Zeolitic Imidazolate Framework Membranes for Light Olefin/Paraffin Separation
Abstract
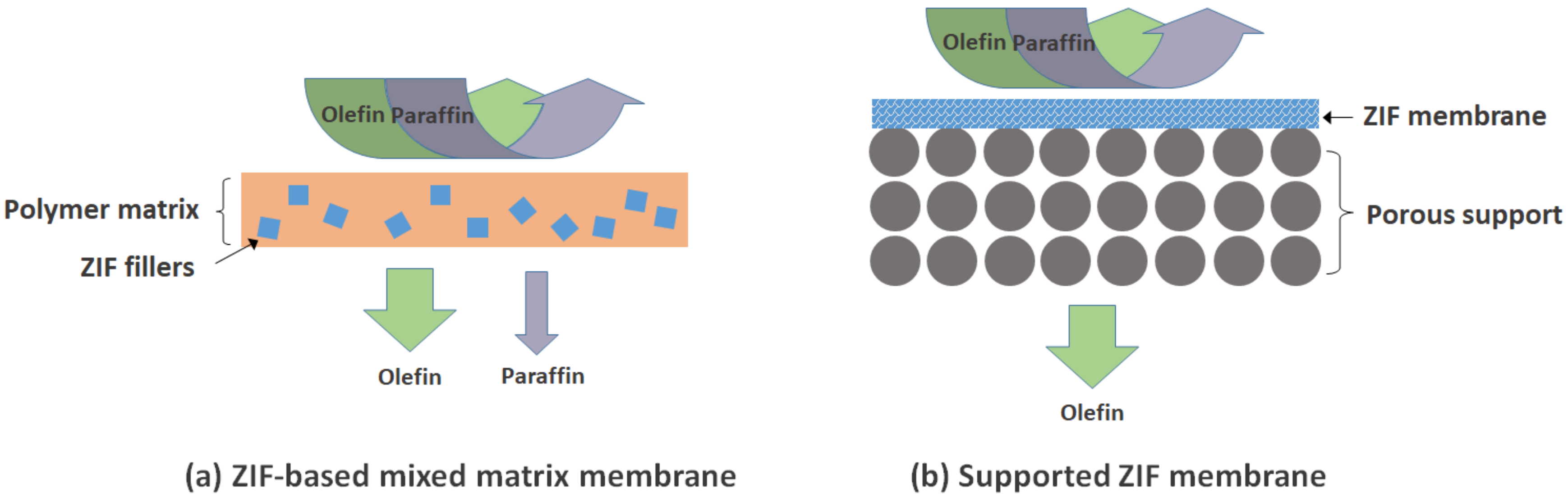
:1. Introduction
2. ZIF Membranes for Propylene/Propane Separation
2.1. ZIF-8 Membranes for C3 Separation: Membrane Synthesis
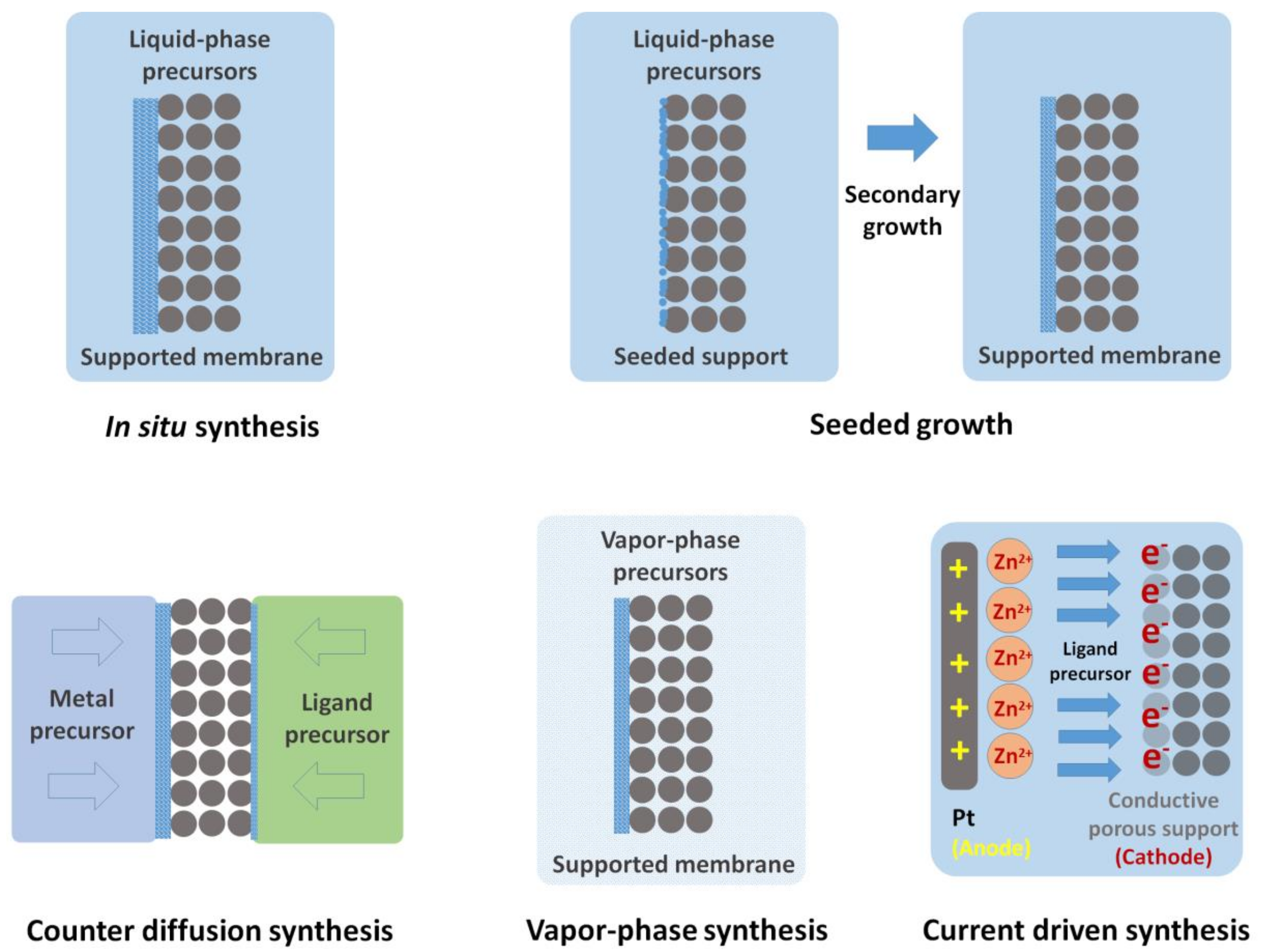
2.1.1. In Situ Crystallization
2.1.2. Seeded Growth
2.1.3. Counter-Diffusion Synthesis
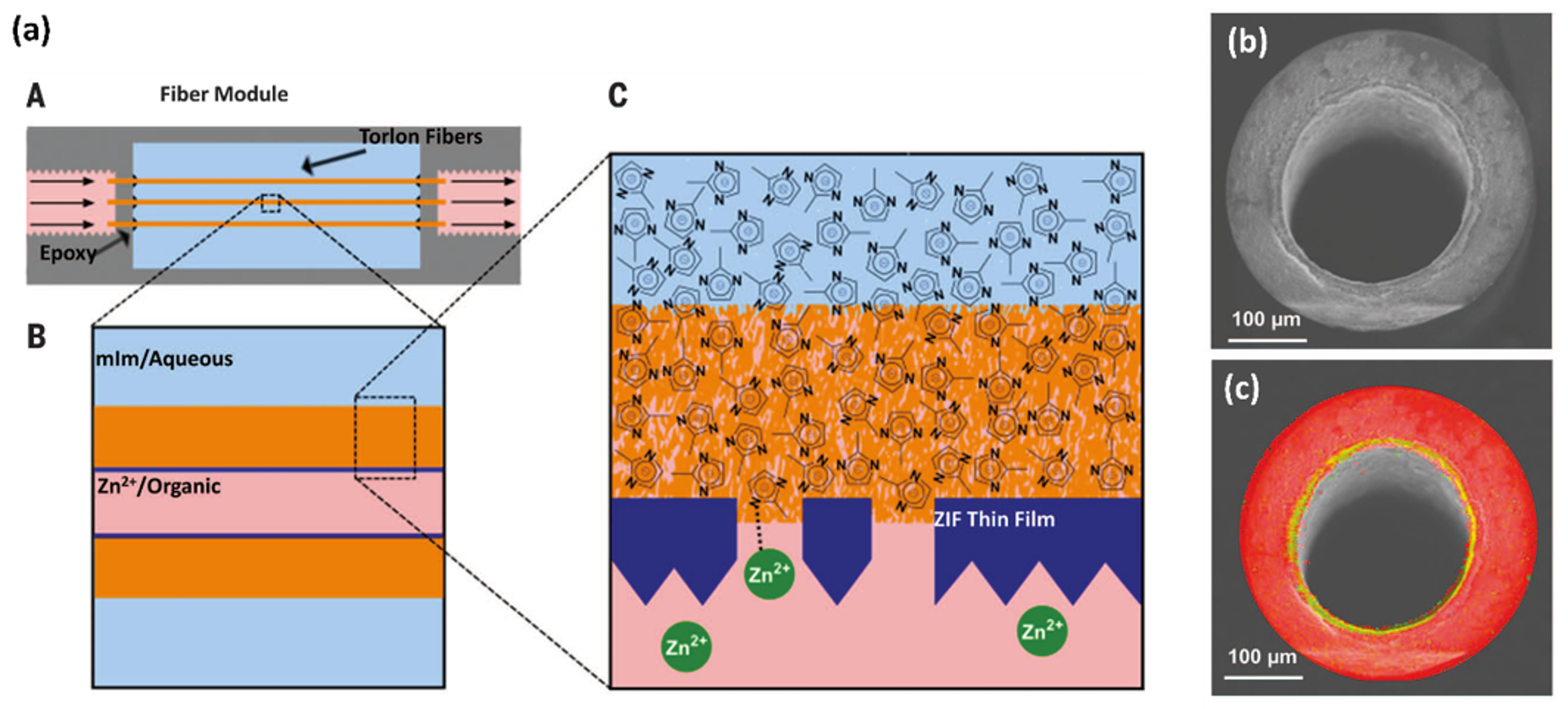
2.1.4. Interfacial Microfluidic Processing
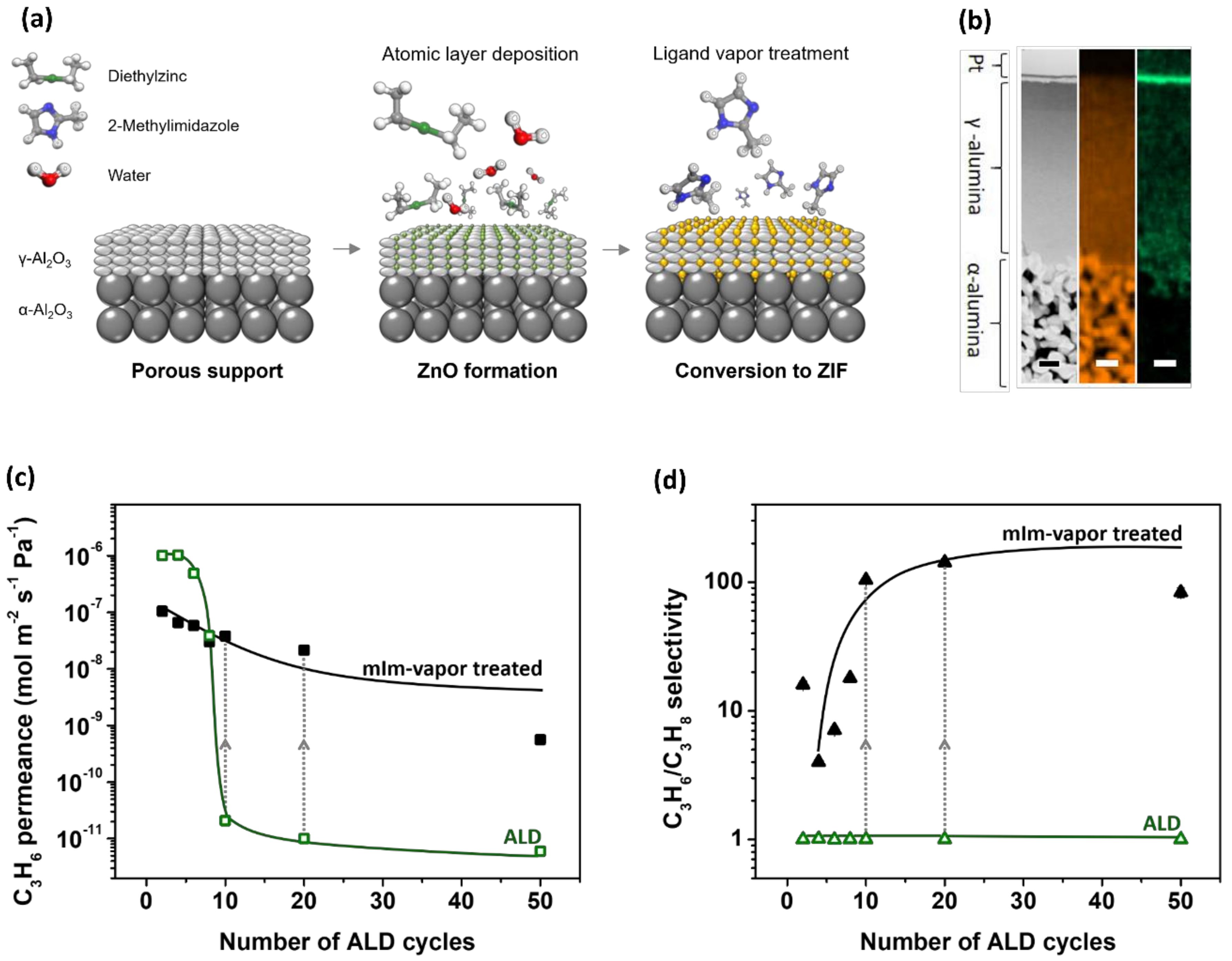
2.1.5. Vapor-Phase Processing
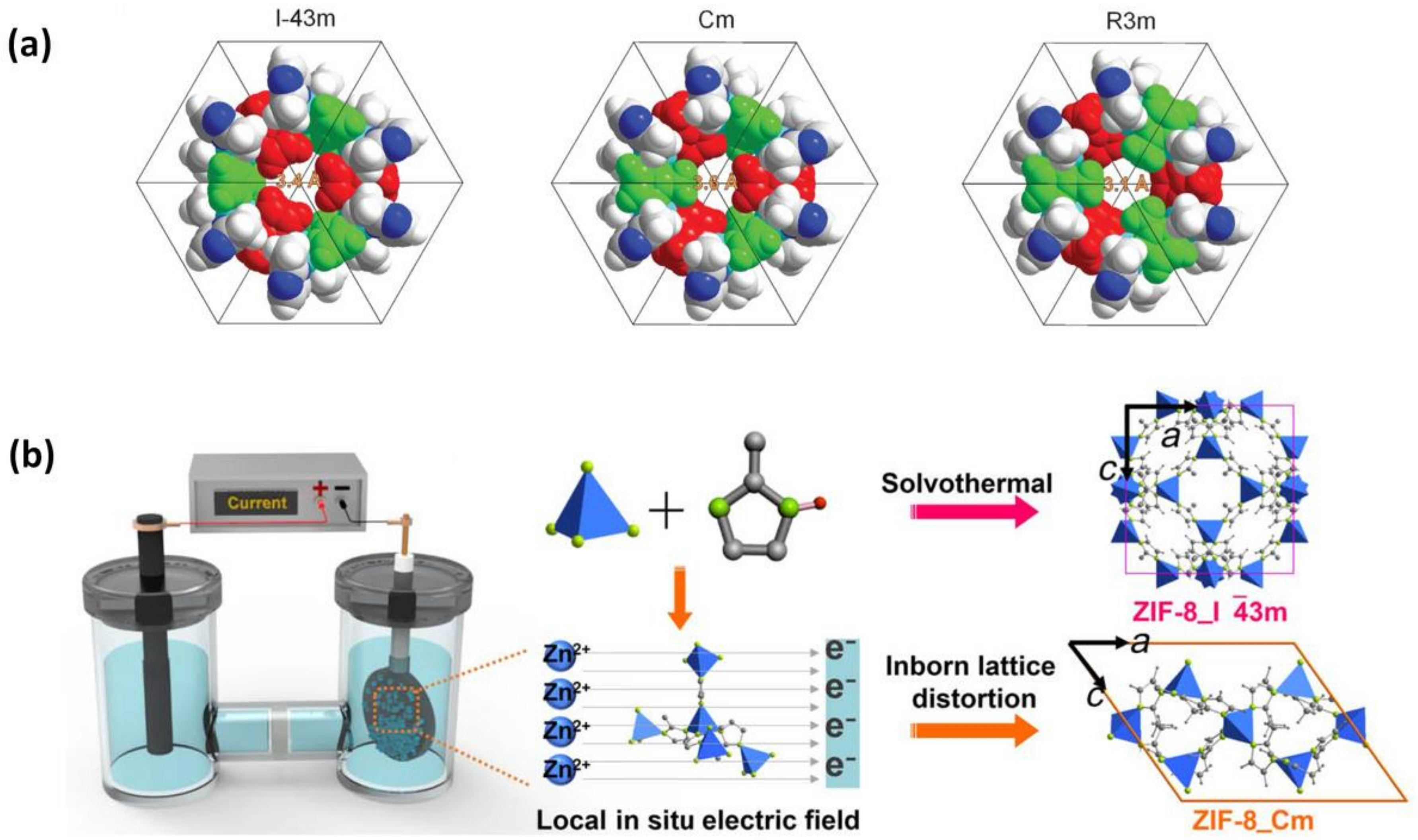
2.1.6. Current-Driven Synthesis
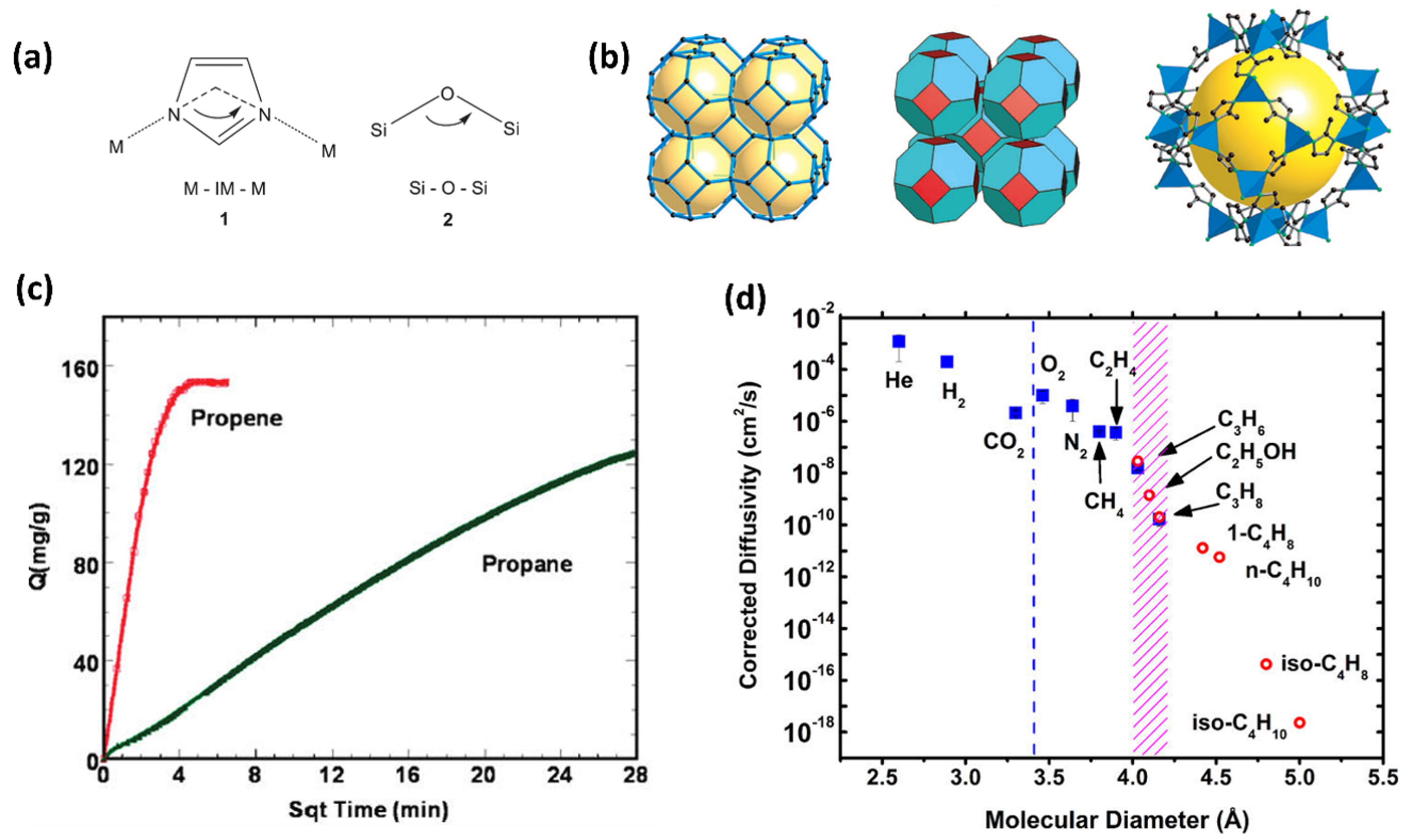
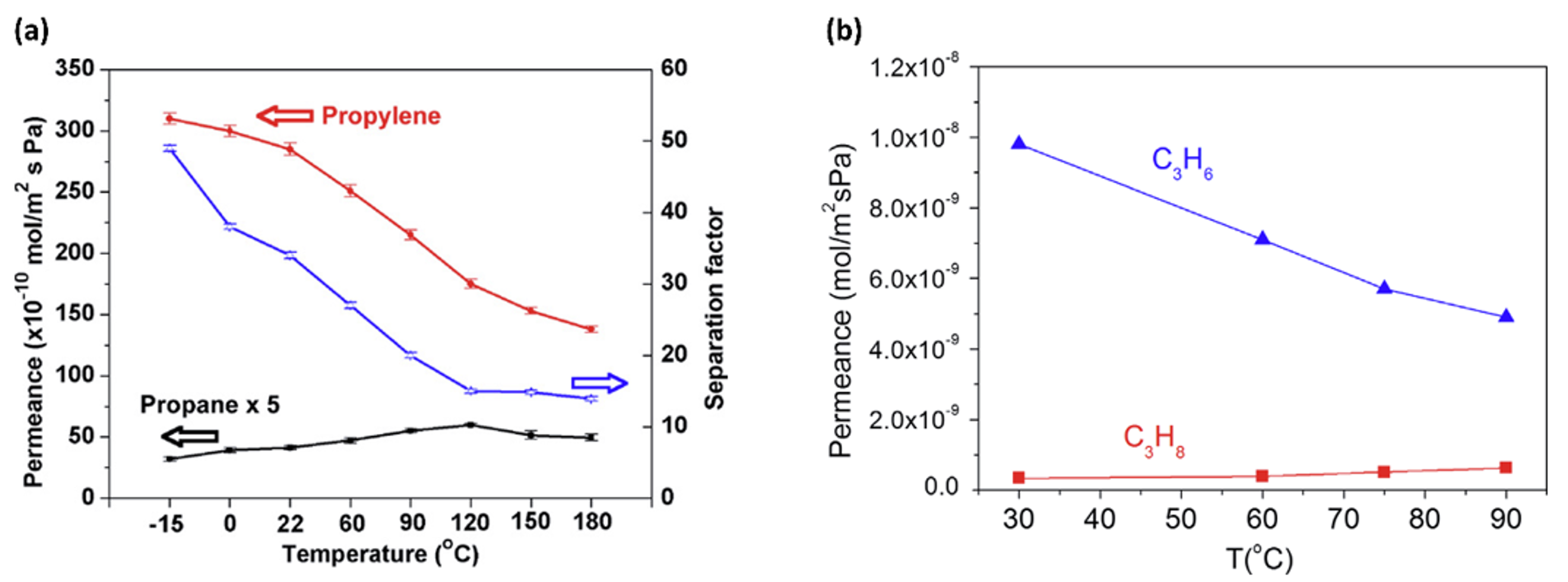
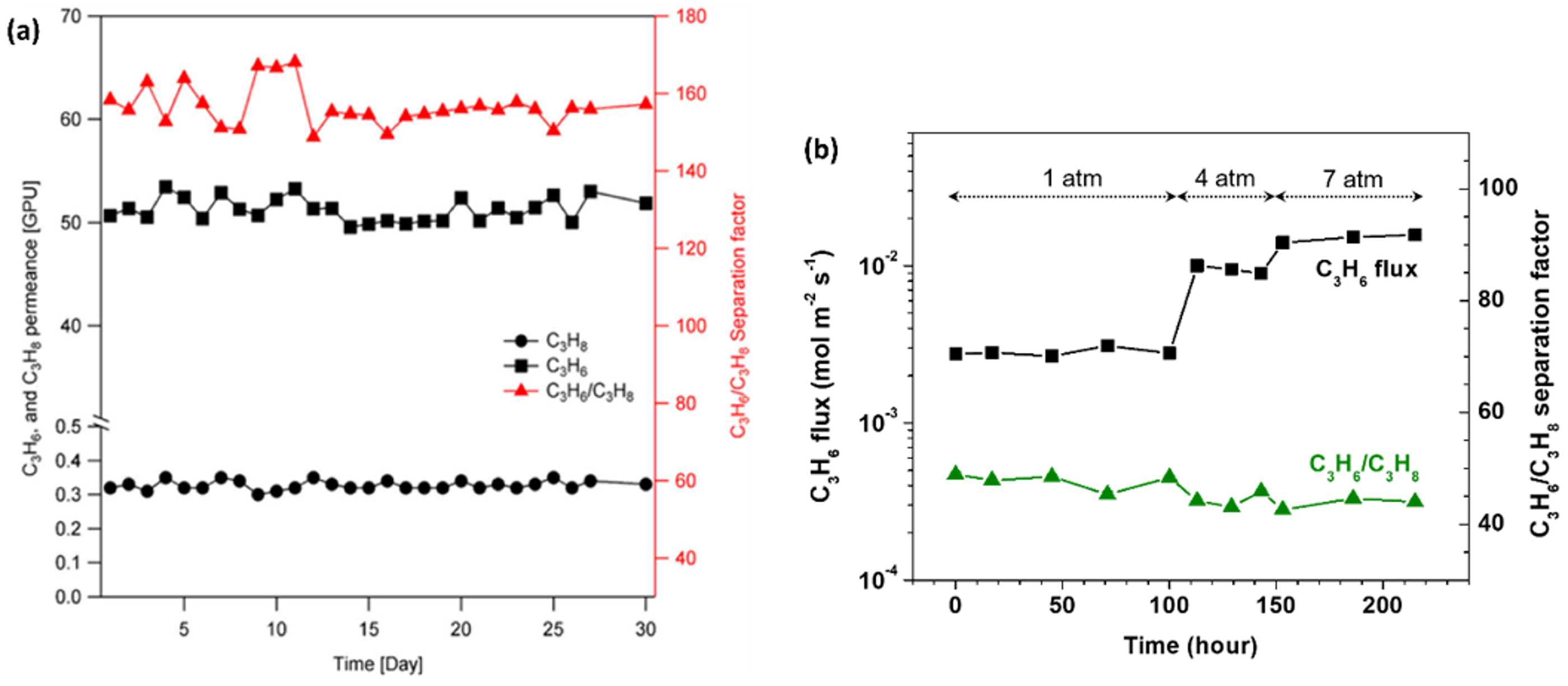
2.2. ZIF-8 Membranes for C3 Separation: Permeation Characteristic and Stability
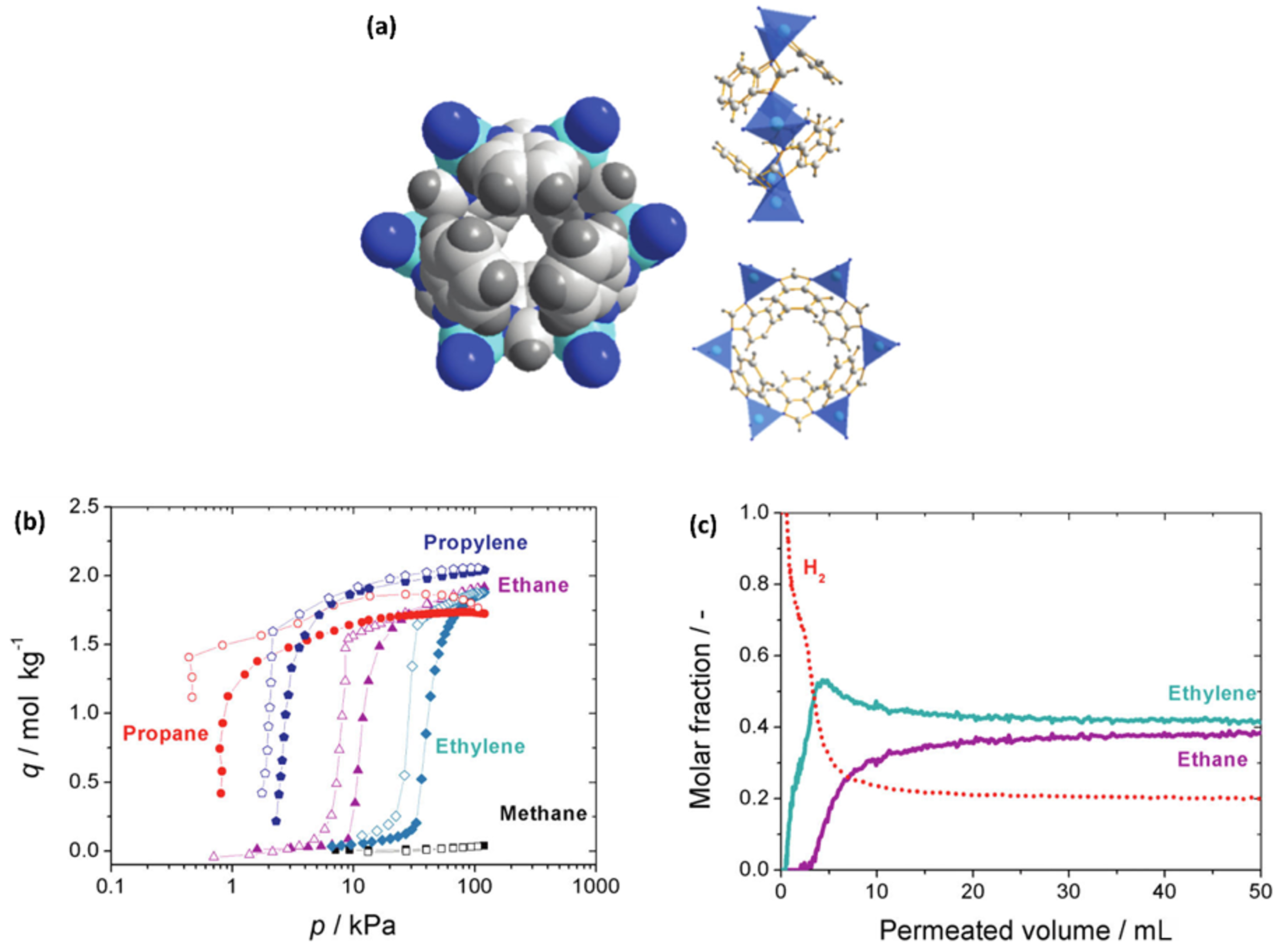
2.3. Other ZIF Membranes for C3 Separation
3. ZIF Membranes for Ethylene/Ethane Separation
3.1. ZIF-8 Membranes for C2 Separation
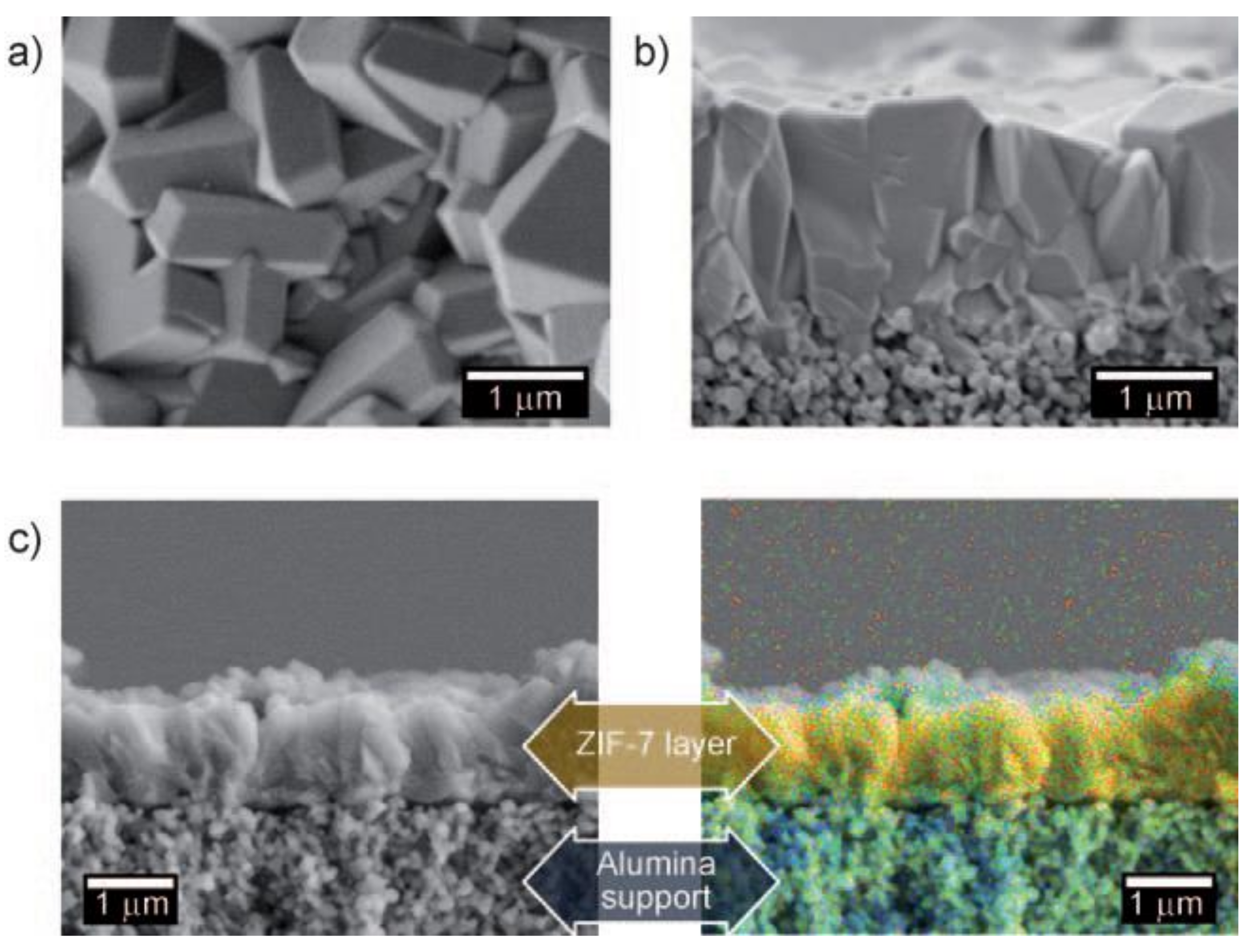
3.2. ZIF-7 Membranes for C2 Separation
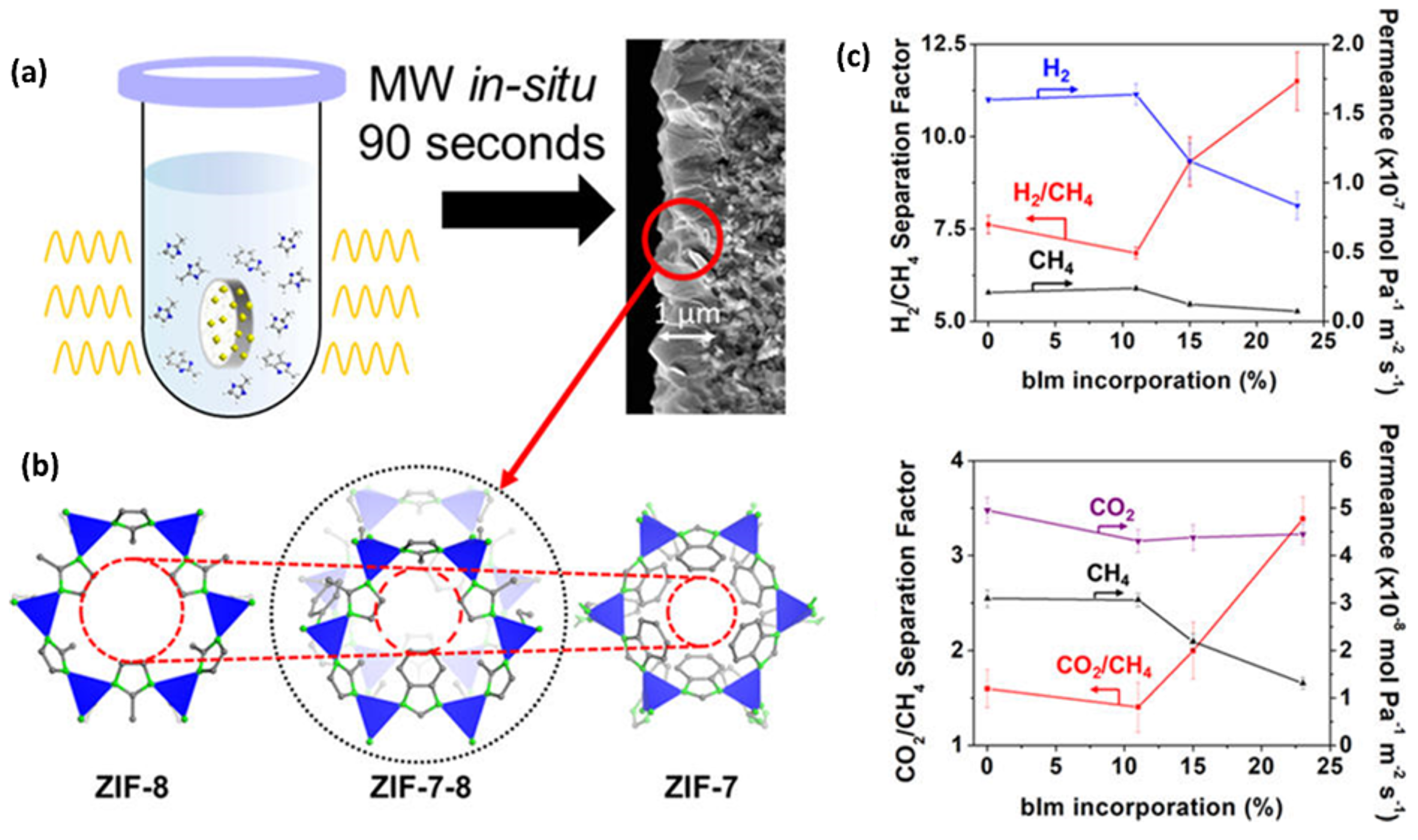
3.3. Mixed-Linker ZIF Membranes
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sholl, D.S.; Lively, R.P. Seven Chemical Separations to Change the World. Nature 2016, 532, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Ryan, L.; Burns, W.J.K. Defining the Challenges for C3H6/C3H8 Separation Using Polymeric Membranes. J. Memb. Sci. 2003, 211, 299–309. [Google Scholar]
- Rungta, M.; Zhang, C.; Koros, W.J.; Xu, L. Membrane-Based Ethylene/Ethane Separation: The Upper Bound and Beyond. AIChE J. 2013, 59, 3475–3489. [Google Scholar] [CrossRef]
- Ma, X.; Lin, B.K.; Wei, X.; Kniep, J.; Lin, Y.S. Gamma-Alumina Supported Carbon Molecular Sieve Membrane for Propylene/Propane Separation. Ind. Eng. Chem. Res. 2013, 52, 4297–4305. [Google Scholar] [CrossRef]
- Eldridge, R.B. Olefin/Paraffin Separation Technology: A Review. Ind. Eng. Chem. Res. 1993, 32, 2208–2212. [Google Scholar] [CrossRef]
- Safarik, D.J.; Eldridge, R.B. Olefin/Paraffin Separations by Reactive Absorption: A Review. Ind. Eng. Chem. Res. 1998, 37, 2571–2581. [Google Scholar] [CrossRef]
- Alshehri, A.K.; Lai, Z. Attainability and Minimum Energy of Multiple-Stage Cascade Membrane Systems. J. Memb. Sci. 2015, 495, 284–293. [Google Scholar] [CrossRef]
- Lee, U.; Kim, J.; Seok Chae, I.; Han, C. Techno-Economic Feasibility Study of Membrane Based Propane/Propylene Separation Process. Chem. Eng. Process. Process Intensif. 2017, 119, 62–72. [Google Scholar] [CrossRef]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-Efficient Polymeric Gas Separation Membranes for a Sustainable Future: A Review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Faiz, R.; Li, K. Polymeric Membranes for Light Olefin/Paraffin Separation. Desalination 2012, 287, 82–97. [Google Scholar] [CrossRef]
- Campos, A.C.C.; Dos Reis, R.A.; Ortiz, A.; Gorri, D.; Ortiz, I. A Perspective of Solutions for Membrane Instabilities in Olefin/Paraffin Separations: A Review. Ind. Eng. Chem. Res. 2018, 57, 10071–10085. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for Next-Generation Molecularly Selective Synthetic Membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Ma, X.L.; Lin, J.Y.S. Preparation Chemistry of Inorganic Membranes. Mod. Inorg. Synth. Chem. Second Ed. 2017, 669–686. [Google Scholar]
- Lin, Y.S.; Kumakiri, I.; Nair, B.N.; Alsyouri, H. Microporous Inorganic Membranes. Sep. Purif. Methods 2002, 31. [Google Scholar] [CrossRef]
- Lin, Y.; Duke, M.C. Recent Progress in Polycrystalline Zeolite Membrane Research. Curr. Opin. Chem. Eng. 2013, 2, 209–216. [Google Scholar] [CrossRef]
- Rangnekar, N.; Mittal, N.; Elyassi, B.; Caro, J.; Tsapatsis, M. Zeolite Membranes—A Review and Comparison with MOFs. Chem. Soc. Rev. 2015, 44, 7128–7154. [Google Scholar] [CrossRef] [PubMed]
- Gascon, J.; Kapteijn, F.; Zornoza, B.; Sebastián, V.; Casado, C.; Coronas, J. Practical Approach to Zeolitic Membranes and Coatings: State of the Art, Opportunities, Barriers, and Future Perspectives. Chem. Mater. 2012, 24, 2829–2844. [Google Scholar] [CrossRef]
