Self-Assembly of Block and Graft Copolymers in Organic Solvents: An Overview of Recent Advances
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis and Molecular Characteristics
2.2. Solvents
2.3. Self-Assembly Techniques
2.4. Characterization Techniques
3. AB and ABA Linear Block Copolymers in Organic Solvents
3.1. Self-Assembly of AB and ABA Block Copolymers in Non-Polar Selective Solvents
3.2. Self-Assembly of AB and ABA Block Copolymers in Polar Selective Solvents
3.3. Self-Assembly of AB and ABA Block Copolymers in Organic Solvent Mixtures
3.4. Self-Assembly of AB and ABA Block Copolymers in Ionic Liquids
3.5. Self-Assembly of AB and ABA Block Copolymers in Biocompatible Organic Solvents
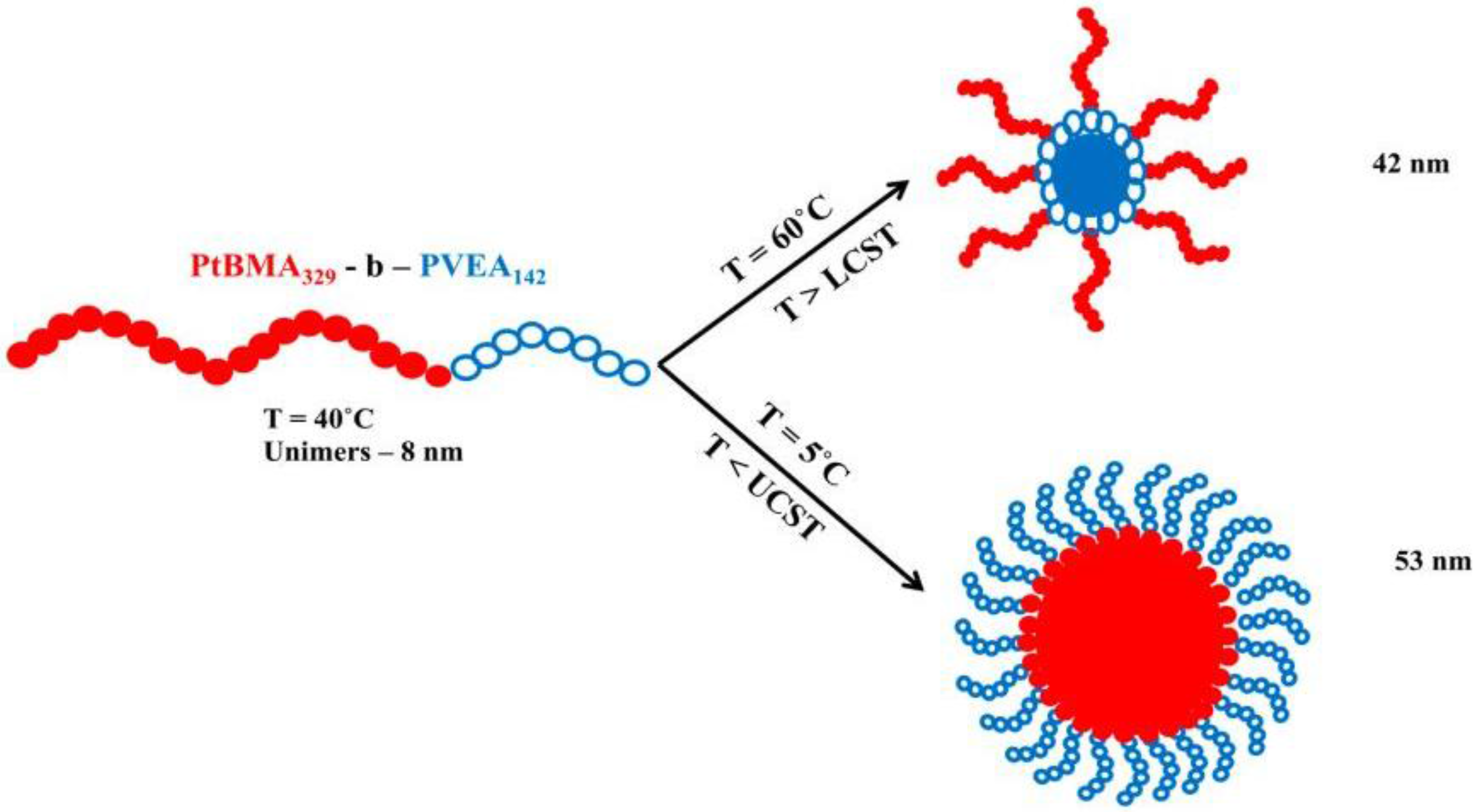
3.6. Crystallization-Induced Self-Assembly of AB and ABA Block Copolymers in Organic Solvents
3.7. Concluding Remarks
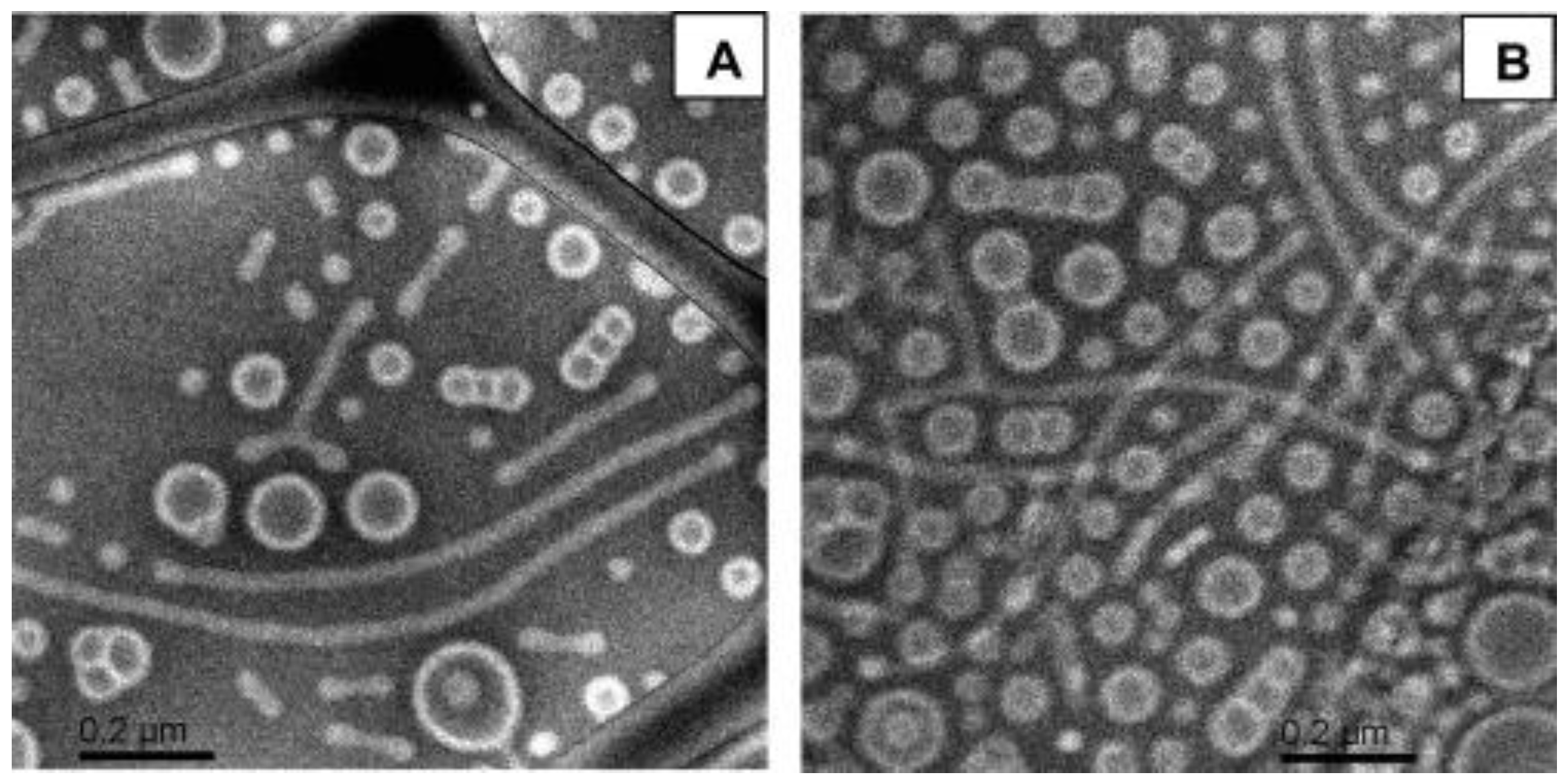
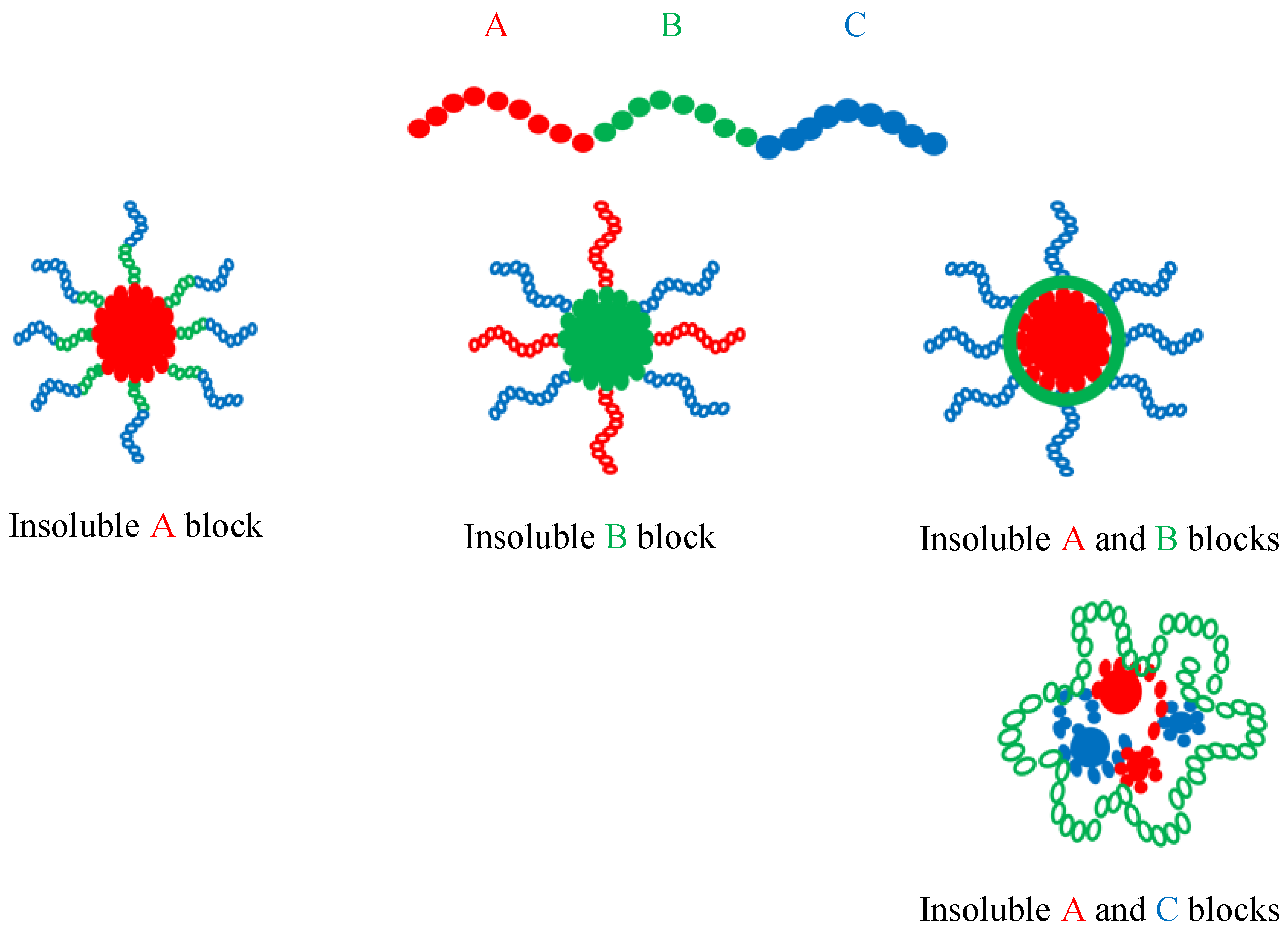
4. Self-Assembly of Linear ABC Triblock Terpolymers in Organic Solvents
4.1. Self-Assembly of Linear ABC Triblock Terpolymers in Non-Polar Selective Solvents
4.2. Self-Assembly of Linear ABC Triblock Terpolymers in Polar Selective Solvents
4.3. Self-Assembly of Linear ABC Triblock Terpolymers in Organic Solvent Mixtures
4.4. Miscellaneous Self-Assembly Studies of Linear ABC Triblock Terpolymers
4.5. Concluding Remarks
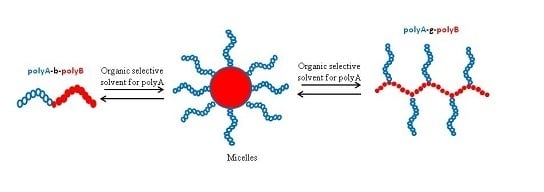
5. Self-Assembly of AB and ABC Graft Copolymers in Organic Solvents
5.1. Self-Assembly of AB Graft Copolymers
5.2. Self-Assembly of ABC Graft Copolymers
5.3. Concluding Remarks
6. General Conclusions and Perspectives
Author Contributions
Conflicts of Interest
References
- Tuzar, Z.; Kratochvil, P. Block and graft copolymer micelles in solution. Adv. Colloid Interface Sci. 1976, 6, 201–232. [Google Scholar] [CrossRef]
- Nakashima, K.; Bahadur, P. Aggregation of water-soluble block copolymers in aqueous solutions: Recent trends. Adv. Colloid Interface Sci. 2006, 123–126, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.K.; Cheong, I.W.; Lee, J.M.; Kim, J.H. Polymeric nanoparticles, micelles and polymersomes from amphiphilic block copolymer. Korean J. Chem. Eng. 2010, 27, 731–740. [Google Scholar] [CrossRef]
- Kulthe, S.S.; Choudhari, Y.M.; Inamdar, N.N.; Mourya, V. Polymeric micelles: Authoritative aspects for drug delivery. Des. Monomers Polym. 2012, 15, 465–521. [Google Scholar] [CrossRef]
- Xu, W.; Ling, P.; Zhang, T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J. Drug Deliv. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Gohy, J.F. Block copolymer micelles. Adv. Polym. Sci. 2005, 190, 65–136. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Misra, B.N. Grafting: A versatile means to modify polymers. Techniques, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Uhrig, D.; Mays, J. Synthesis of well-defined multigraft copolymers. Polym. Chem. 2011, 2, 69–76. [Google Scholar] [CrossRef]
- Feng, C.; Li, Y.; Yang, D.; Hu, J.; Zhang, X.; Huang, X. Well-defined graft copolymers: From controlled synthesis to multipurpose applications. Chem. Soc. Rev. 2011, 40, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, S.; Lu, G.; Huang, X. Constructing well-defined star graft copolymers. Polym. Chem. 2013, 4, 1289–1299. [Google Scholar] [CrossRef]
- Matsuo, Y.; Konno, R.; Ishizone, T.; Goseki, R.; Hirao, A. Precise synthesis of block polymers composed of three or more blocks by specially designed linking methodologies in conjunction with living anionic polymerization system. Polymers 2013, 5, 1012–1040. [Google Scholar] [CrossRef]
- Keddie, D.J. A guide to the synthesis of block copolymers using reversible-addition fragmentation chain transfer (RAFT) polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.; He, G.; Howdle, S.M.; Zetterlund, P.B. Block copolymer synthesis by controlled/living radical polymerization in heterogeneous systems. Chem. Soc. Rev. 2016, 45, 5055–5084. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Oliva, A.P.; Warren, N.J.; Rajkumar, A.; Mykhaylyk, O.O.; Derry, M.J.; Doncom, K.E.B.; Rymaruk, M.J.; Armes, S.P. Polydimethylsiloxane-based diblock copolymer nano-objects prepared in nonpolar media via RAFT-mediated polymerization-induced self-assembly. Macromolecules 2015, 48, 3547–3555. [Google Scholar] [CrossRef]
