers and Sulfur-Containing Poly(urea-urethane)s
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
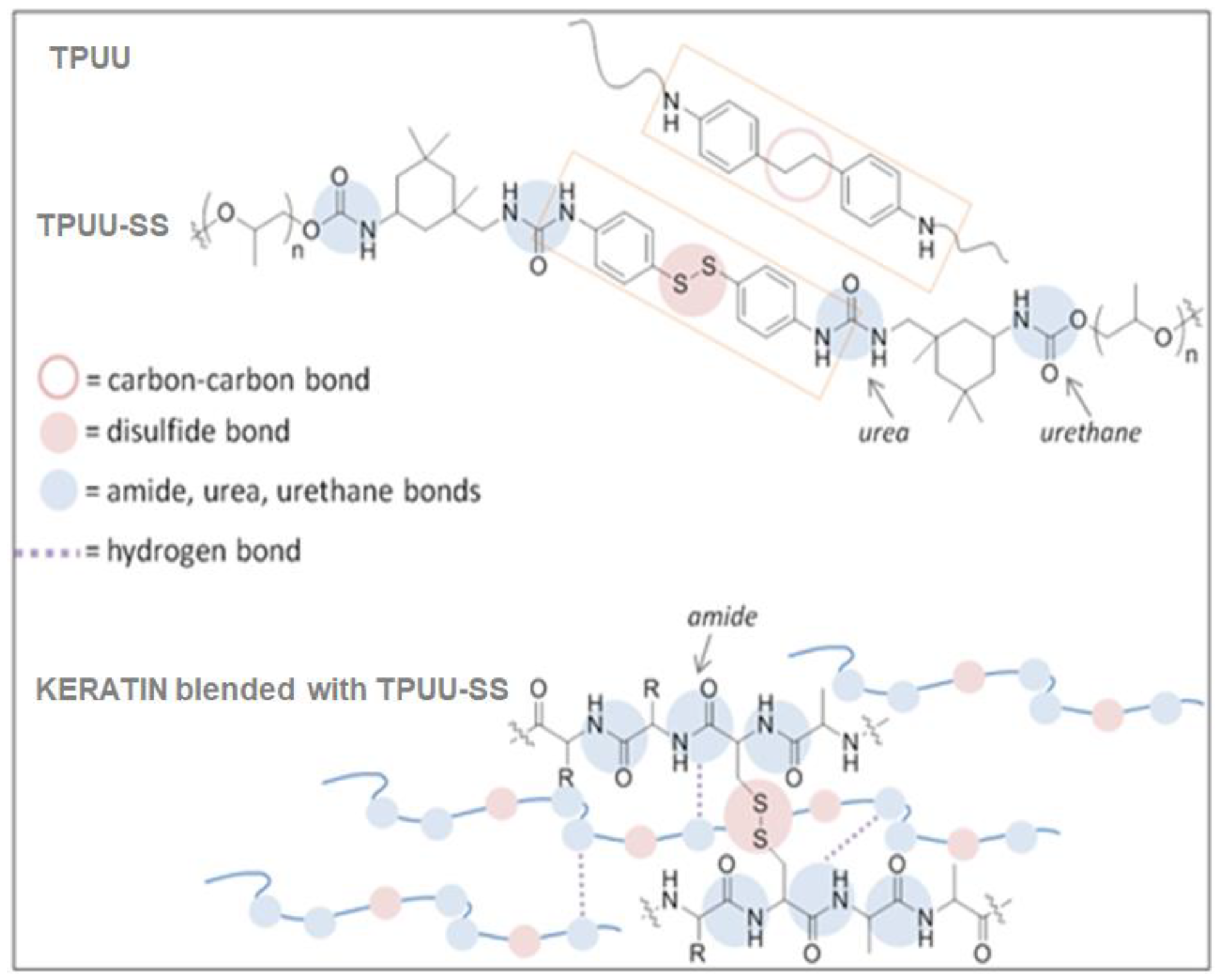
2.2. Synthesis of TPUUs
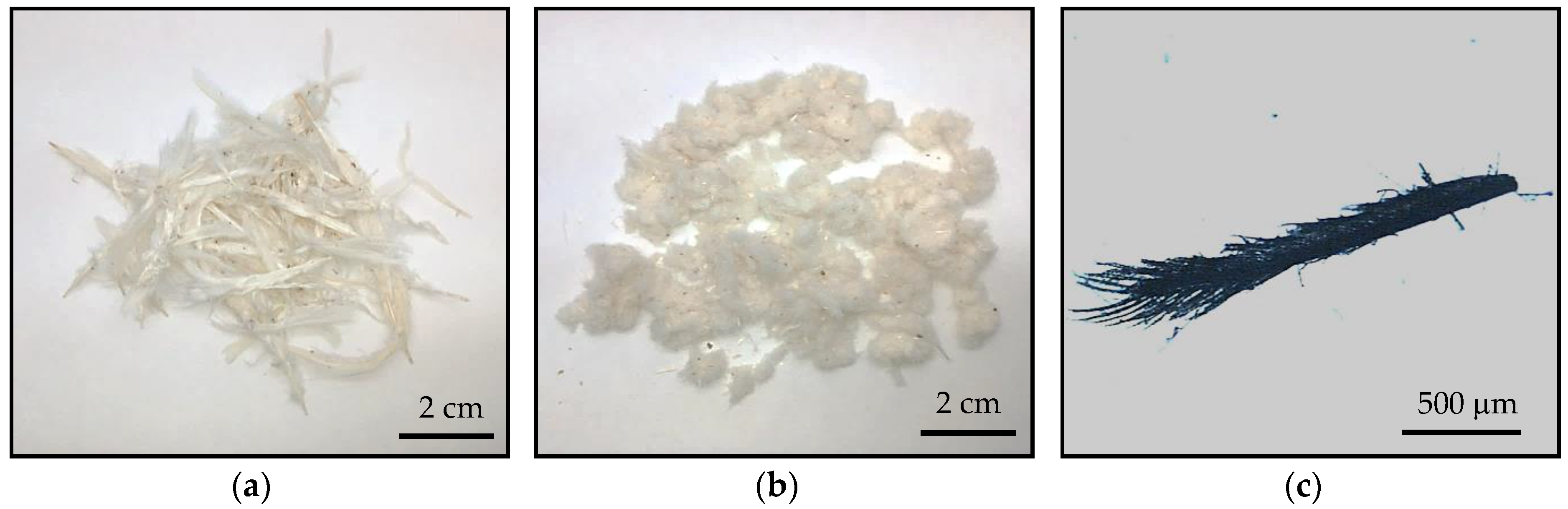
2.3. Biocomposite Preparation
2.4. Characterization of the Biocomposites
2.4.1. Density of the Biocomposites
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Water Absorption and Thickness Swelling
2.4.4. Mechanical Properties
2.4.5. Field Emission Scanning Electron Microscopy (FESEM)
3. Results
3.1. Density
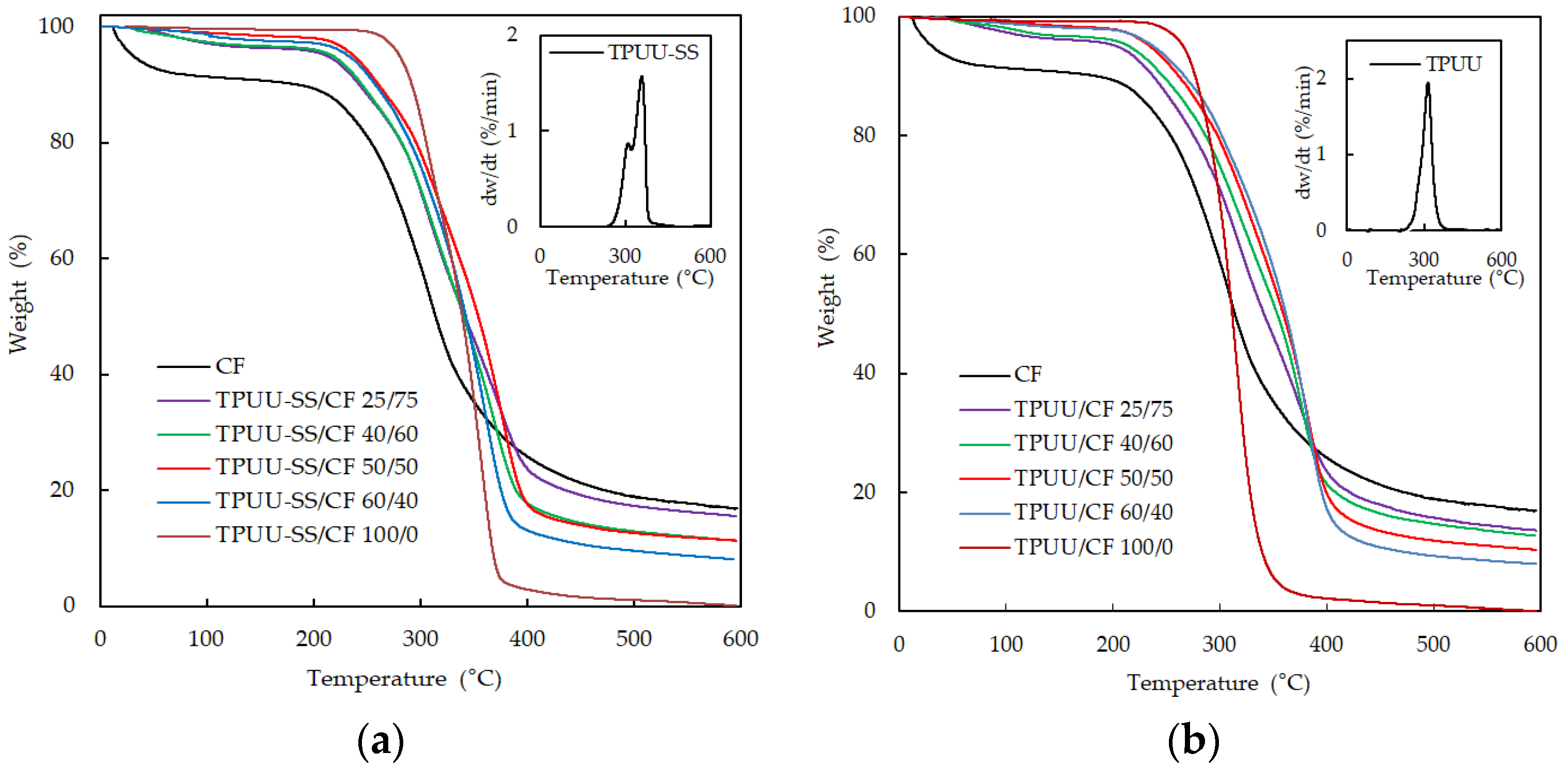
3.2. Thermogravimetric Analysis (TGA)
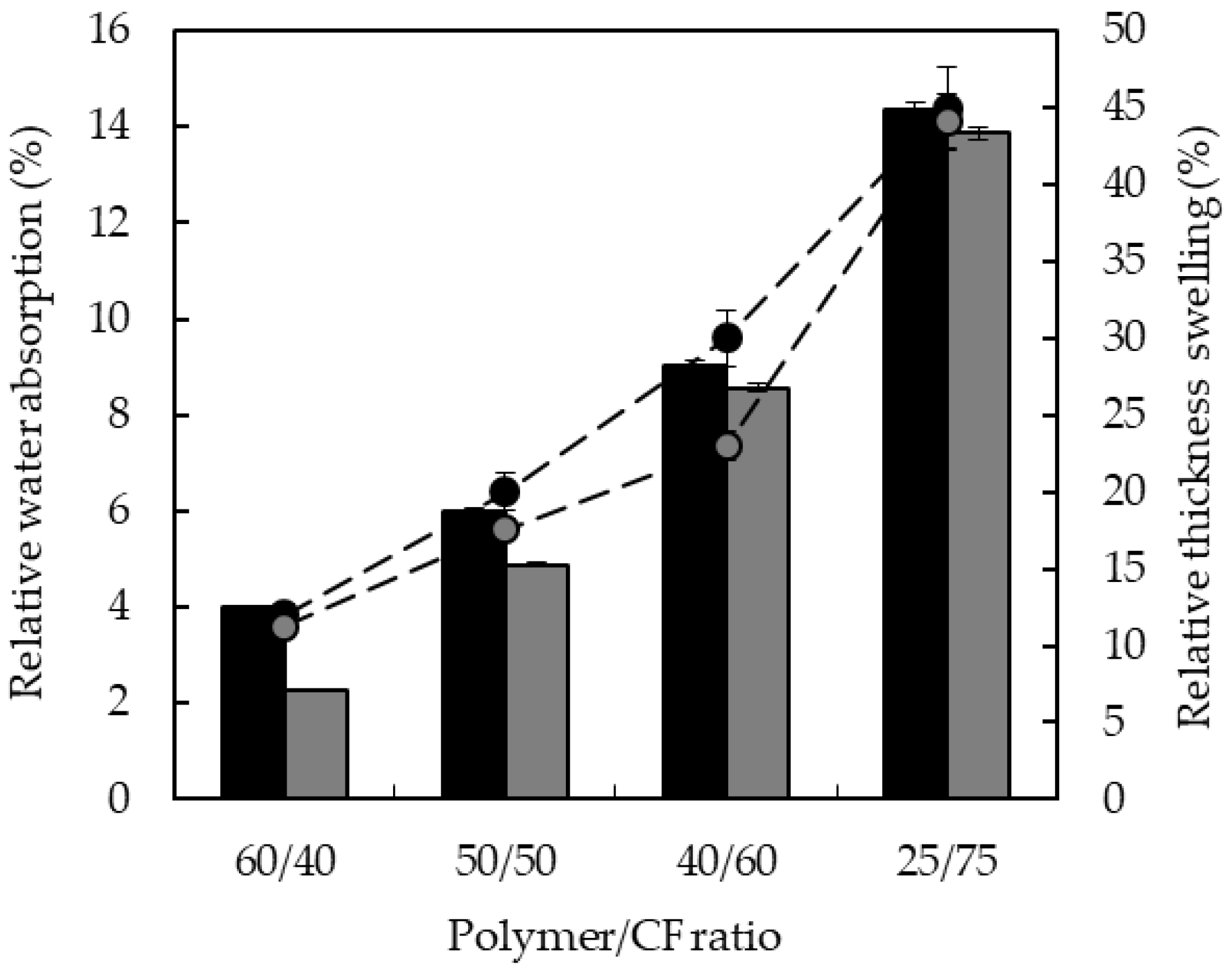
3.3. Water Absorption
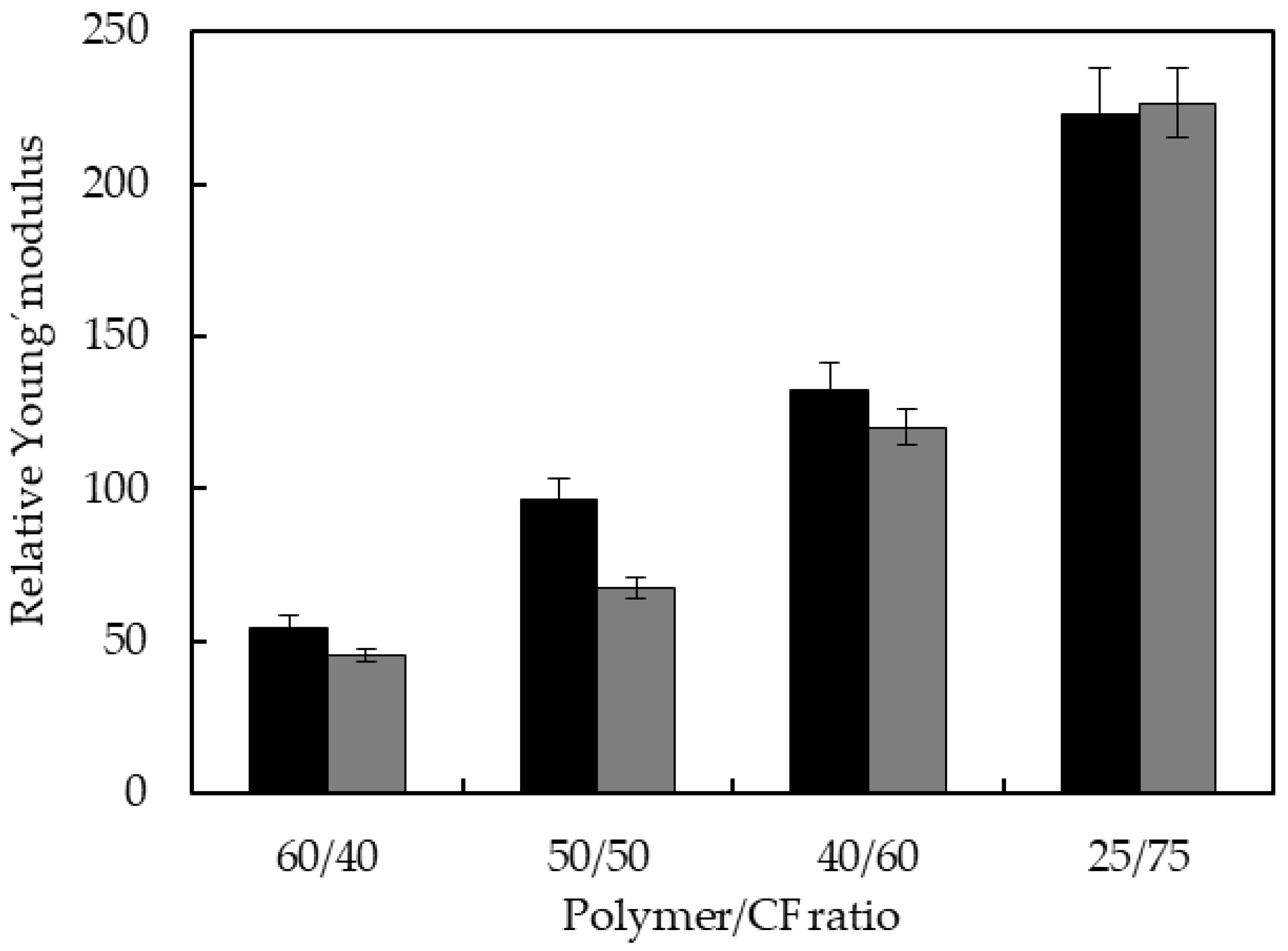
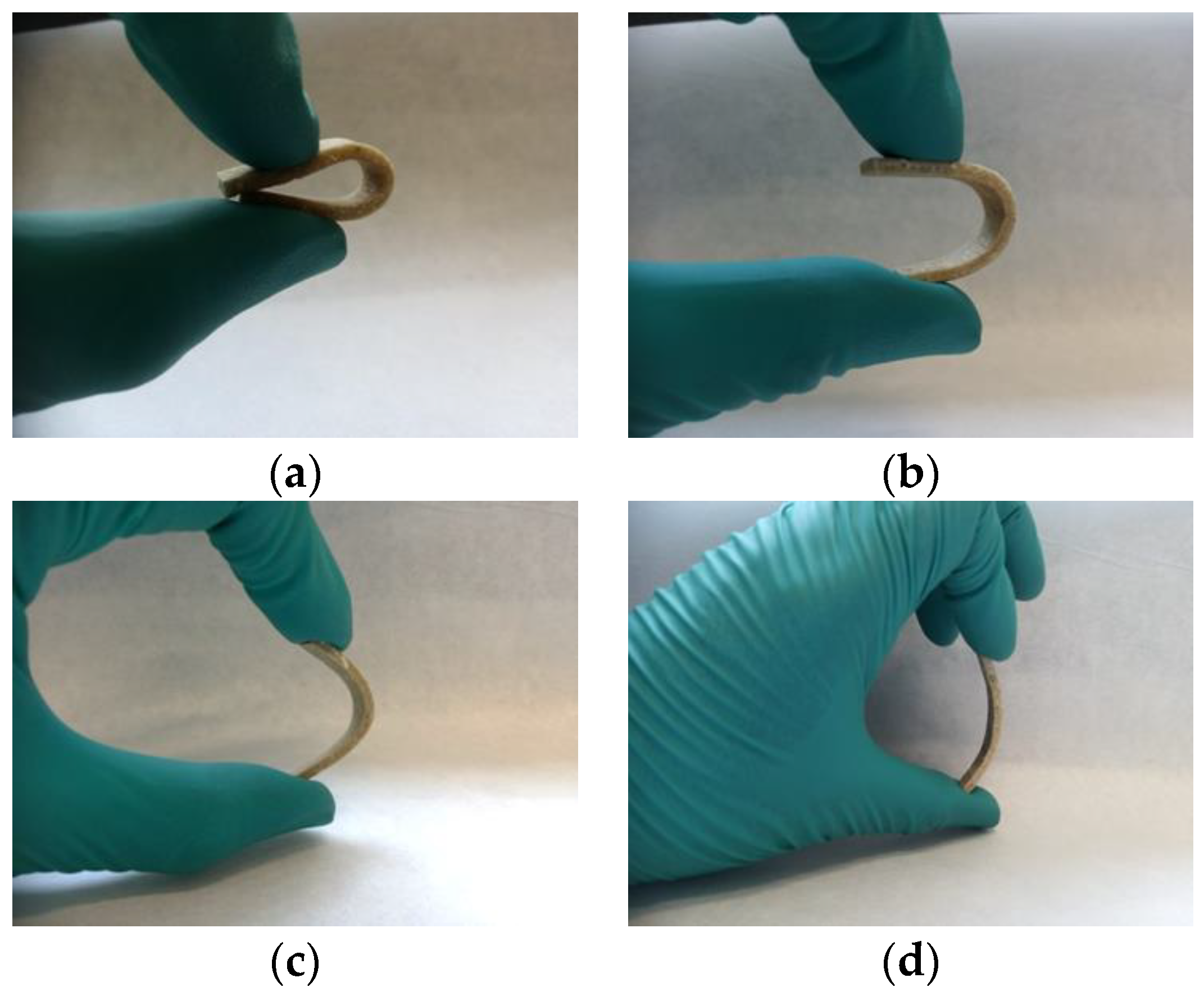
3.4. Mechanical Properties of the Biocomposites
3.5. Morphology of the TPUU/CF and TPUU-SS/CF Biocomposites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conzatti, L.; Giunco, F.; Stagnaro, P.; Patrucco, A.; Marano, C.; Rink, M.; Marsano, E. Composites based on polypropylene and short wool fibres. Compos. Part A Appl. Sci. Manuf. 2013, 47, 165–171. [Google Scholar] [CrossRef]
- Kumar, D.; Boopathy, S.R.; Sangeetha, D.; Bharathiraja, G. Investigation of Mechanical Properties of Horn Powder-Filled Epoxy Composite. J. Mech. Eng. 2017, 63, 138–147. [Google Scholar] [CrossRef]