- Gascon, J.; Kapteijn, F. Metal-Organic Framework Membranes-High Potential, Bright Future? Angew. Chemie Int. Ed. 2010, 49, 1530–1532. [Google Scholar] [CrossRef]
- Caro, J. Are MOF Membranes Better in Gas Separation than Those Made of Zeolites? Curr. Opin. Chem. Eng. 2011, 1, 77–83. [Google Scholar] [CrossRef]
- Duan, J.; Pan, Y.; Liu, G.; Jin, W. Metal-Organic Framework Adsorbents and Membranes for Separation Applications. Curr. Opin. Chem. Eng. 2018, 20, 122–131. [Google Scholar] [CrossRef]
- Liu, G.; Chernikova, V.; Liu, Y.; Zhang, K.; Belmabkhout, Y.; Shekhah, O.; Zhang, C.; Yi, S.; Eddaoudi, M.; Koros, W.J. Mixed Matrix Formulations with MOF Molecular Sieving for Key Energy-Intensive Separations. Nat. Mater. 2018, 17, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Li, J.-R.; Sculley, J.; Zhou, H. Metal-Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon Dioxide Capture by Metal Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ban, Y.; Yang, W. Microstructural Engineering and Architectural Design of Metal–Organic Framework Membranes. Adv. Mater. 2017, 29, 1–17. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Ma, X.; Liu, J.; Lin, Y.S. Mechanism of CO2 adsorption on Mg/DOBDC with Elevated CO2 loading. Fuel 2016, 181, 340–346. [Google Scholar] [CrossRef]
- Shete, M.; Kumar, P.; Bachman, J.E.; Ma, X.; Smith, Z.P.; Xu, W.; Mkhoyan, K.A.; Long, J.R.; Tsapatsis, M. On the Direct Synthesis of Cu(BDC) MOF Nanosheets and Their Performance in Mixed Matrix Membranes. J. Memb. Sci. 2018, 549, 312–320. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Li, Z.; Lin, Y.S. Synthesis, Characterization and Gas Transport Properties of MOF-5 Membranes. J. Memb. Sci. 2011, 382, 82–90. [Google Scholar] [CrossRef]
- Kang, Z.; Ding, J.; Fan, L.; Xue, M.; Zhang, D.; Gao, L.; Qiu, S. Preparation of a MOF Membrane with 3-Aminopropyltriethoxysilane as Covalent Linker for Xylene Isomers Separation. Inorg. Chem. Commun. 2013, 30, 74–78. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Zhang, C.; Qiu, W.; Yi, S.; Chernikova, V.; Chen, Z.; Belmabkhout, Y.; Shekhah, O.; Eddaoudi, M.; et al. Enhanced CO2/CH4 Separation Performance of a Mixed Matrix Membrane Based on Tailored MOF-Polymer Formulations. Adv. Sci. 2018, 5, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Demir, N.K.; Wu, Z.; Li, K. Highly Water-Stable Zirconium Metal-Organic Framework UiO-66 Membranes Supported on Alumina Hollow Fibers for Desalination. J. Am. Chem. Soc. 2015, 137, 6999–7002. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of Water Stable Metal-Organic Frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Mu, S.; Jiang, C.; Song, M.; Fang, Q.; Xue, M.; Qiu, S.; Chen, B. UiO-66-Coated Mesh Membrane with Underwater Superoleophobicity for High-Efficiency Oil-Water Separation. ACS Appl. Mater. Interfaces 2018, 10, 17301–17308. [Google Scholar] [CrossRef]
- Cao, J.; Su, Y.; Liu, Y.; Guan, J.; He, M.; Zhang, R.; Jiang, Z. Self-Assembled MOF Membranes with Underwater Superoleophobicity for Oil/Water Separation. J. Memb. Sci. 2018, 566, 268–277. [Google Scholar] [CrossRef]
- Campbell, J.; Székely, G.; Davies, R.P.; Braddock, D.C.; Livingston, A.G. Fabrication of hybrid polymer/metal organic framework membranes: Mixed matrix membranes versus in situ growth. J. Mater. Chem. A 2014, 2, 9260–9271. [Google Scholar] [CrossRef]
- Campbell, J.; Burgal, J.D.S.; Szekely, G.; Davies, R.P.; Braddock, D.C.; Livingston, A. Hybrid Polymer/MOF Membranes for Organic Solvent Nanofiltration (OSN): Chemical Modification and the Quest for Perfection. J. Memb. Sci. 2016, 503, 166–176. [Google Scholar] [CrossRef]
- Dong, X.; Lin, Y.S. Synthesis of an Organophilic ZIF-71 Membrane for Pervaporation Solvent Separation. Chem. Commun. 2013, 49, 1196–1198. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Lin, Y.S. Pervaporation Separation of Organic Mixtures by MOF-5 Membranes. Ind. Eng. Chem. Res. 2016, 55, 8652–8658. [Google Scholar] [CrossRef]
- Jeong, G.Y.; Singh, A.K.; Kim, M.G.; Gyak, K.W.; Ryu, U.; Choi, K.M.; Kim, D.P. Metal-Organic Framework Patterns and Membranes with Heterogeneous Pores for Flow-Assisted Switchable Separations. Nat. Commun. 2018, 9, 3968. [Google Scholar] [CrossRef]
