Green and Facile Synthesis of Highly Stable Gold Nanoparticles via Hyperbranched Polymer In-Situ Reduction and Their Application in Ag+ Detection and Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Thermoresponsive HPEI-IBAm
2.4. In-Situ Preparation of HPEI-IBAm Functionalized AuNPs
2.5. Preparation of Citrate-Capped AuNPs
2.6. Stability Testing of HPEI-IBAm Functionalized AuNPs
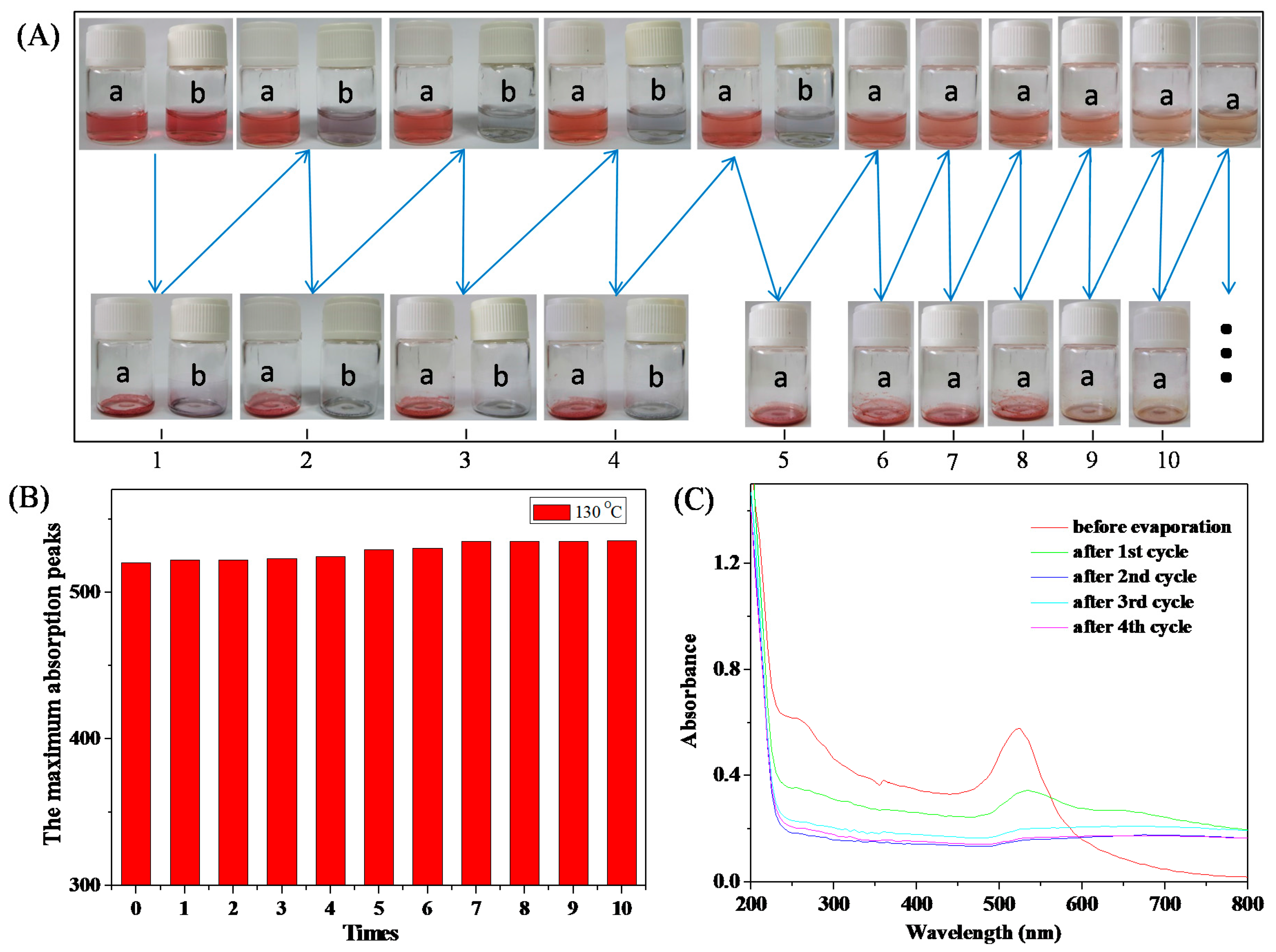
2.6.1. Thermal Stability Testing
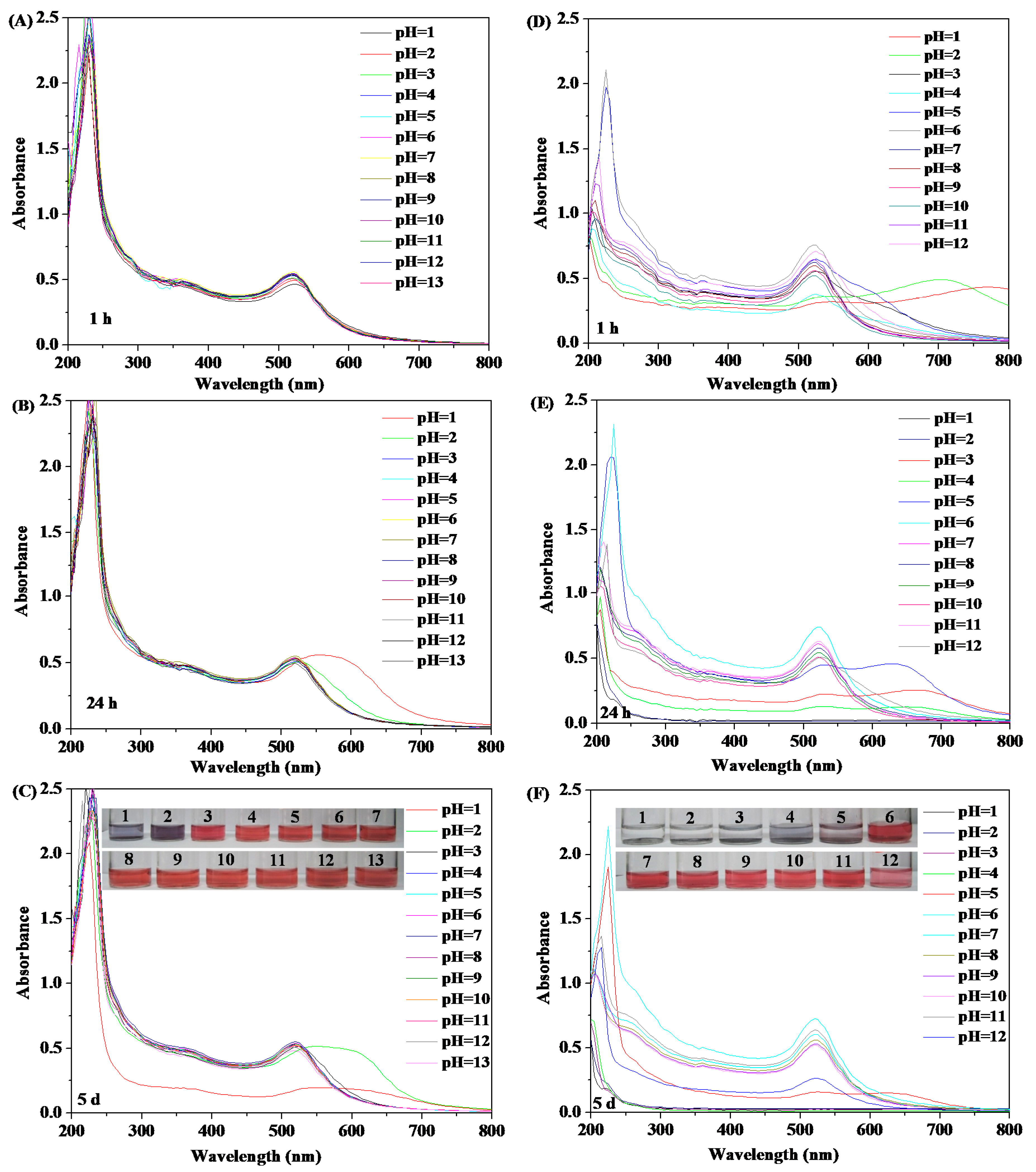
2.6.2. pH Stability Testing
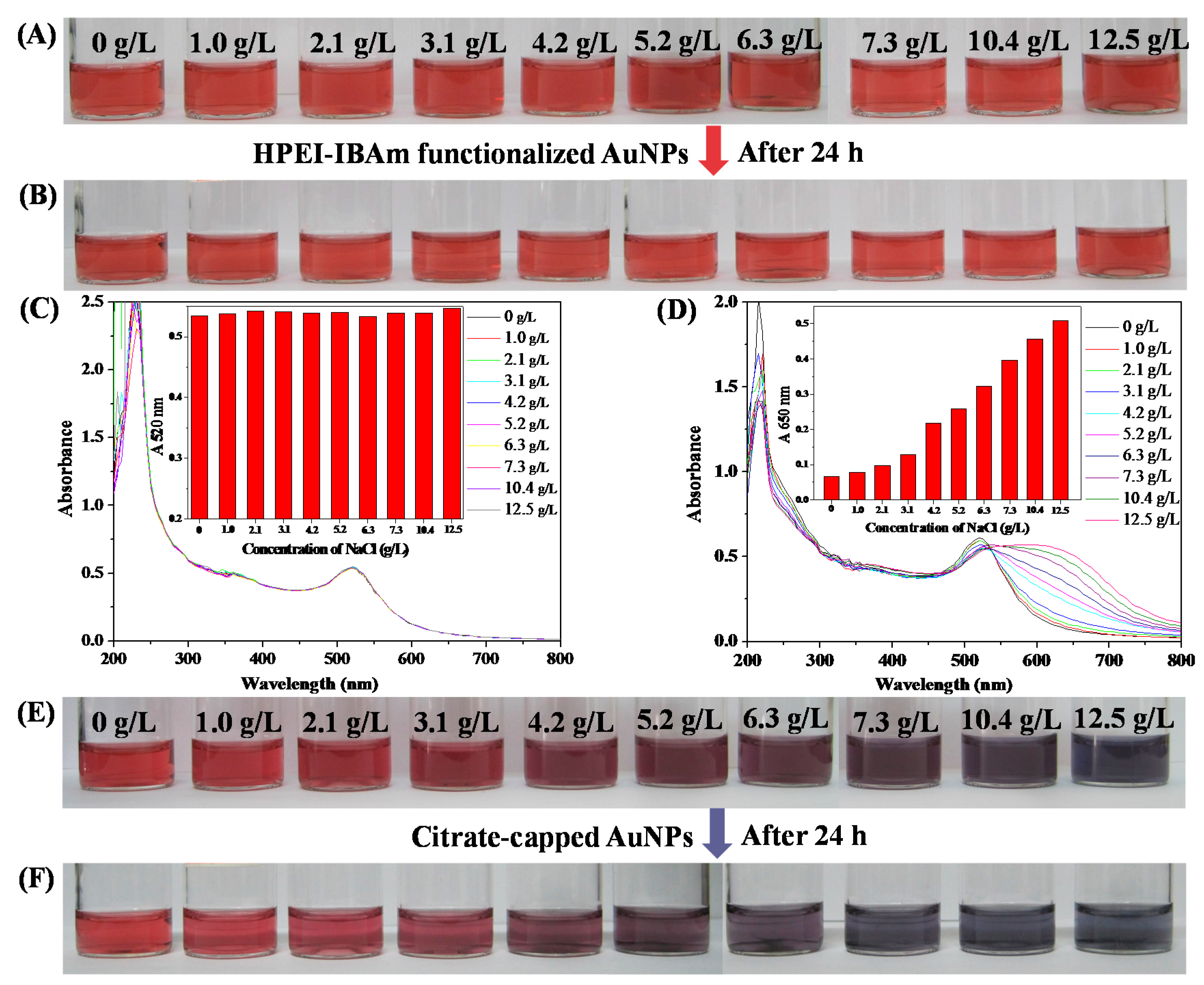
2.6.3. The Salt Tolerance Testing
2.7. Colorimetric Sensing of Ag+
3. Results
3.1. Synthesis and Characterization of HPEI-IBAm
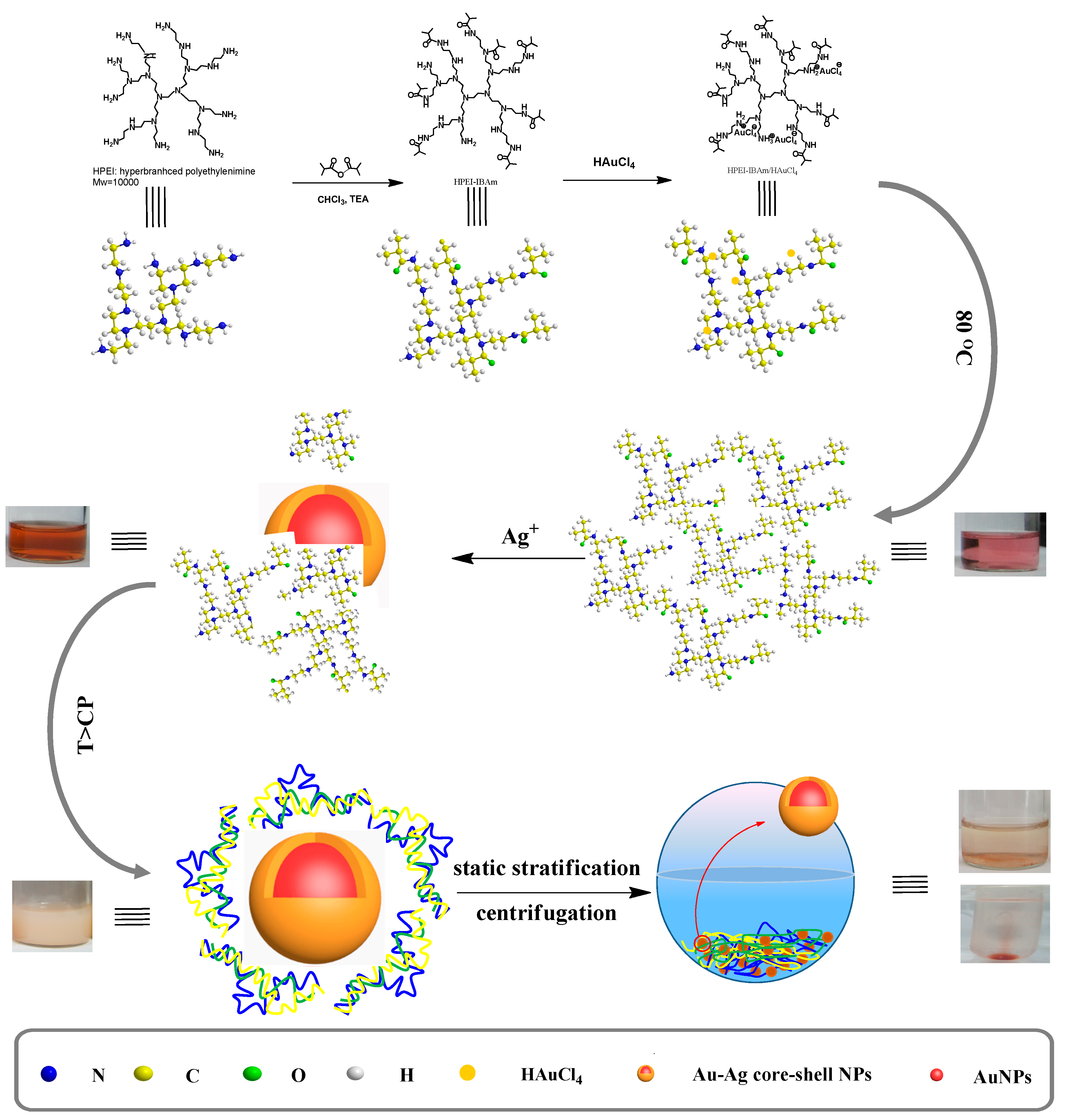
3.2. In-Situ Preparation of HPEI-IBAm Functionalized AuNPs
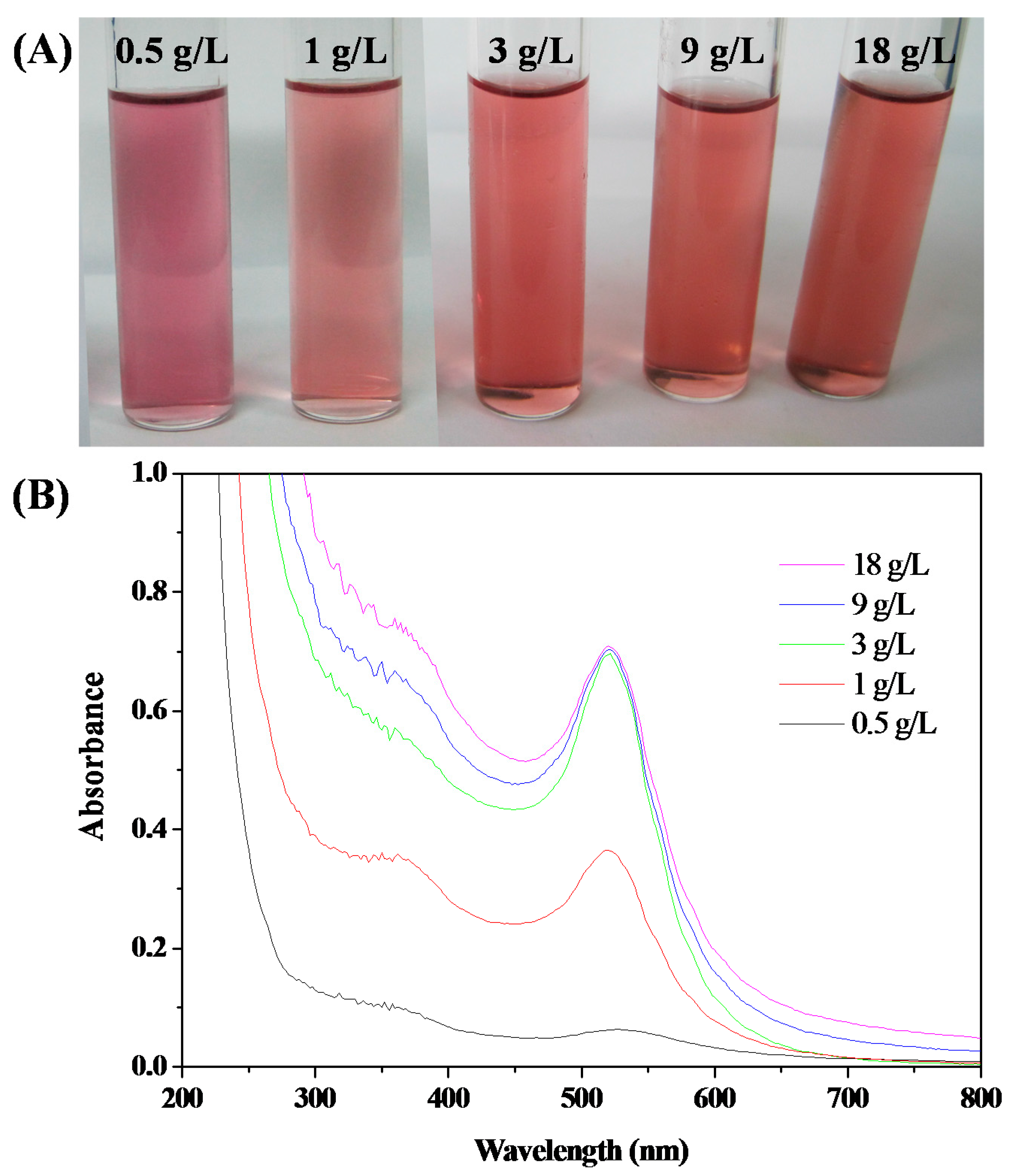
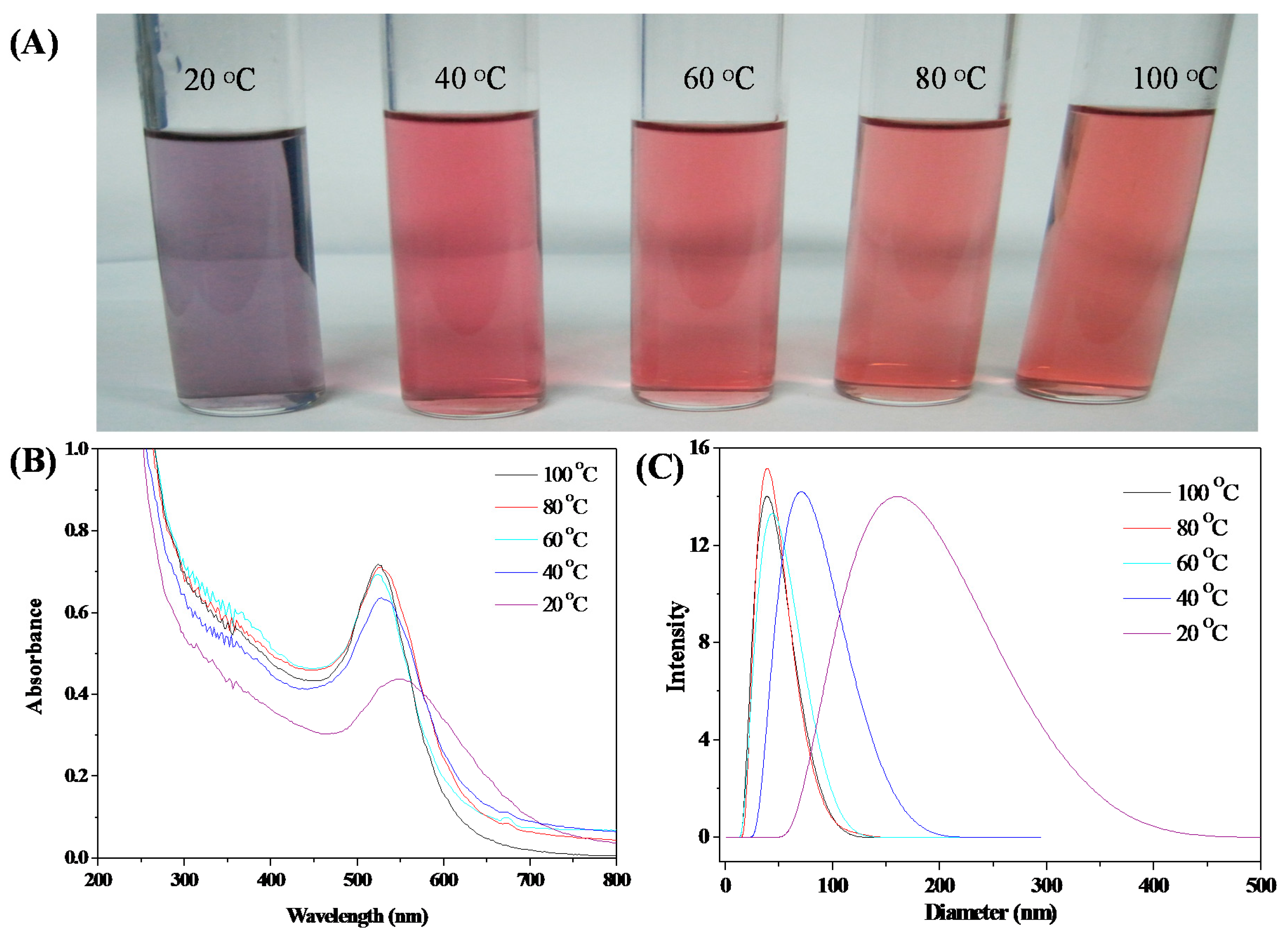
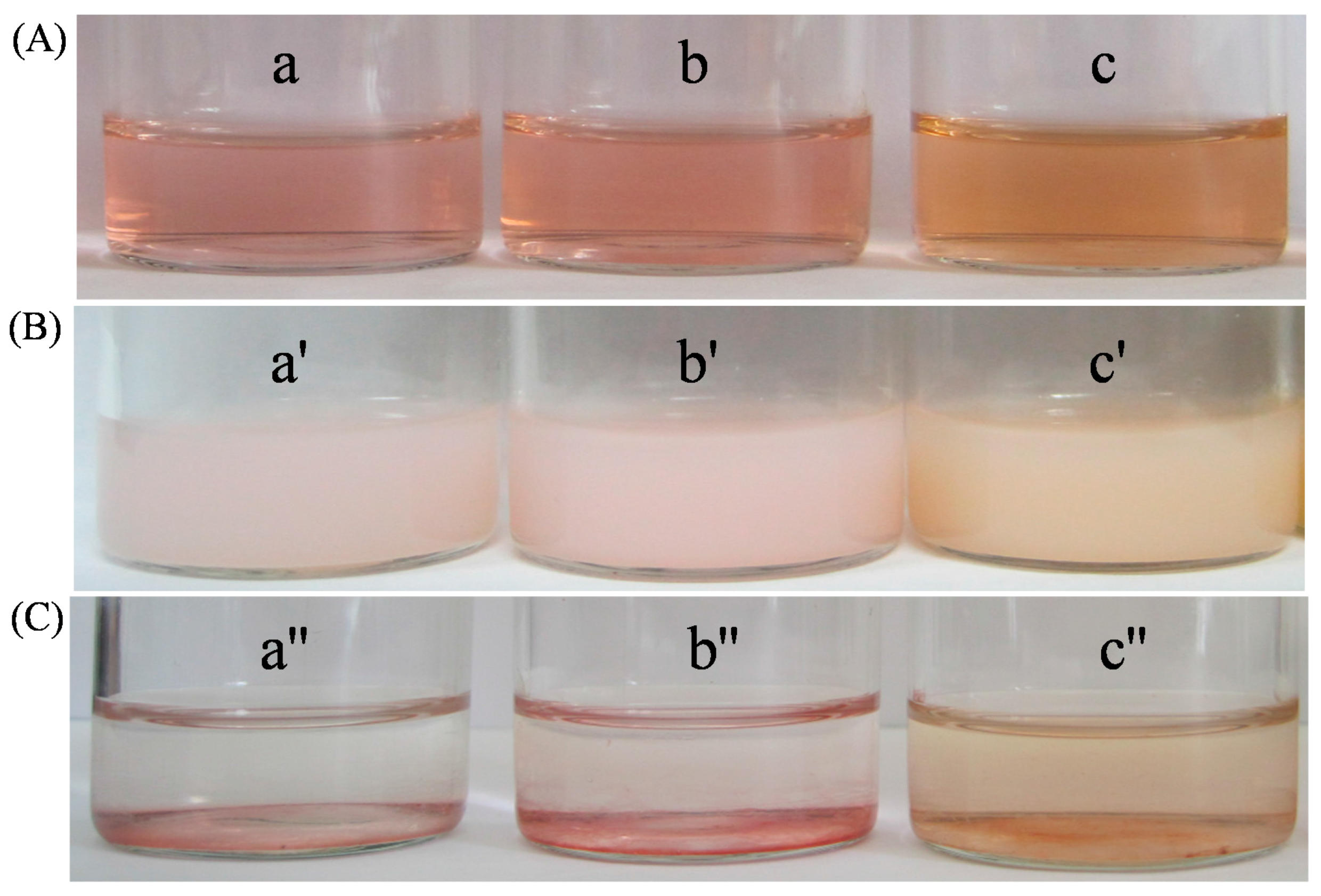
3.3. Stability of HPEI-IBAm Functionalized AuNPs
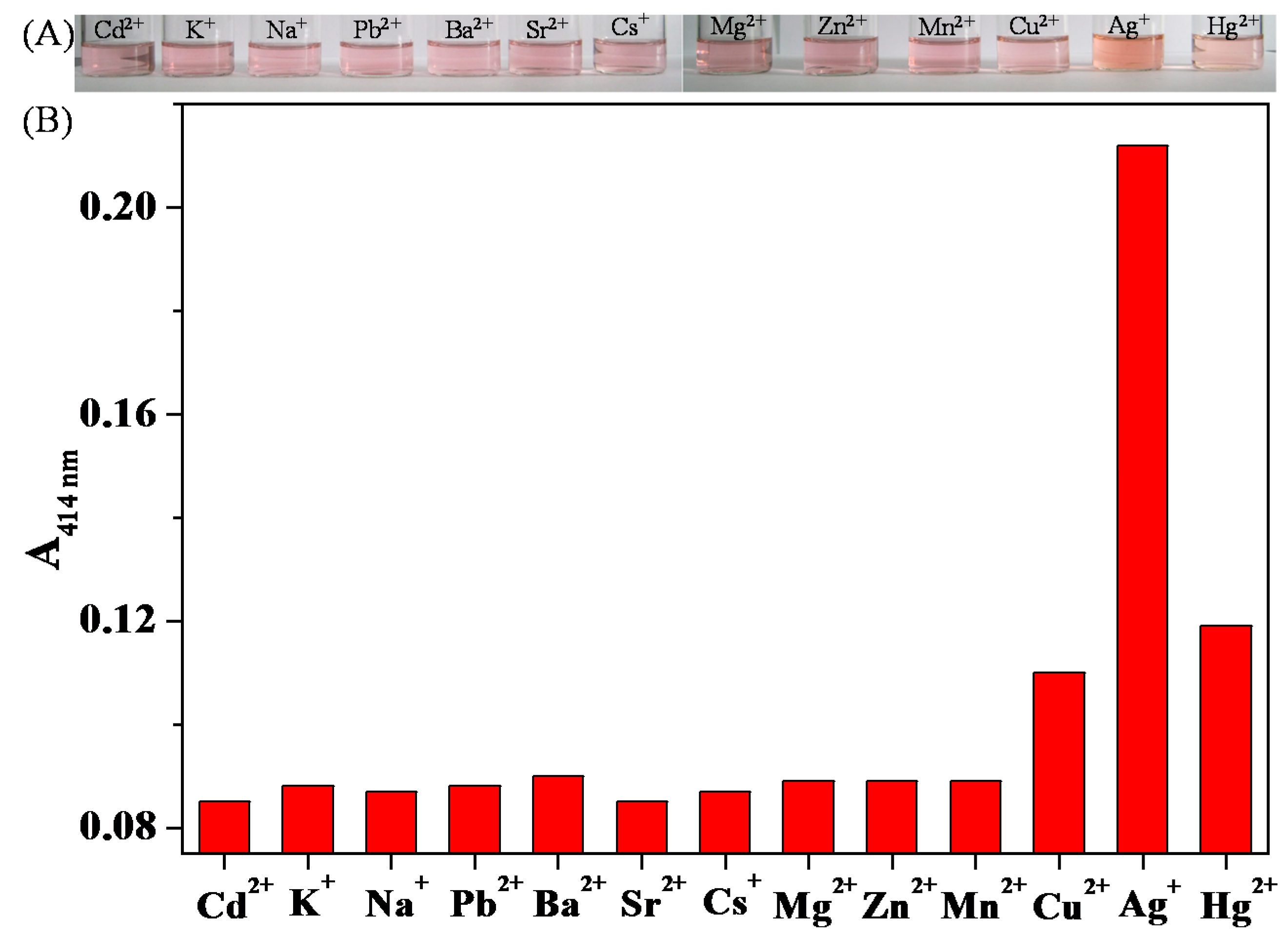
3.4. Colorimetric Sensing of Ag+
3.5. Ag+ Detection and Separation in Drinking Water