- Jones, E.R.; Semsarilar, M.; Wyman, P.; Boerakker, M.; Armes, S.P. Addition of water to an alcoholic RAFT PISA formulation leads to faster kinetics but limits the evolution of copolymer morphology. Polym. Chem. 2016, 7, 851–859. [Google Scholar] [CrossRef]
- Ratcliffe, L.P.D.; McKenzie, B.E.; Le Bouedec, G.M.D.; Williams, C.N.; Brown, S.L.; Armes, S.P. Polymerization-induced self-assembly of all-acrylic diblock copolymers via RAFT dispersion polymerization in alkanes. Macromolecules 2015, 48, 8594–8607. [Google Scholar] [CrossRef]
- Canning, S.L.; Smith, G.N.; Armes, S.P. A critical appraisal of RAFT-mediated polymerization-induced self-assembly. Macromolecules 2016, 49, 1985–2001. [Google Scholar] [CrossRef] [PubMed]
- Canning, S.L.; Cunningham, V.J.; Ratcliffe, L.P.D.; Armes, S.P. Phenyl acrylate is a versatile monomer for the synthesis of acrylic diblock copolymer nano-objects via polymerization-induced self-assembly. Polym. Chem. 2017, 8, 4811–4821. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Properties of Polymers, 3rd ed.; Elsevier Sci.: Amsterdam, The Netherlands, 2003; pp. 189–224. ISBN 044482877X. [Google Scholar]
- Atanase, L.I.; Desbrieres, J.; Riess, R. Micellization of synthetic and polysaccharides-based graft copolymers in aqueous media. Prog. Polym. Sci. 2017, 73, 32–60. [Google Scholar] [CrossRef]
- Kulikov, O.V.; Siriwardane, D.A.; McCandless, G.T.; Mahmood, S.F.; Novak, B.M. Self-assembly studies on triazolepolycarbodiimide-g-polystyrene copolymers. Polymer 2016, 92, 94–101. [Google Scholar] [CrossRef]
- Hudson, Z.M.; Boott, C.E.; Robinson, M.E.; Rupar, P.A.; Winnik, M.A.; Manners, I. Tailored hierarchical micelle architectures using living crystallization-driven self-assembly in two dimensions. Nat. Chem. 2014, 6, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Hailes, R.L.N.; Oliver, A.M.; Gwyther, J.; Whittell, G.R.; Manners, I. Polyferrocenylsilanes: Synthesis, properties and applications. Chem. Soc. Rev. 2016, 45, 5358–5407. [Google Scholar] [CrossRef] [PubMed]
- Tritschler, U.; Pearce, S.; Gwyther, J.; Whittell, G.R.; Manners, I. Functional nanoparticles from the solution self-assembly of block copolymers. Macromolecules 2017, 50, 3439–3463. [Google Scholar] [CrossRef]
- Winnik, F.M.; Regismond, S.T.A.; Anghel, D.F. Fluorescent labels: Versatile tools for studying the association of amphiphilic polymers in water. In Associative Polymers in Aqueous Media; Glass, J.E., Ed.; American Chemical Society: Washington, DC, USA, 2010; Volume 17, pp. 286–302. ISBN 9780841236592. [Google Scholar]
- Štěpánek, M. Fluorescence spectroscopy studies of amphiphilic block copolymer micelles in aqueous solutions. In Fluoresce Studies of Polymer Containing Systems; Procházka, K., Ed.; Springer: Cham, Switzerland, 2016; Volume 16, pp. 203–215. ISBN 978-3-319-26786-9. [Google Scholar]
- Walker, L.M. Scattering from polymer-like micelles. Curr. Opin. Colloid Interface Sci. 2009, 14, 451–454. [Google Scholar] [CrossRef]
- Chavda, S.; Yusa, S.; Inoue, M.; Abezgauz, L.; Kesselman, E.; Danino, D.; Bahadur, P. Synthesis of stimuli responsive PEG47-b-PAA126-b-PSt32 triblock copolymer and its self-assembly in aqueous solutions. Eur. Polym. J. 2013, 49, 209–216. [Google Scholar] [CrossRef]
- Löbling, T.I.; Haataja, J.S.; Synatschke, C.V.; Schacher, F.H.; Müller, M.; Hanisch, A.; Gröschel, A.H.; Müller, A.H.E. Hidden structural features of multicompartment micelles revealed by cryogenic transmission electron tomography. ACS Nano 2014, 8, 11330–11340. [Google Scholar] [CrossRef] [PubMed]
- Franken, L.E.; Boekema, E.J.; Stuart, M.C.A. Transmission electron microscopy as a tool for the characterization of soft materials: Application and interpretation. Adv. Sci. 2017, 4, 1600476. [Google Scholar] [CrossRef] [PubMed]