- Lin, Y.S. Inorganic Membranes for Process Intensification: Challenges and Perspective. Ind. Eng. Chem. Res. 2018. article ASAP. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Kim, J.; Lee, D. Zeolitic Imidazolate Framework Membrane with Marked Thermochemical Stability for High-Temperature Catalytic Processes. Chem. Mater. 2018, 30, 447–455. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z. Development of ZIF-8 Membranes: Opportunities and Challenges for Commercial Applications. Curr. Opin. Chem. Eng. 2018, 20, 78–85. [Google Scholar] [CrossRef]
- Liu, Y.; Kasik, A.; Linneen, N.; Liu, J.; Lin, Y.S. Adsorption and Diffusion of Carbon Dioxide on ZIF-68. Chem. Eng. Sci. 2014, 118, 32–40. [Google Scholar] [CrossRef]
- Dong, X.; Huang, K.; Liu, S.; Ren, R.; Jin, W.; Lin, Y.S. Synthesis of Zeolitic Imidazolate Framework-78 Molecular-Sieve Membrane: Defect Formation and Elimination. J. Mater. Chem. 2012, 22, 19222–19227. [Google Scholar] [CrossRef]
- Li, K.; Olson, D.H.; Seidel, J.; Emge, T.J.; Gong, H.; Zeng, H.; Li, J. Zeolitic Imidazolate Frameworks for Kinetic Separation of Propane and Propene. J. Am. Chem. Soc. 2009, 131, 10368–10369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lively, R.P.; Zhang, K.; Johnson, J.R.; Karvan, O.; Koros, W.J. Unexpected Molecular Sieving Properties of Zeolitic Imidazolate Framework-8. J. Phys. Chem. Lett. 2012, 3, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF Thin Films: Existing and Future Applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Xue, M.; Zhu, G. Metal-Organic Framework Membranes: From Synthesis to Separation Application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S. Metal Organic Framework Membranes for Separation Applications. Curr. Opin. Chem. Eng. 2015, 8, 21–28. [Google Scholar] [CrossRef]
- Krokidas, P.; Castier, M.; Moncho, S.; Brothers, E.; Economou, I.G. Molecular Simulation Studies of the Diffusion of Methane, Ethane, Propane, and Propylene in ZIF-8. J. Phys. Chem. C 2015, 119, 27028–27037. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Düren, T. Opening the Gate: Framework Flexibility in ZIF-8 Explored by Experiments and Simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef] [Green Version]
- Kaewruksa, B.; Vchirawongkwin, V.; Ruangpornvisuti, V. Adsorption of Propane and Propylene in Zeolitic Imidazolate Framework ZIF-8 Pore: Periodic SCC-DFTB Method. Adsorption 2018, 24, 691–701. [Google Scholar] [CrossRef]
- Böhme, U.; Barth, B.; Paula, C.; Kuhnt, A.; Schwieger, W.; Mundstock, A.; Caro, J.; Hartmann, M. Ethene/Ethane and Propene/Propane Separation via the Olefin and Paraffin Selective Metal-Organic Framework Adsorbents CPO-27 and ZIF-8. Langmuir 2013, 29, 8592–8600. [Google Scholar] [CrossRef]
- Bux, H.; Liang, F.; Li, Y.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic Imidazolate Framework Molecular Sieving Membrane Titania Support. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef]
- Pan, Y.; Li, T.; Lestari, G.; Lai, Z. Effective Separation of Propylene/Propane Binary Mixtures by ZIF-8 Membranes. J. Memb. Sci. 2012, 390–391, 93–98. [Google Scholar] [CrossRef]
- Shah, M.; Kwon, H.T.; Tran, V.; Sachdeva, S.; Jeong, H.K. One Step in Situ Synthesis of Supported Zeolitic Imidazolate Framework ZIF-8 Membranes: Role of Sodium Formate. Microporous Mesoporous Mater. 2013, 165, 63–69. [Google Scholar] [CrossRef]
- Wang, J.W.; Li, N.X.; Li, Z.R.; Wang, J.R.; Xu, X.; Chen, C.S. Preparation and Gas Separation Properties of Zeolitic Imidazolate Frameworks-8 (ZIF-8) Membranes Supported on Silicon Nitride Ceramic Hollow Fibers. Ceram. Int. 2016, 42, 8949–8954. [Google Scholar] [CrossRef]
- Huang, A.; Bux, H.; Steinbach, F.; Caro, J. Molecular-Sieve Membrane with Hydrogen Permselectivity: ZIF-22 in LTA Topology Prepared with 3-Aminopropyltriethoxysilane as Covalent Linker. Angew. Chemie Int. Ed. 2010, 49, 4958–4961. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Dou, W.; Caro, J. Steam-Stable Zeolitic Imidazolate Framework ZIF-90 Membrane with Hydrogen Selectivity through Covalent Functionalization. J. Am. Chem. Soc. 2010, 132, 15562–15564. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Okubo, K.; Kida, K.; Sugita, M.; Takewaki, T. Grain Size Control of ZIF-8 Membranes by Seeding-Free Aqueous Synthesis and Their Performances in Propylene/Propane Separation. J. Memb. Sci. 2017, 544, 306–311. [Google Scholar] [CrossRef]
