Recrystallization and Micronization of p-Toluenesulfonamide Using the Rapid Expansion of Supercritical Solution (RESS) Process
Abstract
:1. Introduction
2. Experiment
2.1. Materials
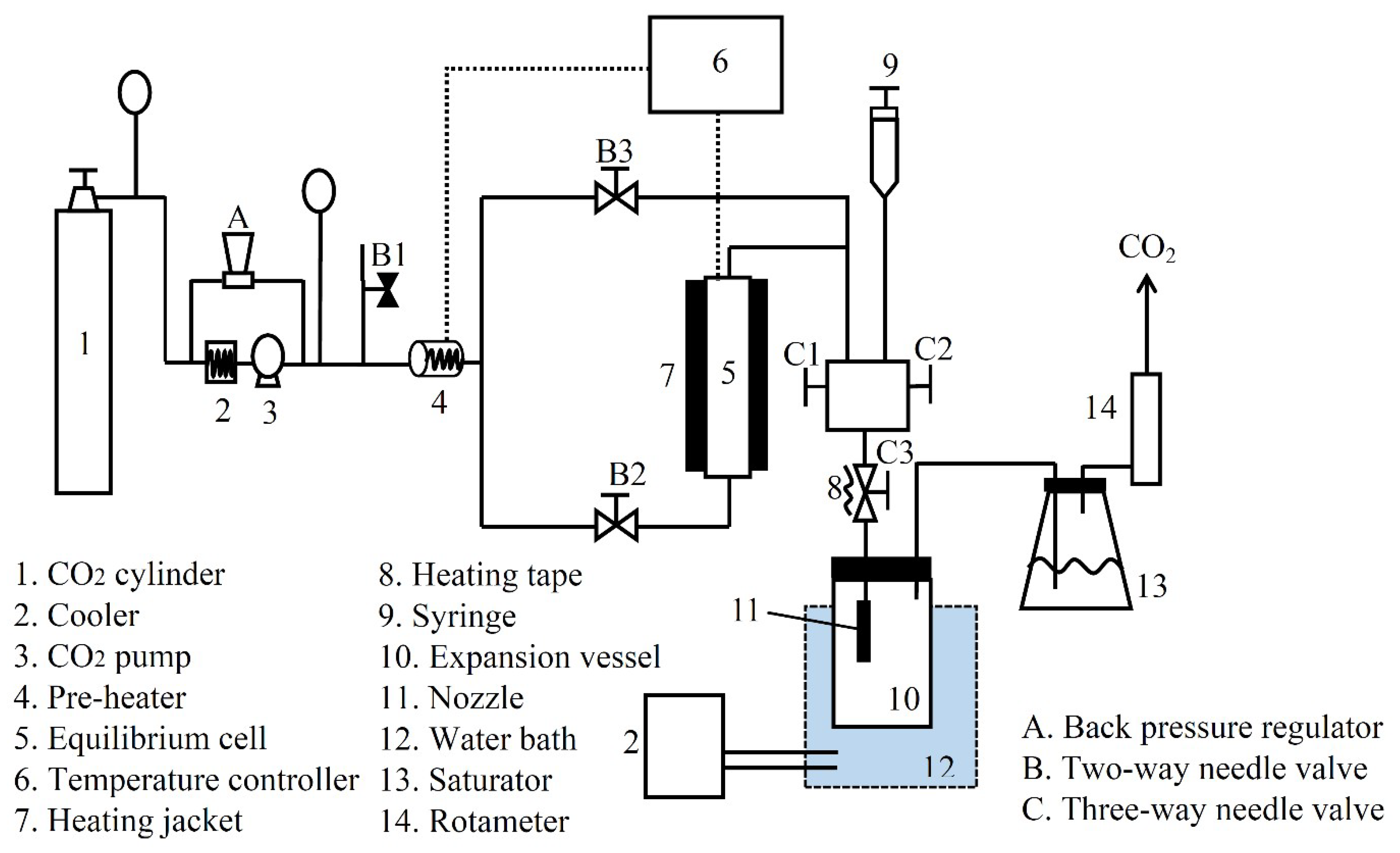
2.2. Apparatus and Procedure
2.3. Design of Experiments
3. Results and Discussion
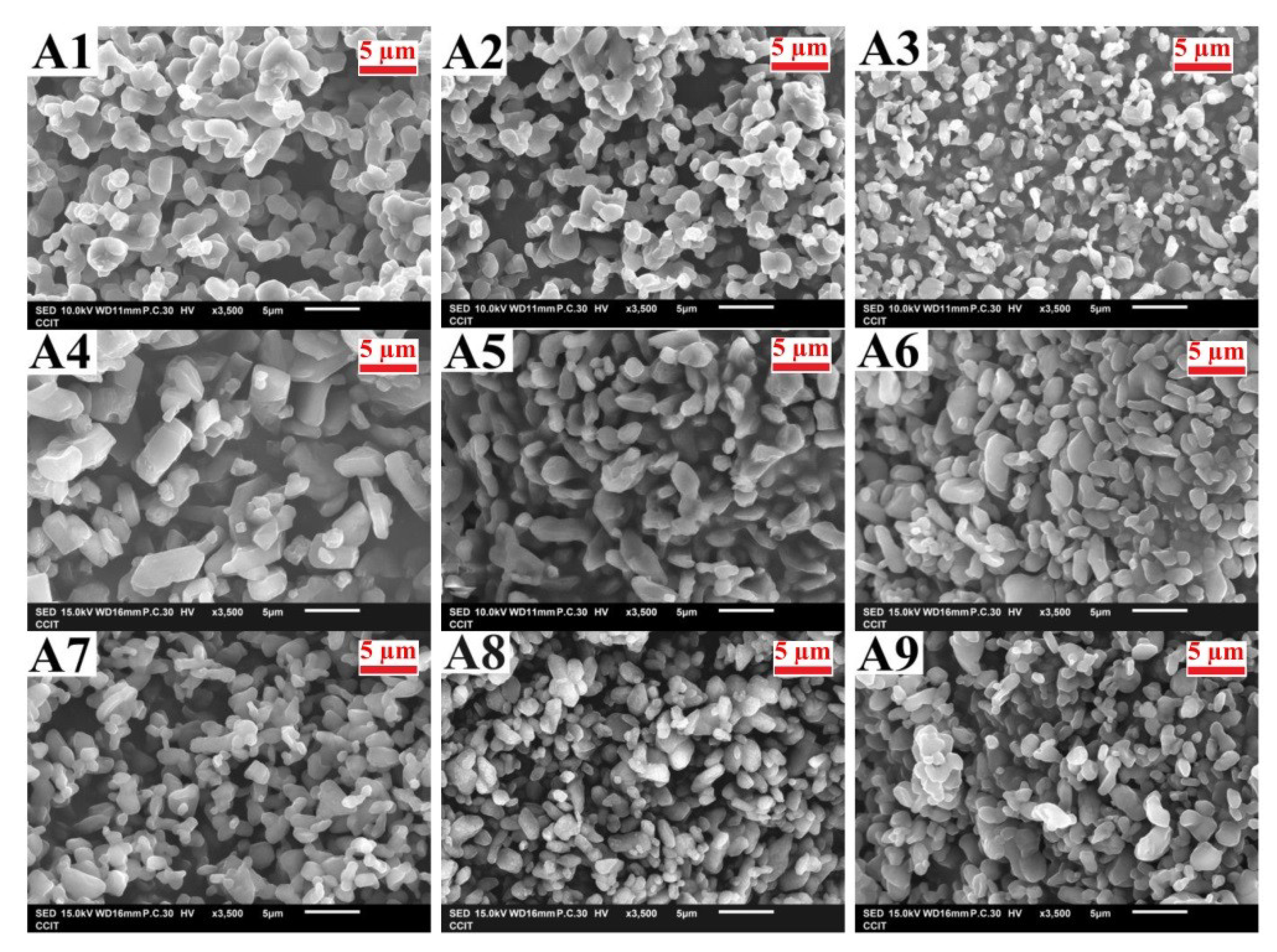
3.1. Analysis and Verification of Taguchi Experiments
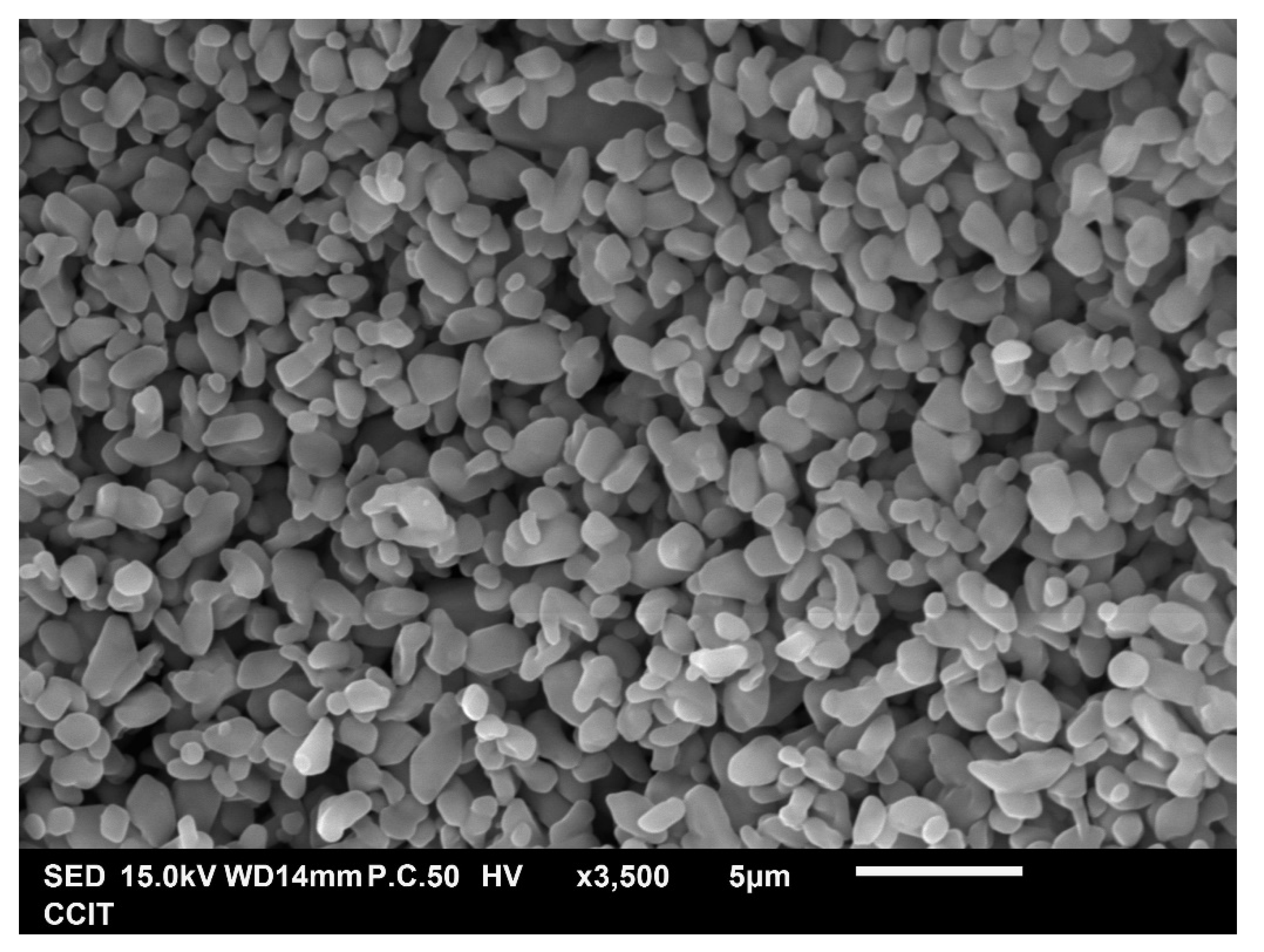
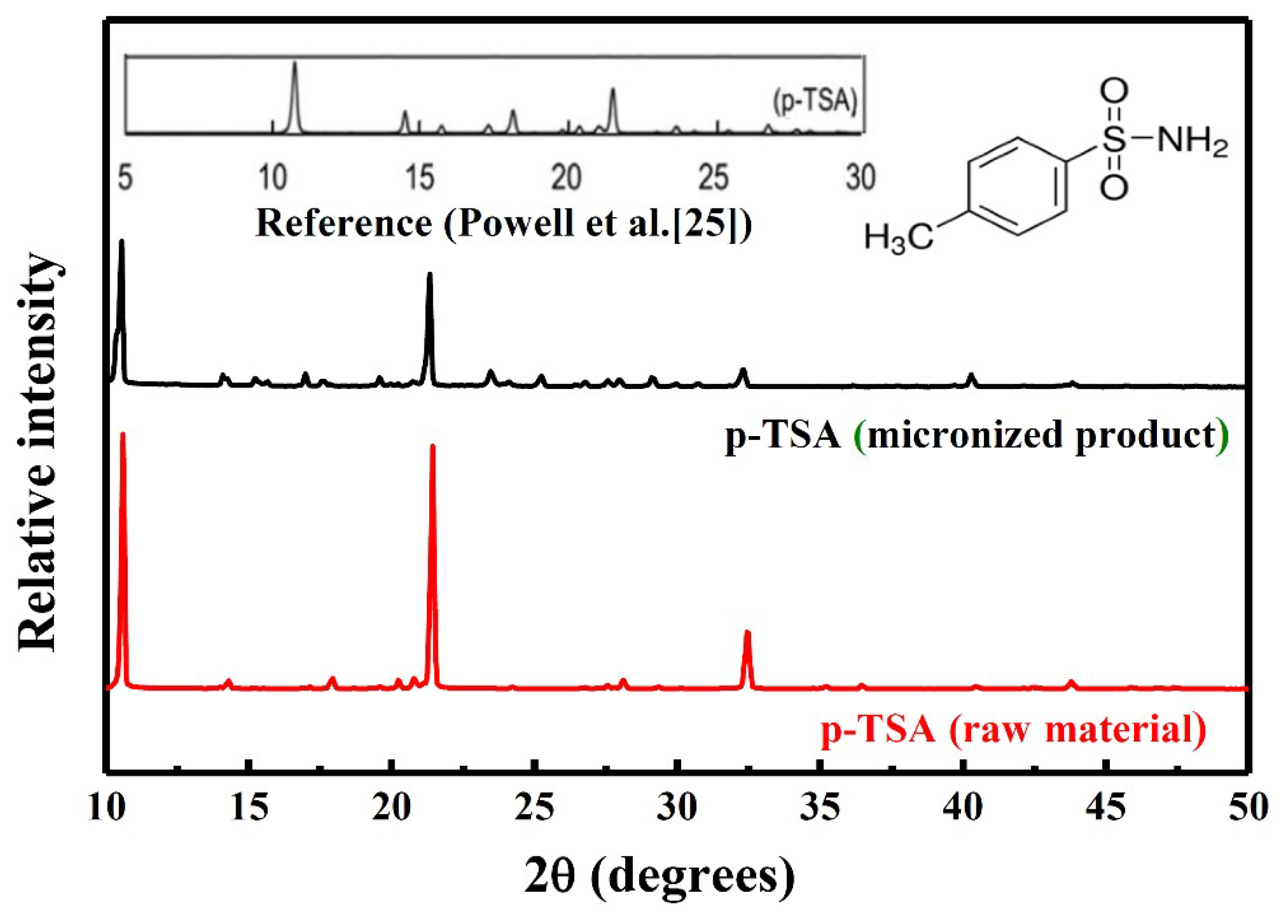
3.2. Identification of Micronized Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Umasish, J.; Maiti, S.; Biswas, S. An efficient FeCl3-catalyzed amidation reaction of secondary benzylic and allylic alcohols with carboxamides or p-toluenesulfonamide. Tetrahedron Lett. 2008, 49, 858–862. [Google Scholar]
- Meinertz, J.R.; Stehly, G.R.; Gingerich, W.H.; Greseth, S.L. Performance of a proposed determinative method for p-TSA in rainbow trout fillet tissue and bridging the proposed method with a method for total chloramine-T residues in rainbow trout fillet tissue. J. AOAC Int. 2001, 84, 1332–1336. [Google Scholar] [PubMed]
- Richter, D.; Dünnbier, U.; Massmann, G.; Pekdeger, A. Quantitative determination of three sulfonamides in environmental water samples using liquid chromatography coupled to electrospray tandem mass spectrometry. J. Chromatogr. A. 2007, 