- Boott, C.E.; Laine, R.F.; Mahou, P.; Finnegan, J.R.; Leitao, E.M.; Webb, S.E.D.; Kaminski, C.F.; Manners, I. In situ visualization of block copolymer self-assembly in organic media by super-resolution fluorescence microscopy. Chem. Eur. J. 2015, 21, 18539–18542. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huo, F.; Li, Q.; Gao, C.; Su, Y.; Zhang, W. Synthesis of a doubly thermo-responsive schizophrenic diblock copolymer based on poly[N-(4-vinylbenzyl)-N,N-diethylamine] and its temperature-sensitive flip-flop micellization. Polym. Chem. 2014, 5, 3910–3918. [Google Scholar] [CrossRef]
- Sotiriou, K.; Nannou, A.; Velis, G.; Pispas, S. Micellization behavior of PS(PI)3 miktoarm star copolymers. Macromolecules 2002, 35, 4106–4112. [Google Scholar] [CrossRef]
- Sotiriou, K.; Pispas, S.; Hadjichristidis, N. Controlling the colloidal behavior of styrene-isoprene diblock copolymers by selective end functionalization. Colloids Surf. Part A Physicochem. Eng. Asp. 2007, 293, 51–57. [Google Scholar] [CrossRef]
- Di Cola, E.; Lefebvre, C.; Deffieux, A.; Narayanan, T.; Borsali, R. Micellar transformations of poly(styrene-b-isoprene) block copolymers in selective solvents. Soft Matter 2009, 5, 1081–1090. [Google Scholar] [CrossRef]
- Lund, R.; Willner, L.; Lindner, P.; Richter, D. Structural properties of weakly segregated PS-PB block copolymer micelles in n-alkanes: Solvent entropy effects. Macromolecules 2009, 42, 2686–2695. [Google Scholar] [CrossRef]
- Cheng, G.; Hammouda, B.; Perahia, D. Polystyrene-block-polyisoprene diblock-copolymer micelles: Coupled pressure and temperature effects. Macromol. Chem. Phys. 2014, 215, 776–782. [Google Scholar] [CrossRef]
- Growney, D.J.; Mykhaylyk, O.O.; Armes, S.P. Micellization and adsorption behavior of a near-monodisperse polystyrene-based diblock copolymer in nonpolar media. Langmuir 2014, 30, 6047–6056. [Google Scholar] [CrossRef] [PubMed]
- Bartels, J.W.; Cauet, S.I.; Billings, P.L.; Lin, L.Y.; Zhu, J.; Fidge, C.; Pochan, D.J.; Wooley, K.L. Evaluation of isoprene chain extension from PEO macromolecular chain transfer agents for the preparation of dual, invertible block copolymer nanoassemblies. Macromolecules 2010, 43, 7128–7138. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Davis, J.L.; Hinestrosa, J.P.; Mays, J.W.; Kilbey, S.M. Control of self-assembled structure through architecturally and compositionally complex block copolymer surfactant mixtures. Macromolecules 2014, 47, 7138–7150. [Google Scholar] [CrossRef]
- Arai, T.; Masaoka, M.; Michitaka, T.; Watanabe, Y.; Hashidzume, A.; Sato, T. Aggregation behavior of polystyrene-based amphiphilic copolymers in organic media. Polym. J. 2014, 46, 189–194. [Google Scholar] [CrossRef]
- Hussain, H.; Tan, B.H.; Gudipati, C.S.; He, C.B.; Liu, Y.; Davis, T.P. Micelle formation of amphiphilic polystyrene-b-poly(N-vinylpyrrolidone) diblock copolymer in methanol and water-methanol binary mixtures. Langmuir 2009, 25, 5557–5564. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Aizhao, P.; Ling, He. POSS end-capped diblock copolymers: Synthesis, micelle self-assembly and properties. J. Colloid Interface Sci. 2014, 425, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.Y.; Henderson, K.J.; Burghardt, W.R.; Wang, J.; Shull, K.R. Micellar morphologies of block copolymer solutions near the sphere/cylinder transition. Macromolecules 2015, 48, 173–183. [Google Scholar] [CrossRef]
- Jena, S.S.; Roy, S.G.; Azmeera, V.; De, P. Solvent-dependent self-assembly behavior of block copolymers having side-chain amino acid and fatty acid block segments. React. Funct. Polym. 2016, 99, 26–34. [Google Scholar] [CrossRef]