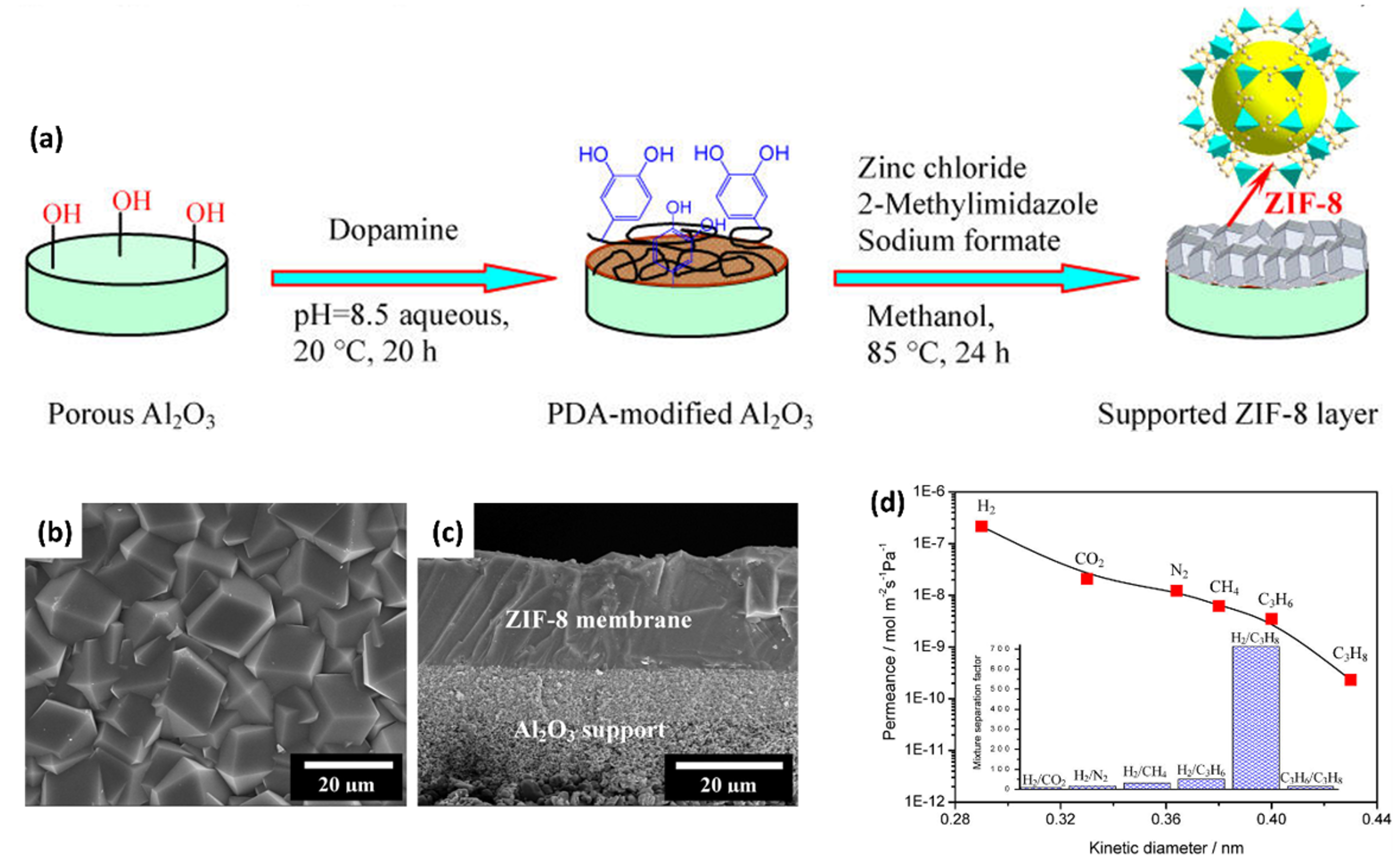
- Liu, Q.; Wang, N.; Caro, J.; Huang, A. Bio-Inspired Polydopamine: A Versatile and Powerful Platform for Covalent Synthesis of Molecular Sieve Membranes. J. Am. Chem. Soc. 2013, 135, 17679–17682. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pan, Y.; Wang, C.; Lai, Z. ZIF-8 Membranes with Improved Reproducibility Fabricated from Sputter-Coated ZnO/Alumina Supports. Chem. Eng. Sci. 2016, 141, 119–124. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, X.; Liu, H.; Qiu, J. Synthesis of a Highly Stable ZIF-8 Membrane on a Macroporous Ceramic Tube by Manual-Rubbing ZnO Deposition as a Multifunctional Layer. J. Memb. Sci. 2015, 490, 354–363. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, J.; Wang, J.; Bai, J.; Yin, H.; Yuan, B.; Lu, J.; Zhang, Y.; Zhou, L.; Duan, C. Deposition of Chemically Modified α-Al2O3particles for High Performance ZIF-8 Membrane on a Macroporous Tube. Chem. Commun. 2012, 48, 5977–5979. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Q.; Caro, J.; Huang, A. Highly Hydrogen-Permselective Zeolitic Imidazolate Framework ZIF-8 Membranes Prepared on Coarse and Macroporous Tubes through Repeated Synthesis. Sep. Purif. Technol. 2015, 146, 68–74. [Google Scholar] [CrossRef]
- Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Controlling Zeolitic Imidazolate Framework Nano- and Microcrystal Formation: Insight into Crystal Growth by Time-Resolved in Situ Static Light Scattering. Chem. Mater. 2011, 23, 2130–2141. [Google Scholar] [CrossRef]
- Zhang, K.; Lively, R.P.; Zhang, C.; Koros, W.J.; Chance, R.R. Investigating the Intrinsic Ethanol/Water Separation Capability of ZIF-8: An Adsorption and Diffusion Study. J. Phys. Chem. C 2013, 117, 7214–7225. [Google Scholar] [CrossRef]
- Zhang, K.; Lively, R.P.; Dose, M.E.; Brown, A.J.; Zhang, C.; Chung, J.; Nair, S.; Koros, W.J.; Chance, R.R. Alcohol and Water Adsorption in Zeolitic Imidazolate Frameworks. Chem. Commun. 2013, 49, 3245–3247. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid Synthesis of Zeolitic Imidazolate Framework-8 (ZIF-8) Nanocrystals in an Aqueous System. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar]
- Pan, Y.; Heryadi, D.; Zhou, F.; Zhao, L.; Lestari, G.; Su, H.; Lai, Z. Tuning the Crystal Morphology and Size of Zeolitic Imidazolate Framework-8 in Aqueous Solution by Surfactants. CrystEngComm 2011, 13, 6937–6940. [Google Scholar]
- Pan, Y.; Lai, Z. Sharp Separation of C2/C3 Hydrocarbon Mixtures by Zeolitic Imidazolate Framework-8 (ZIF-8) Membranes Synthesized in Aqueous Solutions. Chem. Commun. 2011, 47, 10275–10277. [Google Scholar] [CrossRef]
- Liu, D.; Ma, X.; Xi, H.; Lin, Y.S. Gas Transport Properties and Propylene/Propane Separation Characteristics of ZIF-8 Membranes. J. Memb. Sci. 2014, 451, 85–93. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, W.; Zhao, Y.; Wang, C.; Lai, Z. Improved ZIF-8 Membrane: Effect of Activation Procedure and Determination of Diffusivities of Light Hydrocarbons. J. Memb. Sci. 2015, 493, 88–96. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.K. Highly Propylene-Selective Supported Zeolite-Imidazolate Framework (ZIF-8) Membranes Synthesized by Rapid Microwave-Assisted Seeding and Secondary Growth. Chem. Commun. 2013, 49, 3854–3856. [Google Scholar] [CrossRef]
- Yin, H.; Lee, T.; Choi, J.; Yip, A.C.K. On the Zeolitic Imidazolate Framework-8 (ZIF-8) Membrane for Hydrogen Separation from Simulated Biomass-Derived Syngas. Microporous Mesoporous Mater. 2016, 233, 70–77. [Google Scholar] [CrossRef]
- Sun, J.; Yu, C.; Jeong, H.-K. Propylene-Selective Thin Zeolitic Imidazolate Microwave Seeding and Solvothermal. Crystals 2018, 8, 373. [Google Scholar] [CrossRef]