- European Commission. Available online: http://ec.europa.eu/agriculture/poultry_en (accessed on 20 September 2018).
- Aranberri, I.; Montes, S.; Azcune, I.; Rekondo, A.; Grande, H.-J. Fully Biodegradable Biocomposites with High Chicken Feather Content. Polymers 2017, 9, 593. [Google Scholar] [CrossRef]
- Cañavate, J.; Aymerich, J.; Garrido, N.; Colom, X.; Macanás, J.; Molins, G.; Álvarez, M.D.; Carrillo, F. Properties and optimal manufacturing conditions of chicken feather/poly(lactic acid) biocomposites. J. Compos. Mater. 2016, 50, 1671–1683. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, W. Thermally processed keratin films. J. Appl. Pol. Sci. 2005, 97, 1644–1651. [Google Scholar]
- Martelli, S.M.; Laurindo, J.B. Chicken Feather Keratin Films Plasticized with Polyethylene Glycol. Int. J. Polym. Mater. Polym. Biomater. 2012, 61, 17–29. [Google Scholar] [CrossRef]
- Shi, Z.; Reddy, N.; Hou, X.; Yang, Y. Tensile Properties of Thermoplastic Feather Films Grafted with Different Methacrylates. ACS Sustain. Chem. Eng. 2014, 2, 1849–1856. [Google Scholar] [CrossRef]
- Zhou, L.T.; Yang, G.; Yang, X.X.; Cao, Z.J.; Zhou, M.H. Preparation of regenerated keratin sponge from waste feathers by a simple method and its potential use for oil adsorption. Environ. Sci. Pollut. Res. Int. 2014, 2, 5730–5736. [Google Scholar] [CrossRef] [PubMed]