1157, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Meffe, R.; Kohfahl, C.; Holzbecher, E.; Massmann, G.; Richter, D.; Dünnbier, U.; Pekdeger, A. Modelling the removal of p-TSA (para-toluenesulfonamide) during rapid sand filtration used for drinking water treatment. Water Res. 2010, 44, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Massmann, G.; Dünnbier, U. Behaviour and biodegradation of sulfonamides (p-TSA, o-TSA, BSA) during drinking water treatment. Chemosphere 2008, 71, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ying, W.; Yang, H.; Xu, X.; Shao, W.; Guan, Y.; Jiang, M.; Wu, Y.; Zhong, B.; Wang, D.; et al. Gemcitabine plus cisplatin chemotherapy with concurrent para-toluenesulfonamide local injection therapy for peripherally advanced nonsmall cell lung cancer larger than 3 cm in the greatest dimension. Anticancer Drugs 2009, 20, 838–844. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Y.H.; Guan, W.J.; Huang, L.Y.; Xu, X.M.; Zhang, C.T.; Chen, X.Q.; Wu, Y.H.; Zeng, G.Q.; Zhong, N.S. Antitumor effect of para-toluenesulfonamide against lung cancer xenograft in a mouse model. J. Thorac. Dis. 2013, 5, 472–483. [Google Scholar]
- Hsu, J.L.; Leu, W.J.; Hsu, L.C.; Liu, S.P.; Zhong, N.S.; Guh, J.H. Para-Toluenesulfonamide induces anti-tumor activity through akt-dependent and -independent mTOR/p70S6K pathway: Roles of lipid raft and cholesterol contents. Front. Pharmacol. 2018, 9, 1223. [Google Scholar] [CrossRef]