- Tao, K.; Kong, C.; Chen, L. High Performance ZIF-8 Molecular Sieve Membrane on Hollow Ceramic Fiber via Crystallizing-Rubbing Seed Deposition. Chem. Eng. J. 2013, 220, 1–5. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, B.; Lai, Z. Synthesis of Ceramic Hollow Fiber Supported Zeolitic Imidazolate Framework-8 (ZIF-8) Membranes with High Hydrogen Permeability. J. Memb. Sci. 2012, 421–422, 292–298. [Google Scholar] [CrossRef]
- Huang, K.; Dong, Z.; Li, Q.; Jin, W. Growth of a ZIF-8 Membrane on the Inner-Surface of a Ceramic Hollow Fiber via Cycling Precursors. Chem. Commun. 2013, 49, 10326–10328. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Dong, D.; Li, D.; He, L.; Xu, G.; Wang, H. Contra-Diffusion Synthesis of ZIF-8 Films on a Polymer Substrate. Chem. Commun. 2011, 47, 2559–2561. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.T.; Jeong, H. In Situ Synthesis of Thin Zeolitic–Imidazolate Framework ZIF-8 Membranes Exhibiting Exceptionally High Propylene/Propane Separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Taek, H.; Jeong, H. Improving Propylene/Propane Separation Performance of Zeolitic-Imidazolate Framework ZIF-8 Membranes. Chem. Eng. Sci. 2015, 124, 20–26. [Google Scholar]
- Jang, E.; Kim, E.; Kim, H.; Lee, T.; Yeom, H.J.; Kim, Y.W.; Choi, J. Formation of ZIF-8 Membranes inside Porous Supports for Improving Both Their H2/CO2 separation Performance and Thermal/Mechanical Stability. J. Memb. Sci. 2017, 540, 430–439. [Google Scholar] [CrossRef]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. Microporous and Mesoporous Materials ZIF-8 Membranes Prepared at Miscible and Immiscible Liquid—Liquid Interfaces. Microporous Mesoporous Mater. 2015, 206, 75–80. [Google Scholar] [CrossRef]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. Thickness Reduction of the Zeolitic Imidazolate Framework-8 Membrane by Controlling the Reaction Rate during the Membrane Preparation. J. Chem. Eng. of Jpn. 2014, 47, 770–776. [Google Scholar] [CrossRef]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. Effect of Temperature on Synthesis of ZIF-8 Membranes for Propylene/Propane Separation by Counter Diffusion Method. J. Jpn. Petrol. Inst. 2015, 58, 237–244. [Google Scholar]
- Brown, A.J.; Brunelli, N.A.; Eum, K.; Rashidi, F.; Johnson, J.R.; Koros, W.J.; Jones, C.W.; Nair, S.; Gascon, J.; Kapteijn, F.; et al. Interfacial Microfluidic Processing of Metal-Organic Framework Hollow Fiber Membranes. Science 2014, 345, 72–75. [Google Scholar] [PubMed]
- Eum, K.; Ma, C.; Rownaghi, A.; Jones, C.W.; Nair, S. ZIF-8 Membranes via Interfacial Microfluidic Processing in Polymeric Hollow Fibers: Efficient Propylene Separation at Elevated Pressures. ACS Appl. Mater. Interfaces 2016, 8, 25337–25342. [Google Scholar] [CrossRef] [PubMed]
- Eum, K.; Rownaghi, A.; Choi, D.; Bhave, R.R.; Jones, C.W.; Nair, S. Fluidic Processing of High-Performance ZIF-8 Membranes on Polymeric Hollow Fibers: Mechanistic Insights and Microstructure Control. Adv. Funct. Mater. 2016, 26, 5011–5018. [Google Scholar]
- Eum, K.; Ma, C.; Koh, D.Y.; Rashidi, F.; Li, Z.; Jones, C.W.; Lively, R.P.; Nair, S. Zeolitic Imidazolate Framework Membranes Supported on Macroporous Carbon Hollow Fibers by Fluidic Processing Techniques. Adv. Mater. Interfaces 2017, 4, 1–6. [Google Scholar] [CrossRef]
- Cacho-Bailo, F.; Catalán-Aguirre, S.; Etxeberría-Benavides, M.; Karvan, O.; Sebastian, V.; Téllez, C.; Coronas, J. Metal-Organic Framework Membranes on the Inner-Side of a Polymeric Hollow Fiber by Microfluidic Synthesis. J. Memb. Sci. 2015, 476, 277–285. [Google Scholar] [CrossRef]
- Marti, A.M.; Wickramanayake, W.; Dahe, G.; Sekizkardes, A.; Bank, T.L.; Hopkinson, D.P.; Venna, S.R. Continuous Flow Processing of ZIF-8 Membranes on Polymeric Porous Hollow Fiber Supports for CO2 Capture. ACS Appl. Mater. Interfaces 2017, 9, 5678–5682. [Google Scholar] [PubMed]
- Lee, M.J.; Abdul Hamid, M.R.; Lee, J.; Kim, J.S.; Lee, Y.M.; Jeong, H.K. Ultrathin Zeolitic-Imidazolate Framework ZIF-8 Membranes on Polymeric Hollow Fibers for Propylene/Propane Separation. J. Memb. Sci. 2018, 559, 28–34. [Google Scholar] [CrossRef]