- Tronina, P.; Bubel, F. Production of organic fertilizer from poultry feather wastes excluding the composting process. PJCT 2008, 10, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Reddy, N. Potential of using plant proteins and chicken feathers for cotton warp sizing. Cellulose 2013, 20, 2163–2174. [Google Scholar] [CrossRef]
- Gregg, K.; Rogers, G.E. Feather Keratin: Composition, Structure and Biogenesis. In Biology of the Integument, 1st ed.; Bereiter-Hahn, J., Matoltsy, A.G., Richards, K.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; Volume 2, pp. 666–695. ISBN 978-3-662-00991-8. [Google Scholar]
- Ward, W.H.; Binkley, C.H.; Snell, N.S. Amino Acid Composition of Normal Wools, Wool Fractions, Mohair, Feather, and Feather Fractions. Text. Res. J. 1955, 25, 314–325. [Google Scholar] [CrossRef]
- Schmidt, W.F. Microcrystalline keratin: From feathers to composite products. In Proceedings of the MRS Symposium, Boston, MA, USA, 26–29 November 2001; Volume 702, pp. 25–29. [Google Scholar]
- Saucedo-Rivalcoba, V.; Martínez-Hernández, A.L.; Martínez-Barrera, G.; Velasco-Santos, C.; Castaño, V.M. (Chicken feathers keratin)/polyurethane membranes. Appl. Phys. A 2011, 104, 219–228. [Google Scholar] [CrossRef]