- Jana, S.; Saha, A.; Paira, T.K.; Mandal, T.K. Synthesis and self-aggregation of poly(2-ethyl-2-oxazoline)-based photo-cleavable block copolymer: Micelle, compound micelle, reverse micelle, and dye encapsulation/release. J. Phys. Chem. B 2016, 120, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Demarteau, J.; Ameduri, B.; Ladmiral, V.; Mees, M.A.; Hoogenboom, R.; Debuigne, A.; Detrembleur, C. Controlled synthesis of fluorinated copolymers via cobalt-mediated radical copolymerization of perfluorohexylethylene and vinyl acetate. Macromolecules 2017, 50, 3750–3760. [Google Scholar] [CrossRef]
- Pospiech, D.; Jehnichen, D.; Eckstein, K.; Scheibe, P.; Komber, H.; Sahre, K.; Janke, A.; Reuter, U.; Häuβler, L.; Schellkopf, L.; et al. Semifluorinated PMMA block copolymers: Synthesis, nanostructure, and thin film properties. Macromol. Chem. Phys. 2017, 218, 1600599. [Google Scholar] [CrossRef]
- Cho, H.; Park, H.; Park, S.; Choi, H.; Huang, H.; Chang, T. Development of various PS-b-P4VP micellar morphologies: Fabrication of inorganic nanostructures from micellar templates. J. Colloid Interface Sci. 2011, 356, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.N.; Cheng, H.; Luo, Z.H. Fluorinated AB diblock copolymers and their aggregates in organic solvents. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3647–3657. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Yang, S.; Zheng, J.X.; Van Horn, R.M.; Zhang, W.B.; Xu, J.; Cheng, S.Z.D. Polystyrene-block-poly(ethylene oxide) reverse micelles and their temperature-driven morphological transitions in organic solvents. Macromolecules 2012, 45, 3634–3638. [Google Scholar] [CrossRef]
- Rao, J.; Zhang, H.; Gaan, S.; Salentinig, S. Self-assembly of polystyrene-b-poly(2-vinylpyridine) micelles: From solutions to silica particles surfaces. Macromolecules 2016, 49, 5978–5984. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, W.B.; Lodge, T.P.; Bates, F.S. Structure of poly(styrene-b-ethylene-alt-propylene) diblock copolymer micelles in binary solvent mixtures. J. Polym. Sci. Part A Polym. Phys. 2016, 54, 22–31. [Google Scholar] [CrossRef]
- Derry, M.J.; Fielding, L.A.; Armes, S.P. Polymerization-induced self-assembly of block copolymer nanoparticles via RAFT non-aqueous dispersion polymerization. Prog. Polym. Sci. 2016, 52, 1–18. [Google Scholar] [CrossRef]
- Lee, H.N.; Bai, Z.; Newell, N.; Lodge, T.P. Micelle/inverse micelle self-assembly of a PEO-PNIPAm block copolymer in ionic liquids with double thermoresponsivity. Macromolecules 2010, 43, 9522–9528. [Google Scholar] [CrossRef]
- Lu, H.; Akgun, B.; Wei, X.; Li, L.; Satija, S.K.; Russel, T.P. Temperature-triggered micellization of block copolymers on an ionic liquid surface. Langmuir 2011, 27, 12443–12450. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T.; Nakamura, Y.; Lodge, T.P.; Watanabe, M. Light-controlled reversible micellization of a diblock copolymer in an ionic liquid. Macromolecules 2012, 45, 7566–7573. [Google Scholar] [CrossRef]
- Hoarfrost, M.L.; Lodge, T.P. Effects of solvent quality and degree of polymerization on the critical micelle temperature of poly(ethylene oxide-b-n-butyl methacrylate) in ionic liquids. Macromolecules 2014, 47, 1455–1461. [Google Scholar] [CrossRef]
- Chen, Z.; FitzGerald, P.A.; Kobayashi, Y.; Ueno, K.; Watanabe, M.; Warr, G.G.; Atkin, R. Micelle structure of novel diblock polyethers in water and two protic ionic liquids (EAN and PAN). Macromolecules 2015, 48, 1843–1851. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kitazawa, Y.; Komori, T.; Ueno, K.; Kokubo, H.; Watanabe, M. Self-assembly of polyether diblock copolymers in water and ionic liquids. Macromol. Rapid Commun. 2016, 37, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lodge, T.P. Chain exchange kinetics in diblock copolymer micelles in ionic liquids: The role of χ. Macromolecules 2016, 49, 9542–9552. [Google Scholar] [CrossRef]
- Ma, Y.; Lodge, T.P. Poly(methyl methacrylate)-block-poly(n-butyl methacrylate) diblock copolymer micelles in an ionic liquid: Scalling of core and corona size with core block length. Macromolecules 2016, 49, 3639–3646. [Google Scholar] [CrossRef]