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold nanoparticles for in vitro diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [PubMed]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-encapsulated metal nanoparticles: Synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Min, B.K.; Friend, C.M. Heterogeneous gold-based catalysis for green chemistry: Low-temperature co oxidation and propene oxidation. Chem. Rev. 2007, 107, 2709–2724. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, J.; Xu, L.; Liu, X.; Liu, J.; Li, G. Red to brown to green colorimetric detection of Ag+ based on the formation of Au-Ag core-shell NPs stabilized by a multi-sulfhydryl functionalized hyperbranched polymer. Sens. Actuators B 2016, 237, 216–223. [Google Scholar] [CrossRef]
- Lou, T.T.; Chen, Z.P.; Wang, Y.Q.; Chen, L.X. Blue-to-red colorimetric sensing strategy for Hg2+ and Ag+ via redox-regulated surface chemistry of gold nanoparticles. ACS Appl. Mater. Interfaces 2011, 3, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Yu, C.J.; Lin, Y.H.; Tseng, W.L. Colorimetric sensing of silver(I) and mercury(II) ions based on an assembly of tween 20-stabilized gold nanoparticles. Anal. Chem. 2010, 82, 6830–6837. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Li, Z.; Liu, J.; Xu, L.; Liu, X. An unusual red-to-brown colorimetric sensing method for ultrasensitive silver(I) ion detection based on a non-aggregation of hyperbranched polyethylenimine derivative stabilized gold nanoparticles. Analyst 2015, 140, 5335–5343. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Xu, F.; Wang, S.; Xu, K.; Hou, X.; Wu, P. Gold nanoparticle-based colorimetric assay for selenium detection via hydride generation. Anal. Chem. 2017, 89, 4695–4700. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, O.; Bonardd, S.; Saldías, C.; Radic, D.; Leiva, Á. Biobased chitosan nanocomposite films containing gold nanoparticles: Obtainment, characterization, and catalytic activity assessment. ACS Appl. Mater. Interfaces 2017, 9, 16561–16570. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, X.; Hu, J.; Shao, N.; Wang, F.; Zhang, Q.; Xiao, J.; Cheng, Y. Glutathione-triggered “off-on” release of anticancer drugs from dendrimer-encapsulated gold nanoparticles. J. Am. Chem. Soc. 2013, 135, 9805–9810. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, J.Y.; Ying, J.Y. Phase transfer and its applications in nanotechnology. Chem. Soc. Rev. 2011, 40, 1672–1696. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Wu, Z.T.; Zhou, X.D.; Hu, J.M. Colorimetric response of peptide modified gold nanoparticles: An original assay for ultrasensitive silver detection. Biosens. Bioelectron. 2017, 92, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Chinwangso, P.; Jamison, A.C.; Lee, T.R. Multidentate adsorbates for self-assembled monolayer films. Acc. Chem. Res. 2011, 44, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.F.; Yan, Y.H.; Mangadlao, J.D.; Rong, L.H.; Advincula, R. Star-like copolymer stabilized noble-metal nanoparticle powders. Nanoscale 2016, 8, 7435–7442. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.F.; Rong, L.H.; de Leon, A.; Su, Z.; Advincula, R.C. A supramolecular polyethylenimine-cored carbazole dendritic polymer with dual applications. Macromolecules 2015, 48, 6801–6809. [Google Scholar] [CrossRef]
- Liu, X.Y.; Cheng, F.; Liu, Y.; Li, W.G.; Chen, Y.; Pan, H.; Liu, H.J. Thermoresponsive gold nanoparticles with adjustable lower critical solution temperature as colorimetric sensors for temperature, pH and salt concentration. J. Mater. Chem. 2010, 20, 278–284. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Liu, J.; Liu, X. Simultaneous enrichment, separation and detection of mercury(II) ions using cloud point extraction and colorimetric sensor based on thermoresponsive hyperbranched polymer-gold nanocomposite. Anal. Methods 2015, 7, 10151–10161. [Google Scholar] [CrossRef]
- Liu, X.Y.; Mu, X.R.; Liu, Y.; Liu, H.J.; Chen, Y.; Cheng, F.; Jiang, S.C. Hyperbranched polymers with thermoresponsive property highly sensitive to ions. Langmuir 2012, 28, 4867–4876. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Cheng, F.; Liu, Y.; Liu, H.J.; Chen, Y. Preparation and characterization of novel thermoresponsive gold nanoparticles and their responsive catalysis properties. J. Mater. Chem. 2010, 20, 360–368. [Google Scholar] [CrossRef]