- Gokce, O.; Kasap, M.; Akpinar, G.; Ozkoc, G. Preparation, characterization, and in vitro evaluation of chicken feather fiber–thermoplastic polyurethane composites. J. Appl. Polym. Sci. 2017, 134, 45338–45347. [Google Scholar] [CrossRef]
- Wrześniewska-Tosik, K.; Zajchowski, S.; Bryśkiewicz, A.; Ryszkowska, J. Feathers as a Flame-Retardant in Elastic Polyurethane Foam. Fibres Text. East Eur. 2014, 22, 119–128. [Google Scholar]
- Martin, R.; Rekondo, A.; Ruiz de Luzuriaga, A.; Casuso, P.; Dupin, D.; Cabañero, G.; Grande, H.J.; Odriozola, I. Dynamic sulfur chemistry as a key tool on the design of self-healing polymers. Smart Mater. Struct. 2016, 25, 084017. [Google Scholar] [CrossRef]
- Azcune, I.; Odriozola, I. Aromatic disulfide crosslinks in polymer systems: Self-healing, reprocessability, recyclability and more. Eur Polym. J. 2016, 84, 147–160. [Google Scholar] [CrossRef]
- Rekondo, A.; Martin, R.; Ruiz de Luzuriaga, A.; Cabañero, G.; Grande, H.J.; Odriozola, I. Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horiz. 2014, 1, 237–240. [Google Scholar] [CrossRef]
- ISO 9427:2003. Wood-Based Panels. Determination of Density; International Organization for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- ASTM D570-98. Standard Test Method for Water Absorption of Plastics; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- ISO 