- Simone, P.M.; Lodge, T.P. Micellization of PS-PMMA diblock copolymers in an ionic liquid. Macromol. Chem. Phys. 2007, 208, 339–348. [Google Scholar] [CrossRef]
- Mok, M.M.; Thiagarajan, R.; Flores, M.; Morse, D.C.; Lodge, T.P. Apparent critical micelle concentrations in block copolymer/ionic liquid solutions: Remarkably weak dependence on solvophobic block molecular weight. Macromolecules 2012, 45, 4818–4829. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Simone, P.; Lodge, T.P. Self-assembly of block copolymer micelles in an ionic liquid. J. Am. Chem. Soc. 2006, 128, 2745–2750. [Google Scholar] [CrossRef] [PubMed]
- Meli, L.; Santiago, J.M.; Lodge, T.P. Path-dependent morphology and relaxation kinetics of highly amphiphilic diblock copolymer micelles in ionic liquids. Macromolecules 2010, 43, 2018–2027. [Google Scholar] [CrossRef]
- Zhang, S.; Li, N.; Zheng, L.; Li, X.; Gao, Y.; Yu, L. Aggregation behavior of pluronic triblock copolymer in 1-butyl-3-methylimidazolium type ionic liquids. J. Phys. Chem. B 2008, 112, 10228–10233. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Barron, C.R.; Li, D.; Wagner, N.J.; Caplan, J.L. Triblock copolymer self-assembly in ionic liquids: Effect of PEO block length on the self-assembly of PEO-PPO-PEO in ethylammonium nitrate. Macromolecules 2014, 47, 7484–7495. [Google Scholar] [CrossRef]
- Miller, A.C.; Bershteyn, A.; Tan, W.; Hammond, P.T.; Cohen, R.E.; Irvine, D.J. Block copolymer micelles as nanocontainers for controlled release of proteins from biocompatible oil phases. Biomacromolecules 2009, 10, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I.; Riess, G. Block copolymer stabilized nonaqueous biocompatible sub-micron emulsions for topical applications. Int. J. Pharm. 2013, 448, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I.; Riess, G. Stabilization of non-aqueous emulsions by poly(2-vinylpyridine)-b-poly(butadiene) block copolymers. Colloids Surf. Part A Physicochem. Eng. Asp. 2014, 458, 19–24. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. PEG 400/Paraffin oil non-aqueous emulsions stabilized by PBut-block-P2VP block copolymers. J. Appl. Polym. Sci. 2015, 132, 41390–41397. [Google Scholar] [CrossRef]
- He, W.N.; Xu, J.T. Crystallization assisted self-assembly of semicrystalline block copolymers. Prog. Polym. Sci. 2012, 37, 1350–1400. [Google Scholar] [CrossRef]
- Crassous, J.J.; Schurtenberger, P.; Ballauff, M.; Mihut, A.M. Design of block copolymer micelles via crystallization. Polymer 2015, 62, 1–13. [Google Scholar] [CrossRef]
- Mihut, A.M.; Crassous, J.J.; Schmalz, H.; Ballauf, M. Crystallization-induced aggregation of block copolymer micelles: Influence of crystallization kinetics on morphology. Colloid Polym. Sci. 2010, 288, 573–578. [Google Scholar] [CrossRef]
- Wang, H.; Wu, C.; Xia, G.; Ma, Z.; Mo, G.; Song, R. Semi-crystalline polymethylene-b-poly(acrylic acid) diblock copolymers: Aggregation behavior, confined crystallization and controlled growth of semycrystalline micelles from dilute DMF solution. Soft Matter 2015, 11, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Li, X.; Gao, X.; Song, L. Crystallization and morphology transition of P2VP-b-PEO block copolymer micelles composed of an amorphous core and a crystallizable corona. Polym. Bull. 2015, 73, 773–789. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, Y.; Zhang, M.; Guerin, G.; Manners, I.; Winnik, M.A. PFS-b-PNIPAM: A first step toward polymeric nanofibrillar hydrogels based on uniform fiber-like micelles. Macromolecules 2016, 49, 4265–4276. [Google Scholar] [CrossRef]
- Groves, P. Diffusion ordered spectroscopy (DOSY) as applied to polymers. Polym. Chem. 2017, 8, 6700–6708. [Google Scholar] [CrossRef]
- Wyman, I.W.; Liu, G. Micellar structures of linear triblock terpolymers: Three blocks but many possibilities. Polymer 2013, 54, 1950–1978. [Google Scholar] [CrossRef]
- Gröschel, A.H.; Müller, A.H.E. Sell-assembly concepts for multicompartment nanostructures. Nanoscale 2015, 7, 11841–11876. [Google Scholar] [CrossRef] [PubMed]