- Pham, T.C.T.; Nguyen, T.H.; Yoon, K.B. Gel-Free Secondary Growth of Uniformly Oriented Silica MFI Zeolite Films and Application for Xylene Separation. Angew. Chemie Int. Ed. 2013, 52, 8693–8698. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.K.; Lee, A.S.; An, H.S.; Lee, T.; Jang, E.; Lee, J.S.; Choi, J. Defect-Induced Ripening of Zeolitic-Imidazolate Framework ZIF-8 and Its Implication to Vapor-Phase Membrane Synthesis. Chem. Commun. 2016, 52, 11669–11672. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kumar, P.; Mittal, N.; Khlyustova, A.; Daoutidis, P.; Andre Mkhoyan, K.; Tsapatsis, M. Zeolitic Imidazolate Framework Membranes Made by Ligand-Induced Permselectivation. Science 2018, 361, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.Y.; Kim, D.; Kumar, P.; Lee, P.S.; Rangnekar, N.; Bai, P.; Shete, M.; Elyassi, B.; Lee, H.S.; Narasimharao, K.; et al. Ultra-Selective High-Flux Membranes from Directly Synthesized Zeolite Nanosheets. Nature 2017, 543, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.V.; Topuz, B.; Pham, T.C.T.; Nguyen, T.H.; Sauer, N.; Rangnekar, N.; Zhang, H.; Narasimharao, K.; Basahel, S.N.; Francis, L.F.; et al. Oriented MFI Membranes by Gel-Less Secondary Growth of Sub-100 Nm MFI-Nanosheet Seed Layers. Adv. Mater. 2015, 27, 3243–3249. [Google Scholar] [CrossRef]
- Stassen, I.; Styles, M.; Grenci, G.; Van Gorp, H.; Vanderlinden, W.; De Feyter, S.; Falcaro, P.; De Vos, D.; Vereecken, P.; Ameloot, R. Chemical Vapour Deposition of Zeolitic Imidazolate Framework Thin Films. Nat. Mater. 2016, 15, 304–310. [Google Scholar] [CrossRef]
- Li, W.; Su, P.; Li, Z.; Xu, Z.; Wang, F.; Ou, H.; Zhang, J.; Zhang, G.; Zeng, E. Ultrathin Metal-Organic Framework Membrane Production by Gel-Vapour Deposition. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Koros, W.J. Zeolitic Imidazolate Framework-Enabled Membranes: Challenges and Opportunities. J. Phys. Chem. Lett. 2015, 6, 3841–3849. [Google Scholar] [CrossRef]
- Knebel, A.; Geppert, B.; Volgmann, K.; Kolokolov, D.I.; Stepanov, A.G.; Twiefel, J.; Heitjans, P.; Volkmer, D.; Caro, J. Defibrillation of Soft Porous Metal-Organic Frameworks with Electric Fields. Science 2017, 358, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wei, Y.; Li, L.; Duan, Y.; Hou, Q.; Zhang, L.; Ding, L.X.; Xue, J.; Wang, H.; Caro, J. Paralyzed Membrane: Current-Driven Synthesis of a Metal-Organic Framework with Sharpened Propene/Propane Separation. Sci. Adv. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Hou, Q.; Wu, Y.; Zhou, S.; Wei, Y.; Caro, J.; Wang, H. Ultra-Tuning of the Aperture Size in Stiffened ZIF-8_Cm Frameworks with Mixed-Linker Strategy for Enhanced CO2/CH4 Separation. Angew. Chemie Int. Ed. 2019, 58, 327–331. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Bonnin-Paris, J.; Bostyn, S.; Ruiz-Cabrera, M.A.; Moscosa-Santillán, M. Assessment of Sugars Separation from a Model Carbohydrates Solution by Nanofiltration Using a Design of Experiments (DoE) Methodology. Sep. Purif. Technol. 2014, 131, 84–93. [Google Scholar] [CrossRef]
- Didaskalou, C.; Kupai, J.; Cseri, L.; Barabas, J.; Vass, E.; Holtzl, T.; Szekely, G. Membrane-Grafted Asymmetric Organocatalyst for an Integrated Synthesis-Separation Platform. ACS Catal. 2018, 8, 7430–7438. [Google Scholar] [CrossRef]
- Valtcheva, I.B.; Marchetti, P.; Livingston, A.G. Crosslinked polybenzimidazole membranes for organic solvent nanofiltration (OSN): Analysis of crosslinking reaction mechanism and effects of reaction parameters. J. Memb. Sci. 2015, 493, 568–579. [Google Scholar] [CrossRef]
- Marchetti, P.; Butté, A.; Livingston, A.G. Quality by Design for Peptide Nanofiltration: Fundamental Understanding and Process Selection. Chem. Eng. Sci. 2013, 101, 200–212. [Google Scholar] [CrossRef]
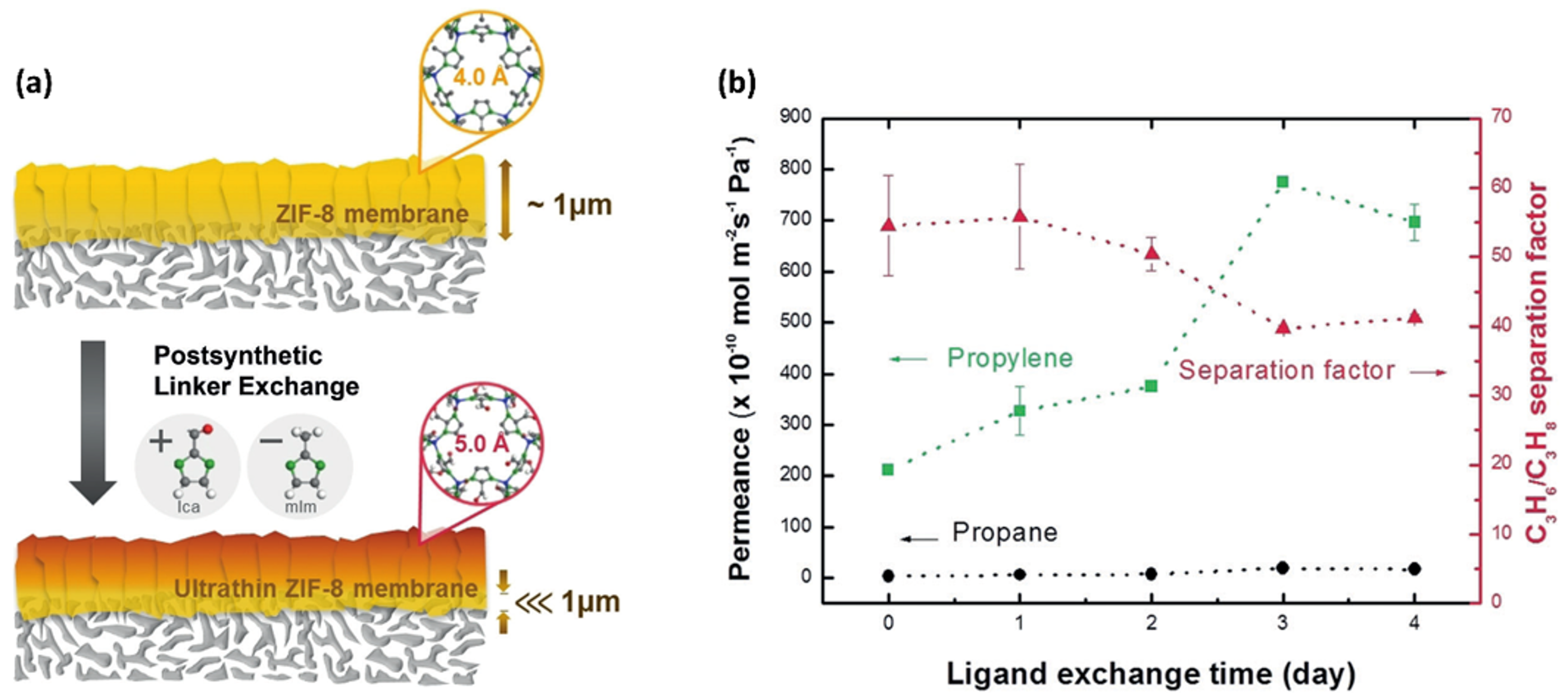
- Lee, M.J.; Kwon, H.T.; Jeong, H.K. High-Flux Zeolitic Imidazolate Framework Membranes for Propylene/Propane Separation by Postsynthetic Linker Exchange. Angew. Chemie Int. Ed. 2018, 57, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lin, Y.S.; Wei, X.; Kniep, J. Ultrathin Carbon Molecular Sieve Membrane for Propylene/Propane Separation. AIChE J. 2016, 62, 491–499. [Google Scholar] [CrossRef]