527-1:2012. Plastics—Determination of Tensile Properties; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Barone, J.R.; Schmidt, W.F. Polyethylene reinforced with keratin fibers obtained from chicken feathers. Compos. Sci. Technol. 2005, 65, 173–181. [Google Scholar] [CrossRef]
- Carrillo, F.; Rahalli, A.; Cañavate, J.; Colom, X. Composites from keratin biofibers. Study of compatibility using polyolephinic matrices. In Proceedings of the 15th European Conference on Composite Materials (ECCM-15) Conference, Venice, Italy, 24–28 June 2012; pp. 1–15. [Google Scholar]
- Yin, X.-C.; Li, F.-Y.; He, Y.-F.; Wang, Y.; Wang, R.-M. Study on Effective extraction of chicken feather keratins and their films for controlling drug release. Biomater. Sci. 2013, 1, 528–536. [Google Scholar] [CrossRef]
- Starón, P.; Banach, M.; Kowalski, Z. Keratin—Origins, properties, application. Chemik 2011, 65, 1019–1026. [Google Scholar]
- Martínez-Hernández, A.L.; Velasco-Santos, C.; de Icaza, M.; Castaño, V.M. Microstructural characterization of keratin fibres from chicken feathers. Int. J. Environ. Pollut. 2005, 23, 162–178. [Google Scholar] [CrossRef]