- Marsat, J.N.; Heydenreich, M.; Kleinpeter, E.; Berlepsch, H.; Böttcher, C.; Laschewsky, A. Self-assembly into multicompartment micelles and selective solubilization by hydrophilic-lipophilic-flurophilic block copolymers. Macromolecules 2011, 44, 2092–2105. [Google Scholar] [CrossRef]
- Kyeremateng, S.O.; Busse, K.; Kohlbrecher, J.; Kressler, J. Synthesis and self-organization of poly(propylene oxide)-based amphiphilic and triphilic block copolymers. Macromolecules 2011, 44, 583–593. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, W.; Jing, R.; Huang, J. Effect of block sequence on the self-assembly of ABC terpolymers in selective solvent. J. Phys. Chem. B 2008, 112, 16455–16460. [Google Scholar] [CrossRef] [PubMed]
- Lerch, J.P.; Atanase, L.I.; Purcar, V.; Riess, G. Self-aggregation of poly(butadiene)-b-poly(2-vinylpyridine0-b-poly(ethylene oxide) triblock copolymers in heptanes studied by viscometry and dynamic light scattering. C. R. Chim. 2017, 20, 724–729. [Google Scholar] [CrossRef]
- Lerch, J.P.; Atanase, L.I.; Riess, G. Adsorption of non-ionic ABC triblock copolymers: Surface modification of TiO2 suspensions in aqueous and non-aqueous medium. Appl. Surf. Sci. 2017, 419, 713–719. [Google Scholar] [CrossRef]
- Noolandi, J.; Hong, K.M. Theory of block copolymer micelles in solution. Macromolecules 1983, 16, 1443–1448. [Google Scholar] [CrossRef]
- Tsitsilianis, C.; Sfika, V. Heteroarm star-like micelles formed from polystyrene-block-poly(2-vinyl pyridine)-block-poly(methyl methacrylate) ABC triblock copolymers in toluene. Macromol. Rapid Commun. 2001, 22, 647–651. [Google Scholar] [CrossRef]
- Bütün, V.; Taktak, F.F.; Tuncer, C. Tertiary amine methacrylate-based ABC triblock copolymers: Synthesis, characterization, and self-assembly in both aqueous and nonaqueous media. Macromol. Chem. Phys. 2011, 212, 1115–1128. [Google Scholar] [CrossRef]
- Njikang, G.; Han, D.; Wang, J.; Liu, G. ABC triblock micelle-like aggregates in selective solvents for A and C. Macromolecules 2008, 41, 9727–9735. [Google Scholar] [CrossRef]
- Schacher, F.; Walther, A.; Ruppel, M.; Drechsler, M.; Müller, A.H.E. Multicompartment core micelles of triblock terpolymers in organic media. Macromolecules 2009, 42, 3540–3548. [Google Scholar] [CrossRef]
- Dupont, J.; Liu, G. ABC triblock copolymer hamburger-like micelles, segmented cylinders, and Janus particles. Soft Matter 2010, 6, 3654–3661. [Google Scholar] [CrossRef]
- Muslim, A.; Shi, Y.; Yan, Y.; Yao, D.; Rexit, A.A. Preparation of cylindrical multi-compartment micelles by the hierarchical self-assembly of ABC triblock polymer in solution. RSC Adv. 2015, 5, 85446–85452. [Google Scholar] [CrossRef]
- Löbling, T.I.; Ikkala, O.; Gröschel, A.H.; Müller, A.H.E. Controlling multicompartment morphologies using solvent conditions and chemical modification. ACS Macro Lett. 2016, 5, 1044–1048. [Google Scholar] [CrossRef]
- Cong, Y.; Zhou, Q.; Fang, J.; Zhu, K. Morphology transformation of multicompartment self-assemblies of ABC triblock copolymers. Polymer 2017, 116, 173–177. [Google Scholar] [CrossRef]
- Wang, L.; Huang, H.; He, T. ABC triblock terpolymer self-assembled core-shel-corona nanotubes with high aspect ratios. Macromol. Rapid. Commun. 2014, 35, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Löbling, T.I.; Borisov, O.; Haataja, J.S.; Ikkala, O.; Gröschel, A.H.; Müller, A.H.E. Rational design of ABC triblock terpolymer solution nanostructures with controlled patch morphology. Nat. Commun. 2016, 7, 12097. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, Y.; Ueki, T.; McIntosh, L.D.; Tamura, S.; Niitsuma, K.; Imaizumi, S.; Lodge, T.P.; Watanabe, M. Hierarchical sol-gel transition induced by thermosensitive self-assembly of an ABC triblock polymer in an ionic liquid. Macromolecules 2016, 49, 1414–1423. [Google Scholar] [CrossRef]
- Wang, X.S.; Winnik, M.A.; Manners, I. Synthesis and solution self-assembly of coil-crystalline-coil polyferrocenylphosphine-b-polyferrocenylsilane-b-polysiloxane triblock copolymers. Macromolecules 2002, 35, 9146–9150. [Google Scholar] [CrossRef]