- Rasenack, N.; Müller, B.W. Micron-Size drug particles: Common and novel micronization techniques. Pharm. Dev. Technol. 2004, 9, 1–13. [Google Scholar] [CrossRef]
- Hezave, A.Z.; Lashkarbolooki, M.; Esmaeilzadeh, F. Micronization of cetirizine using rapid expansion of supercritical carbon dioxide. Open Access Libr. J. 2015, 2, 1–14. [Google Scholar] [CrossRef]
- Hiendrawan, S.; Veriansyah, B.; Tjandrawinata, R.R. Preparation of fenofibrate microparticles using top-down and bottom-up processes. Procedia. Chem. 2014, 9, 257–264. [Google Scholar] [CrossRef]
- Chang, C.J.; Randolph, A.D. Precipitation of microsize organic particles from supercritical fluids. AIChE J. 1989, 35, 1876–1882. [Google Scholar] [CrossRef]
- Su, C.S.; Tang, M.; Chen, Y.P. Micronization of nabumetone using the rapid expansion of supercritical solution (RESS) process. J. Supercrit. Fluids 2009, 50, 69–76. [Google Scholar] [CrossRef]
- Hezave, A.Z.; Esmaeilzadeh, F. Micronization of drug particles via RESS process. J. Supercrit. Fluids 2010, 52, 84–98. [Google Scholar] [CrossRef]
- Keshavarz, A.; Karimi-Sabet, J.; Fattahi, A.; Golzary, A.A.; Rafiee-Tehrani, M.; Dorkoosh, F.A. Preparation and characterization of raloxifene nanoparticles using rapid expansion of supercritical solution (RESS). J. Supercrit. Fluids 2012, 63, 169–179. [Google Scholar] [CrossRef]