- Cruz, S.; Viana, J.C. Melt blending and characterization of carbon nanoparticles-filled thermoplastic polyurethane elastomers. J. Elastom. Plast. 2014, 27, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Spoljaric, S.; Shanks, R.A. Novel polyhedral oligomeric silsesquioxane-substituted dendritic polyester tougheners for linear thermoplastic polyurethane. J. Appl. Polym. Sci. 2012, 126, 440–454. [Google Scholar] [CrossRef]
- Xia, H.; Song, M. Preparation and characterization of polyurethane carbon nanotube composites. Soft Matter. 2005, 1, 386–394. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Dean, C.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Jiménez-Cervantes Amieva, E.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Rivera-Armenta, J.L.; Mendoza-Martínez, A.M.; Castaño, V.M. Composites from chicken feathers quill and recycled polypropylene. J. Comp. Mater. 2015, 49, 275–283. [Google Scholar] [CrossRef]
- Barone, J.R.; Gregoire, N.T. Characterization of fibre-polymer interactions and transcrystallity in short keratin fiber-polypropylene composites. Plast. Rubber Compos. 2006, 35, 287–293. [Google Scholar] [CrossRef]
- Pourjavaheri, F.; Jones, O.; Mohaddes, F.; Sherkat, F.; Gupta, A.; Shanks, R.A. Green plastics: Utilizing chicken feather keratin in thermoplastic polyurethane composites to enhance thermo-mechanical properties. In Proceedings of the 74th Annual Technical Conference of the Society of Plastics Engineers 2016, Indianapolis, Indiana, 23–25 May 2016; pp. 1–8. [Google Scholar]