- Huang, Y.; Merkel, T.C.; Baker, R.W. Pressure Ratio and Its Impact on Membrane Gas Separation Processes. J. Memb. Sci. 2014, 463, 33–40. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power Plant Post-Combustion Carbon Dioxide Capture: An Opportunity for Membranes. J. Memb. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Ma, X.; Williams, S.; Wei, X.; Kniep, J.; Lin, Y.S. Propylene/Propane Mixture Separation Characteristics and Stability of Carbon Molecular Sieve Membranes. Ind. Eng. Chem. Res. 2015, 54, 9824–9831. [Google Scholar] [CrossRef]
- James, J.B.; Lin, Y.S. Kinetics of ZIF-8 Thermal Decomposition in Inert, Oxidizing, and Reducing Environments. J. Phys. Chem. C 2016, 120, 14015–14026. [Google Scholar] [CrossRef]
- James, J.B.; Lin, Y.S. Thermal Stability of ZIF-8 Membranes for Gas Separations. J. Memb. Sci. 2017, 532, 9–19. [Google Scholar] [CrossRef]
- Zhang, H.; James, J.; Zhao, M.; Yao, Y.; Zhang, Y.; Zhang, B.; Lin, Y.S. Improving Hydrostability of ZIF-8 Membranes via Surface Ligand Exchange. J. Memb. Sci. 2017, 532, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, D.; Yao, Y.; Zhang, B.; Lin, Y.S. Stability of ZIF-8 Membranes and Crystalline Powders in Water at Room Temperature. J. Memb. Sci. 2015, 485, 103–111. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.K.; Lee, A.S.; An, H.S.; Lee, J.S. Heteroepitaxially Grown Zeolitic Imidazolate Framework Membranes with Unprecedented Propylene/Propane Separation Performances. J. Am. Chem. Soc. 2015, 137, 12304–12311. [Google Scholar] [CrossRef] [PubMed]
- Krokidas, P.; Castier, M.; Moncho, S.; Sredojevic, D.N.; Brothers, E.N.; Kwon, H.T.; Jeong, H.K.; Lee, J.S.; Economou, I.G. ZIF-67 Framework: A Promising New Candidate for Propylene/Propane Separation. Experimental Data and Molecular Simulations. J. Phys. Chem. C 2016, 120, 8116–8124. [Google Scholar] [CrossRef]
- Bux, H.; Chmelik, C.; Krishna, R.; Caro, J. Ethene/Ethane Separation by the MOF Membrane ZIF-8: Molecular Correlation of Permeation, Adsorption, Diffusion. J. Memb. Sci. 2011, 369, 284–289. [Google Scholar] [CrossRef]
- James, J.B.; Wang, J.; Meng, L.; Lin, Y.S. ZIF-8 Membrane Ethylene/Ethane Transport Characteristics in Single and Binary Gas Mixtures. Ind. Eng. Chem. Res. 2017, 56, 7567–7575. [Google Scholar] [CrossRef]
- Li, Y.S.; Liang, F.Y.; Bux, H.; Feldhoff, A.; Yang, W.S.; Caro, J. Molecular Sieve Membrane: Supported Metal-Organic Framework with High Hydrogen Selectivity. Angew. Chemie Int. Ed. 2010, 49, 548–551. [Google Scholar] [CrossRef]
- Li, Y.; Liang, F.; Bux, H.; Yang, W.; Caro, J. Zeolitic Imidazolate Framework ZIF-7 Based Molecular Sieve Membrane for Hydrogen Separation. J. Memb. Sci. 2010, 354, 48–54. [Google Scholar] [CrossRef]
- Aceituno Melgar, V.M.; Kwon, H.T.; Kim, J. Direct Spraying Approach for Synthesis of ZIF-7 Membranes by Electrospray Deposition. J. Memb. Sci. 2014, 459, 190–196. [Google Scholar] [CrossRef]
- Gücüyener, C.; van den Bergh, J.; Gascon, J.; Kapteijn, F.; Gücüyener, C.; van den Bergh, J.; Gascon, J.; Kapteijn, F. Ethane/Ethene Separation Turned on Its Head: Selective Ethane Adsorption on the Metal−Organic Framework ZIF-7 through a Gate-Opening Mechanism. J. Am. Chem. Soc. 2010, 132, 17704–17706. [Google Scholar] [CrossRef] [PubMed]
- Noro, S.I.; Tanaka, D.; Sakamoto, H.; Shimomura, S.; Kitagawa, S.; Takeda, S.; Uemura, K.; Kita, H.; Akutagawa, T.; Nakamura, T. Selective Gas Adsorption in One-Dimensional, Flexible CuII Coordination Polymers with Polar Units. Chem. Mater. 2009, 21, 3346–3355. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C. Large Breathing Effects in Three-Dimensional Porous Hybrid Matter: Facts, Analyses, Rules and Consequences. Chem. Soc. Rev. 2009, 38, 1380–1399. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Li, Y. Flexibility of Metal-Organic Frameworks for Separations: Utilization, Suppression and Regulation. Curr. Opin. Chem. Eng. 2018, 20, 107–113. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.B.; Liu, Q.; Trickett, C.A.; Gutiérrez-Puebla, E.; Monge, M.Á.; Cong, H.; Aldossary, A.; Deng, H.; Yaghi, O.M. Principles of Designing Extra-Large Pore Openings and Cages in Zeolitic Imidazolate Frameworks. J. Am. Chem. Soc. 2017, 139, 6448–6455. [Google Scholar] [CrossRef]