- Schmelz, J.; Karg, M.; Hellweg, T.; Schmalz, H. General pathway toward crystalline-core micelles with tunable morphology and corona segregation. ACS Nano 2011, 5, 9523–9534. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I.; Lerch, J.P.; Riess, G. Water dispersibility of non-aqueous emulsions stabilized and viscosified by a poly(butadiene)-poly(2-vinylpyridine)-poly(ethylene oxide) (PBut-P2VP-PEO) triblock copolymer. Colloids Surf. Part A Physicochem. Eng. Asp. 2015, 464, 89–95. [Google Scholar] [CrossRef]
- Betthausen, E.; Hanske, C.; Müller, M.; Fery, A.; Schacher, F.H.; Müller, A.H.E.; Pochan, D.J. Self-assembly of amphiphilic triblock terpolymers mediated by multifunctional organic acids: Vesicles, toroids, and (undulated) ribbons. Macromolecules 2014, 47, 1672–1683. [Google Scholar] [CrossRef]
- Kikuchi, A.; Nose, T. Unimolecular micelle formation of poly(methyl methacrylate)-graft-polystyrene in mixed selective solvents of acetonitrile/acetoacetic acid ethyl ether. Macromolecules 1996, 29, 6770–6777. [Google Scholar] [CrossRef]
- Borisov, O.V.; Zhulina, E.B. Amphiphilic graft copolymer in a selective solvent: Intramolecular structures and conformational transitions. Macromolecules 2005, 38, 2506–2514. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Hao, X.; Zeng, G.; Wang, W.; Liu, R.; Huang, Y. Backbone-collapsed intra- and inter-molecular self-assembly of cellulose-based dense graft copolymer. Carbohydr. Polym. 2012, 88, 290–298. [Google Scholar] [CrossRef]
- Francis, R.; Baby, D.K.; Gnanou, Y. Synthesis and self-assembly of chitosan-g-polystyrene copolymer: A new route for the preparation of heavy metal nanoparticles. J. Colloid Interface Sci. 2015, 438, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Jana, S.; Mandal, T.K. Peptide-poly(tert-butyl methacrylate) conjugate into composite micelles in organic solvents versus peptide-poly(methacrylic acid) conjugate into spherical and worm-like micelles in water: Synthesis and self-assembly. J. Polym. Sci. Part A Polym Chem. 2016, 54, 3019–3031. [Google Scholar] [CrossRef]
- Bose, A.; Jana, S.; Saha, A.; Mandal, T.K. Amphiphilic polypeptide-polyoxazoline graft copolymer conjugate with tunable thermoresponsiveness: Synthesis and self-assembly into various micellar structures in aqueous and nonaqueous media. Polymer 2017, 110, 12–24. [Google Scholar] [CrossRef]
- Stepánek, M.; Kosovan, P.; Procházka, K. Self-assembly of poly(4-methylstyrene)-g-poly(methacrylic acid) graft copolymer in selective solvents for grafts: Scattering and molecular dynamics simulation study. Langmuir 2010, 26, 9289–9296. [Google Scholar] [CrossRef] [PubMed]
- Gromadzki, D.; Filippov, S.; Netopilik, M.; Makuska, R.; Jigounov, A.; Plestil, J.; Horsky, J.; Stepánek, P. Combination of “living” nitroxide-mediated and photoiniferter-induced “grafting from” free-radical polymerizations: From branched copolymers to unimolecular micelles and microgels. Eur. Polym. J. 2009, 45, 1748–1758. [Google Scholar] [CrossRef]
- Koda, Y.; Terachima, T.; Sawamoto, M. Multimode self-folding polymers via reversible and thermoresponsive self-assembly of amphiphilic/fluorous random copolymers. Macromolecules 2016, 49, 4534–4543. [Google Scholar] [CrossRef]
- Liu, M.; Fu, Z.; Wang, Q.; Xu, J.; Fan, Z. Study of amphiphilic poly(1-dodecene-co-para-methylstyrene)-graft-poly(ethylene glycol). Part II: Preparation and micellization behavior of the amphiphilic copolymers. Eur. Polym. J. 2008, 44, 4122–4128. [Google Scholar] [CrossRef]
- Alexander, S.; Cosgrove, T.; de Vos, W.M.; Castle, T.C.; Prescott, S.W. Aggregation behavior of polyisoprene-pluronic graft copolymers in selective solvents. Langmuir 2014, 30, 5747–5754. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Liu, G.; Tu, Y.; Lin, S.; Song, J.; Hu, J.; Liu, F. Morphological switching of unimolar micelles of ternary graft copolymers in different solvents. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1021–1030. [Google Scholar] [CrossRef]