- Keshmiri, K.; Vatanara, A.; Tavakoli, O.; Manafi, N. Production of ultrafine clobetasol propionate via rapid expansion of supercritical solution (RESS): Full factorial approach. J. Supercrit. Fluids 2015, 101, 176–183. [Google Scholar] [CrossRef]
- Fattahi, A.; Karimi-Sabet, J.; Keshavarz, A.; Golzary, A.; Rafiee-Tehrani, M.; Dorkoosh, F.A. Preparation and characterization of simvastatin nanoparticles using rapid expansion of supercritical solution (RESS) with trifluoromethane. J. Supercrit. Fluids 2016, 107, 469–478. [Google Scholar] [CrossRef]
- Hiendrawan, S.; Veriansyah, B.; Tjandrawinata, R.R. Micronization of fenofibrate by rapid expansion of supercritical solution. J. Ind. Eng. Chem. 2014, 20, 54–60. [Google Scholar] [CrossRef]
- Li, J.L.; Jin, J.S.; Zhang, Z.T.; Pei, X.M. Solubility of p-Toluenesulfonamide in pure and modified supercritical carbon dioxide. J. Chem. Eng. Data 2009, 54, 1142–1146. [Google Scholar] [CrossRef]
- Taguchi, S. Taguchi Methods and QFD: Hows and Whys for Management; American Supplier Institute: Michigan, MI, USA, 1987. [Google Scholar]
- Cochran, W.G.; Cox, G.M. Experimental Design; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Mitra, A. Fundamentals of Quality Control and Improvement; John Wiley & Sons: New York, NY, USA, 2016. [Google Scholar]
- Roy, R.K. Design of Experiments Using the Taguchi Approach; John Wiley & Sons: New Jersey, NJ, USA, 2001. [Google Scholar]