| Matrix | Matrix/CF Ratio | % of Density Reduction Compared to Neat Polymer Matrix |
|---|---|---|
| 100/0 | - | |
| 60/40 | 7.1 | |
| TPUU-SS/CF | 50/50 | 10.7 |
| 40/60 | 11.6 | |
| 25/75 | 21.4 | |
| 100/0 | - | |
| 60/40 | 6.5 | |
| TPUU/CF | 50/50 | 13.8 |
| 40/60 | 13.3 | |
| 25/75 | 18.2 |
| Sample | Polymer/CF Ratio | T (5%) (°C) | T (25%) (°C) | T (50%) (°C) | % Residual Mass after 600 °C |
|---|---|---|---|---|---|
| TPUU-SS/CF | 100/0 | 280.31 | 310.98 | 339.04 | 0.13 |
| 60/40 | 232.36 | 301.98 | 342.29 | 8.14 | |
| 50/50 | 237.38 | 306.93 | 354.02 | 11.02 | |
| 40/60 | 217.24 | 293.96 | 340.27 | 11.36 | |
| 25/75 | 211.79 | 293.90 | 351.38 | 15.59 | |
| TPUU/CF | 100/0 | 265.78 | 294.85 | 311.36 | 0.01 |
| 60/40 | 237.98 | 314.12 | 361.63 | 8.03 | |
| 50/50 | 234.87 | 310.09 | 359.27 | 10.12 | |
| 40/60 | 215.29 | 299.57 | 353.56 | 12.72 | |
| 25/75 | 204.08 | 289.78 | 341.17 | 13.62 | |
| CF | 0/100 | 30.76 | 268.13 | 313.94 | 16.92 |
| Type of Matrix | Polymer/CF | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| TPUU-SS | 100/0 | 1.24 | 1.03 | 1030.25 |
| 60/40 | 67.45 | 3.82 | 25.25 | |
| 50/50 | 120.12 | 7.73 | 11.37 | |
| 40/60 | 164.05 | 3.98 | 9.11 | |
| 25/75 | 276.06 | 2.78 | 1.92 | |
| TPUU | 100/0 | 1.81 | 4.80 | 450.10 |