| Non-Polar Solvents | δ (MPa1/2) | Polar Solvents | δ (MPa1/2) |
|---|---|---|---|
| n-Hexane | 14.9 | Ethyl acetate | 18.1 |
| n-Heptane | 15.2 | Dichloromethane | 19.8 |
| n-Decane | 15.8 | Acetone | 19.9 |
| Hexadecane | 16.3 | THF | 19.5–20.2 |
| Cyclohexane | 16.7 | Isopropanol | 23.6 |
| Toluene | 18.2 | n-Propanol | 24.4 |
| Chloroform | 18.7 | Acetonitrile | 24.4 |
| 1,4-Dioxane | 19.9–20.5 | DMF | 24.9 |
| DMSO | 26.5 | ||
| Ethanol | 26.5 | ||
| Methanol | 29.7 | ||
| Water | 47.9 | ||
| Ionic liquids | 24.0–32.0 |
| Sample | Nagg | Rh (nm) |
|---|---|---|
| PS60-b-PI203 | 10.2 | 10.4 |
| (SO3Li)-PS60-b-PI203 | 23.4 | 14.2 |
| PS55-b-PI151-(SO3Li) | 17.0 | 660.0 a |
| 11.0 b | 75.0 b |
| AB Block Copolymers | Selective Solvent for A Block | Selective Solvent for B Block | Micellar Characteristics | Ref. |
|---|---|---|---|---|
| PS48-b-PNVP99 | - | CH3–OH | Rh = 16 nm Rg/Rh = 0.65 C.M.C. = 0.13 mg·mL−1 Nagg = 10 | [45] |
| POSS-PMMA144-b-P(MA-POSS)2.6;9.6;11.3 | THF | - | Rh = 85; 148; 80 nm Core-shell micelles | [46] |
| PMMA340;400-b-PtBMA134 | - | 2-ethylhexanol | Rh = 19.4 ÷ 28.2 nm Spheres and cylinders | [47] |
| PSAMA15-b-P(Boc-Phe-HEMA)7;17;37;75 | - | DMF; DMSO; ACN | Rh(DMF) = 119 ÷ 318 nm Rh(DMSO) = 37÷90 nm Rh(ACN) = 24 ÷ 48 nm Spheres | [48] |
| PEtOx10-b-PNBA7;17;31;48 | - | CH2Cl2 | Rh = 45 ÷ 60 nm Spheres | [49] |
| PVAc57-b-(PFHE-stat-PVAc)95 | CH3–OH | - | Rh = 10 ÷ 40 nm Spheres | [50] |
| PMMA41;54;73-b-PsfMA59;46;27 | THF; ACN; CHCl3 | - | Nagg(THF) = 8 Nagg(CHCl3) = 26 Nagg(ACN) = 410 Rh(THF) = 60–70 nm Spheres | [51] |
| AB Block Copolymers | Selective Solvent for A Block | Selective Solvents for B Block | Micellar Characteristics | Ref. |
|---|---|---|---|---|
| PEO432-b-PNIPAM106 | [BMIM][BF4] δ = 24 MPa1/2 | - | Rh = 25 nm L.C.M.T = 200 °C U.C.M.T = 60 °C | [58] |
| PS529;548;981-b-P2VP543;543;923 | - | [BMIM][CF3SO3] δ = 25 MPa1/2 | Spherical micelles | [59] |
| PEO341-b-P(AzoMA-r-NIPAM)177 | [C4MIM][PF6] δ = 23 MPa1/2 | - | Rh ~ 120 nm | [60] |
| PEO432-b-PnBMA99;183 | [BMIM][TFSI] δ = 26 MPa1/2 [EMIM][TFSI] δ = 27 MPa1/2 | - | C.M.T = 120–150 °C | [61] |
| PEGE109;113;104-b-PEO54;115;178 PGPrE98-b-PEO260 | PAN; EAN δ = 25–26 MPa1/2 | - | spherical micelles for PEO178-b-PEGE104 Disk-shape micelles for PEO54-b-PEGE109 | [62] |
| PEGE104-b-PEO178 | [C4MIM][PF6] δ = 23 MPa1/2 | - | Rh = 13 nm | [63] |
| PMMA250-b-PnBMA92;169;218;246;310;373;549 | [EMIM][TFSI] [BMIM][TFSI] | - | Rh = 17.8 ÷ 34.6 nm Rcore = 5.8 ÷ 23.2 nm Nagg = 41 ÷ 432 | [64,65] |
| ABC Triblock Terpolymers | Selective Solvent for A Block | Selective Solvent for B Block | Selective Solvent for C Block | Micellar Characteristics | Ref. |
|---|---|---|---|---|---|
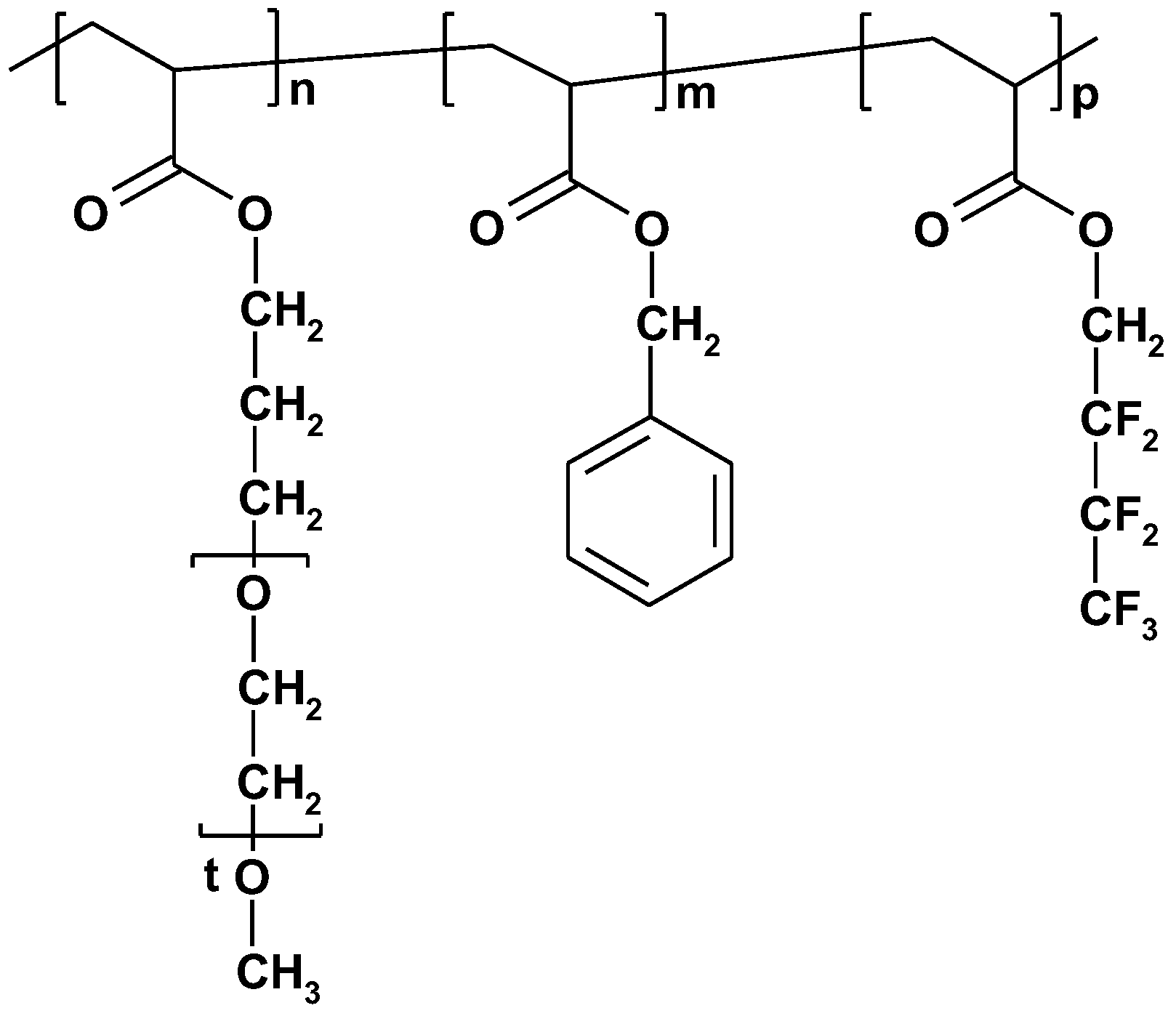
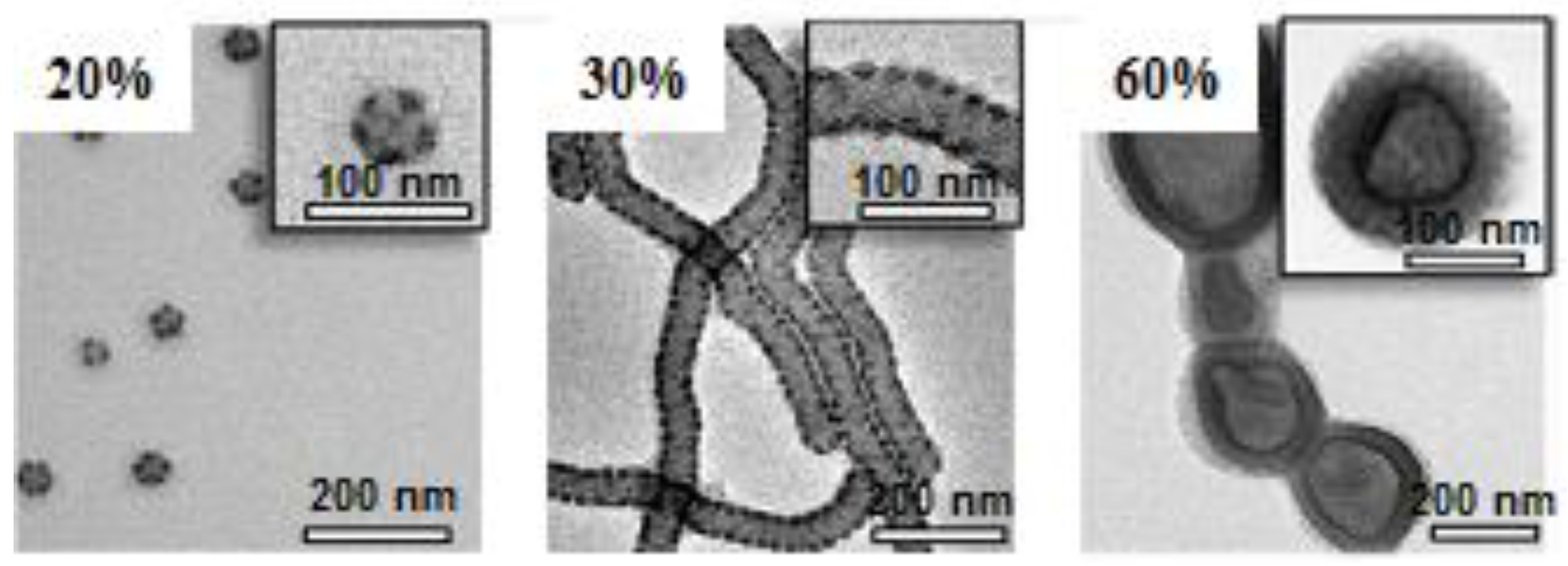
| PtBA107-b-PCEMA193-b-PGMA115 | CH3–OH | - | CH3–OH | Vesicles and cylinders | [93] |
| PB800-b-P2VP190-b-PtBMA380;550 | Acetone | - | - | Rh = 43; 44 nm Rg = 34; 36 nm Nagg = 203; 174 | [94] |
| PtBA110-b-PCEMA195-b-PSGMA115 | Propanol | - | - | Cylinders | [95] |
| PnBA28-b-PS37-b-P2VP73 | - | - | CH3–OH | Rh = 27 nm Spheres | [96] |
| PS306;510;516-b-PB151;258;140-b-PMMA340;260;76 | DMAc | - | DMAc | Spheres | [97] |
| PS385-b-PI485-b-P2VP829 | - | - | C2H5–OH | Ellipsoid | [98] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanase, L.I.; Riess, G. Self-Assembly of Block and Graft Copolymers in Organic Solvents: An Overview of Recent Advances. Polymers 2018, 10, 62. https://doi.org/10.3390/polym10010062
Atanase LI, Riess G. Self-Assembly of Block and Graft Copolymers in Organic Solvents: An Overview of Recent Advances. Polymers. 2018; 10(1):62. https://doi.org/10.3390/polym10010062
Chicago/Turabian StyleAtanase, Leonard Ionut, and Gerard Riess. 2018. "Self-Assembly of Block and Graft Copolymers in Organic Solvents: An Overview of Recent Advances" Polymers 10, no. 1: 62. https://doi.org/10.3390/polym10010062