- Atila, C.; Yildiz, N.; Calimli, A. Particle size design of digitoxin in supercritical fluids. J. Supercrit. Fluids. 2010, 51, 404–411. [Google Scholar] [CrossRef]
- Powell, K.A.; Croker, D.M.; Rielly, C.D.; Nagy, Z.K. PAT-Based design of agrochemical co-crystallization processes: A case-study for the selective crystallization of 1:1 and 3:2 co-crystals of p-toluenesulfonamide/triphenylphosphine oxide. Chem. Eng. Sci. 2016, 152, 95–108. [Google Scholar] [CrossRef]




| Control Factor | Level | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| A. Extraction temperature (°C) | 40 | 45 | 50 |
| B. Extraction pressure (MPa) | 180 | 200 | 220 |
| C. Pre-expansion temperature (°C) | 180 | 200 | 220 |
| D. Post-expansion temperature (°C) | 20 | 30 | 40 |
| Exp. No. | Extraction Temperature (°C) | Extraction Pressure (MPa) | Pre-Expansion Temperature (°C) | Post-Expansion Temperature (°C) |
|---|---|---|---|---|
| A1 | 40 | 180 | 180 | 20 |
| A2 | 40 | 200 | 200 | 30 |
| A3 | 40 | 220 | 220 | 40 |
| A4 | 45 | 180 | 200 | 40 |
| A5 | 45 | 200 | 220 | 20 |
| A6 | 45 | 220 | 180 | 30 |
| A7 | 50 | 180 | 220 | 30 |
| A8 | 50 | 200 | 180 | 40 |
| A9 | 50 | 220 | 200 | 20 |
| Exp. No. | Measured Particle Number (-) | Average Particle Size (µm) | Relative Standard Deviation (%) |
|---|---|---|---|
| A1 | 851 | 1.83 | 3.7 |