| 60/40 | 81.61 | 8.18 | 29.48 | |
| 50/50 | 122.04 | 10.28 | 30.73 | |
| 40/60 | 216.57 | 11.16 | 16.21 | |
| 25/75 | 407.83 | 6.09 | 2.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranberri, I.; Montes, S.; Azcune, I.; Rekondo, A.; Grande, H.-J. Flexible Biocomposites with Enhanced Interfacial Compatibility Based on Keratin Fibers and Sulfur-Containing Poly(urea-urethane)s. Polymers 2018, 10, 1056. https://doi.org/10.3390/polym10101056
Aranberri I, Montes S, Azcune I, Rekondo A, Grande H-J. Flexible Biocomposites with Enhanced Interfacial Compatibility Based on Keratin Fibers and Sulfur-Containing Poly(urea-urethane)s. Polymers. 2018; 10(10):1056. https://doi.org/10.3390/polym10101056
Chicago/Turabian StyleAranberri, Ibon, Sarah Montes, Itxaso Azcune, Alaitz Rekondo, and Hans-Jürgen Grande. 2018. "Flexible Biocomposites with Enhanced Interfacial Compatibility Based on Keratin Fibers and Sulfur-Containing Poly(urea-urethane)s" Polymers 10, no. 10: 1056. https://doi.org/10.3390/polym10101056
APA StyleAranberri, I., Montes, S., Azcune, I., Rekondo, A., & Grande, H.-J. (2018). Flexible Biocomposites with Enhanced Interfacial Compatibility Based on Keratin Fibers and Sulfur-Containing Poly(urea-urethane)s. Polymers, 10(10), 1056. https://doi.org/10.3390/polym10101056