- Eum, K.; Jayachandrababu, K.C.; Rashidi, F.; Zhang, K.; Leisen, J.; Graham, S.; Lively, R.P.; Chance, R.R.; Sholl, D.S.; Jones, C.W.; et al. Highly Tunable Molecular Sieving and Adsorption Properties of Mixed-Linker Zeolitic Imidazolate Frameworks. J. Am. Chem. Soc. 2015, 137, 4191–4197. [Google Scholar] [CrossRef]
- Thompson, J.A.; Brunelli, N.A.; Lively, R.P.; Johnson, J.R.; Jones, C.W.; Nair, S. Tunable CO2 Adsorbents by Mixed-Linker Synthesis and Postsynthetic Modification of Zeolitic Imidazolate Frameworks. J. Phys. Chem. C 2013, 117, 8198–8207. [Google Scholar] [CrossRef]
- Thompson, J.A.; Blad, C.R.; Brunelli, N.A.; Lydon, M.E.; Lively, R.P.; Jones, C.W.; Nair, S. Hybrid Zeolitic Imidazolate Frameworks: Controlling Framework Porosity and Functionality by Mixed-Linker Synthesis. Chem. Mater. 2012, 24, 1930–1936. [Google Scholar] [CrossRef]
- Hillman, F.; Brito, J.; Jeong, H.K. Rapid One-Pot Microwave Synthesis of Mixed-Linker Hybrid Zeolitic-Imidazolate Framework Membranes for Tunable Gas Separations. ACS Appl. Mater. Interfaces 2018, 10, 5586–5593. [Google Scholar] [CrossRef]


| Membrane Fabrication Method | Support | Membrane Thickness | Gas Pair | Permeance (mol·m−2·s−1·Pa−1) | Separation Factor | REF |
|---|---|---|---|---|---|---|
| In situ synthesis | Titania support | ~30 µm | H2/CH4 | 5.08 × 10–8 (H2) | 11.2 | [60] |
| Al2O3 support | ~20 µm | H2/C3H8 | 1.8 × 10–7 (H2) | 712.6 | [67] | |
| Macroporous Al2O3 tube | 25 µm | H2/C3H8 | 1.1 × 10−7 (H2) | 328.6 | [71] | |
| Si3N4 hollow fiber | ~30 µm | H2/CO2 | 8.35 × 10−7 (H2) | 11.7 | [63] | |
| Seeded growth | α-Al2O3 support | ~2.2 µm | C3H6/C3H8 | 2.06 × 10−8 (C3H6) | 45 | [61] |
| 2.77 × 10−8 (C3H6) | 35 | |||||
| α-Al2O3 support | ~0.5–1.6 µm | C3H6/C3H8 | 7.8 × 10−9 (C3H6) | 89 | [79] | |
| 1.56 × 10−8 (C3H6) | 50 | |||||
| α-Al2O3 support | ~1.5 µm | C3H6/C3H8 | 2.08 × 10−8 (C3H6) | 40 | [80] | |
| Commercial ceramic tube | ~1.2 µm | C3H6/C3H8 | 1.90 × 10−8 (C3H6) | 80 | [82] | |
| YSZ hollow fiber | ~2 µm | H2/C3H8 | 1.50 × 10−6 (H2) | >1000 | [84] | |
| α-Al2O3hollow fiber | ~2 µm | H2/CO2 | ~4 × 10−7 (H2) | 3.28 | [85] | |
| Counter diffusion | Nylon support | ~16 µm | H2/N2 | 1.97 × 10−6 (H2) | 4.3 | [86] |
| α-Al2O3 support | ~1.5 µm | C3H6/C3H8 | 2.13 × 10−8 (C3H6) | 50 | [87] | |
| α-Al2O3 support | 1 µm | C3H6/C3H8 | 2.68 × 10−8 (C3H6) | 70.6 | [88] | |
| α-Al2O3 hollow capillary substrate | 20 µm | C3H6/C3H8 | 1.2 × 10−8 (C3H6) | 20 | [90] | |
| α-Al2O3 hollow capillary substrate | 40-50 µm | C3H6/C3H8 | 2.2 × 10−9 (C3H6) | 10 | [92] | |
| Interfacial microfluidic processing | Polymer hollow fiber | ~8.8 µm | C3H6/C3H8 | 9 × 10−9 (C3H6) | 12 | [93] |
| Polymer hollow fiber | ~8 µm | C3H6/C3H8 | 1.51 × 10−8 (C3H6) | 184.4 | [94] | |
| Polymer hollow fiber | 5 µm | C3H6/C3H8 | 2.21 × 10−8 (C3H6) | 65 | [95] | |
| Polymer hollow fiber | 8.5 µm | CO2/N2 | 7.4 × 10−9 (CO2) | 52 | [98] | |
| Vapor-phase ripening | α-Al2O3 support | 300–400 nm | C3H6/C3H8 | 1.25 × 10−8 (C3H6) | 120 | [101] |
| Gel–vapor deposition | PVDF hollow fiber | <20 nm | C3H6/C3H8 | 2.8 × 10−7 (C3H6) | 67.2 | [106] |
| All-vapor ligand-induced permselectivation | γ-Al2O3 coated α-Al2O3 support | <500 nm | C3H6/C3H8 | 8.8 × 10−8 (C3H6) | 71 | [102] |
| 1.6 × 10−7 (C3H6) | 74 | |||||
| Current-driven synthesis | Pt coated AAO support | ~200 nm | C3H6/C3H8 | 1.74 × 10−8 (C3H6) | 300 | [109] |
| Postsynthetic linker exchange | α-Al2O3 support | <1 µm | C3H6/C3H8 | 7.8 × 10−8 (C3H6) | 40 | [115] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Liu, D. Zeolitic Imidazolate Framework Membranes for Light Olefin/Paraffin Separation. Crystals 2019, 9, 14. https://doi.org/10.3390/cryst9010014
Ma X, Liu D. Zeolitic Imidazolate Framework Membranes for Light Olefin/Paraffin Separation. Crystals. 2019; 9(1):14. https://doi.org/10.3390/cryst9010014
Chicago/Turabian StyleMa, Xiaoli, and Defei Liu. 2019. "Zeolitic Imidazolate Framework Membranes for Light Olefin/Paraffin Separation" Crystals 9, no. 1: 14. https://doi.org/10.3390/cryst9010014