- Zhan, C.; Fu, X.B.; Yao, Y.; Liu, H.J.; Chen, Y. Stimuli-responsive hyperbranched poly(amidoamine)s integrated with thermal and pH sensitivity, reducible degradability and intrinsic photoluminescence. RSC Adv. 2017, 7, 5863–5871. [Google Scholar] [CrossRef]
- Jin, R.; Wu, G.; Li, Z.; Mirkin, C.A.; Schatz, G.C. What controls the melting properties of DNA-linked gold nanoparticle assemblies? J. Am. Chem. Soc. 2003, 125, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Shi, X. Dendrimer-based organic/inorganic hybrid nanoparticles in biomedical applications. Nanoscale 2010, 2, 1596–1610. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Locklin, J.; Patton, D.; Baba, A.; Advincula, R.C. Thiophene dendron jacketed poly(amidoamine) dendrimers: Nanoparticle synthesis and adsorption on graphite. J. Am. Chem. Soc. 2005, 127, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Kaewtong, C.; Jiang, G.; Ponnapati, R.; Pulpoka, B.; Advincula, R. Redox nanoreactor dendrimer boxes: In situ hybrid gold nanoparticles via terthiophene and carbazole peripheral dendrimer oxidation. Soft Matter 2010, 6, 5316–5319. [Google Scholar] [CrossRef]