| A2 | 1088 | 1.39 | 4.0 |
| A3 | 1039 | 1.26 | 4.2 |
| A4 | 868 | 2.53 | 3.5 |
| A5 | 856 | 1.89 | 4.1 |
| A6 | 892 | 1.63 | 5.3 |
| A7 | 1285 | 1.45 | 4.1 |
| A8 | 922 | 1.37 | 4.7 |
| A9 | 927 | 1.40 | 4.6 |
| Level | Control Factors | |||
|---|---|---|---|---|
| A (Extraction Temperature) | B (Extraction Pressure) | C (Pre-Expansion Temperature) | D (Post-Expansion Temperature) | |
| 1 | 1.49 | 1.94 | 1.61 | 1.71 |
| 2 | 2.02 | 1.55 | 1.77 | 1.49 |
| 3 | 1.41 | 1.43 | 1.53 | 1.72 |
| Range | 0.61 | 0.51 | 0.24 | 0.23 |
| Rank | 1 | 2 | 3 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.-M.; Su, C.-S.; Li, J.-S.; Lu, K.-T.; Yeh, T.-F. Recrystallization and Micronization of p-Toluenesulfonamide Using the Rapid Expansion of Supercritical Solution (RESS) Process. Crystals 2019, 9, 449. https://doi.org/10.3390/cryst9090449
Yang T-M, Su C-S, Li J-S, Lu K-T, Yeh T-F. Recrystallization and Micronization of p-Toluenesulfonamide Using the Rapid Expansion of Supercritical Solution (RESS) Process. Crystals. 2019; 9(9):449. https://doi.org/10.3390/cryst9090449
Chicago/Turabian StyleYang, Tsung-Mao, Chie-Shaan Su, Jin-Shuh Li, Kai-Tai Lu, and Tsao-Fa Yeh. 2019. "Recrystallization and Micronization of p-Toluenesulfonamide Using the Rapid Expansion of Supercritical Solution (RESS) Process" Crystals 9, no. 9: 449. https://doi.org/10.3390/cryst9090449