- Boisselier, E.; Diallo, A.K.; Salmon, L.; Ornelas, C.; Ruiz, J.; Astruc, D. Encapsulation and stabilization of Gold nanoparticles with “Click” polyethyleneglycol dendrimers. J. Am. Chem. Soc. 2010, 132, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Hermes, J.P.; Sander, F.; Peterle, T.; Urbani, R.; Pfohl, T.; Thompson, D.; Mayor, M. Gold nanoparticles stabilized by thioether dendrimers. Chem. A Eur. J. 2011, 17, 13473–13481. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Khodaei, M.M.; Hamidi, Z.; Shamsuddin, M.B. Naked-eye colorimetric detection of Cu2+ and Ag+ ions based on close-packed aggregation of pyridines-functionalized gold nanoparticles. Sens. Actuators, B 2014, 190, 782–791. [Google Scholar] [CrossRef]
- Huang, H.; Chen, S.; Liu, F.; Zhao, Q.; Liao, B.; Yi, S.; Zeng, Y. Multiplex plasmonic sensor for detection of different metal ions based on a single type of gold nanorod. Anal. Chem. 2013, 85, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- National Secondary Drinking Water Regulation: EPA 816-F-09-004; Environmental Protection Agency: Washington, DC, USA, 2009.





| Added (µM) | Found (µM) | Recovery (%) | Residual Ag (µM) | Enriching efficiency (%) |
|---|---|---|---|---|
| 60 | 62.1 | 103.5 | 1.9 | 96.8 |
| 90 | 88.4 | 98.2 | 3.5 | 96.1 |
| 120 | 126.3 | 105.3 | 3.2 | 97.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhu, C.; Xu, L.; Dai, Y.; Liu, Y.; Liu, Y. Green and Facile Synthesis of Highly Stable Gold Nanoparticles via Hyperbranched Polymer In-Situ Reduction and Their Application in Ag+ Detection and Separation. Polymers 2018, 10, 42. https://doi.org/10.3390/polym10010042
Liu X, Zhu C, Xu L, Dai Y, Liu Y, Liu Y. Green and Facile Synthesis of Highly Stable Gold Nanoparticles via Hyperbranched Polymer In-Situ Reduction and Their Application in Ag+ Detection and Separation. Polymers. 2018; 10(1):42. https://doi.org/10.3390/polym10010042
Chicago/Turabian StyleLiu, Xunyong, Chenxue Zhu, Li Xu, Yuqing Dai, Yanli Liu, and Yi Liu. 2018. "Green and Facile Synthesis of Highly Stable Gold Nanoparticles via Hyperbranched Polymer In-Situ Reduction and Their Application in Ag+ Detection and Separation" Polymers 10, no. 1: 42. https://doi.org/10.3390/polym10010042