Sc2[Se2O5]3: The First Rare-Earth Metal Oxoselenate(IV) with Exclusively [Se2O5]2− Anions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supporting Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Wontcheu, J.; Schleid, T. Ce2[SeO3]3 and Pr2[SeO3]3: Non-Isostructural Oxoselenates(IV) of the Light Lanthanoids. Z. Anorg. Allg. Chem. 2006, 632, 645–654. [Google Scholar] [CrossRef]
- Wontcheu, J.; Schleid, T. Sc2Se3O9: Scandium(III) Oxoselenate(IV) According to Sc2[SeO3]3 with a Hexagonal “Lone-Pair” Channel Structure. Z. Anorg. Allg. Chem. 2003, 629, 1463–1465. [Google Scholar] [CrossRef]
- Harrison, W.T.A. Lanthanum selenite, La2(SeO3)3. Acta Crystallogr. 2000, 56, 627–628. [Google Scholar]
- Wickleder, M.S. Wasserfreie Selenite des Lanthans: Synthese und Kristallstruktur von La2(SeO3)3 und LaFSeO3. Z. Anorg. Allg. Chem. 2000, 626, 547–551. [Google Scholar] [CrossRef]
- Krügermann, I.; Wickleder, M.S. Syntheses and Crystal Structures of Er2(SeO3)3 and Dy3(SeO3)4F. J. Solid State Chem. 2002, 167, 113–118. [Google Scholar] [CrossRef]
- Krügermann, I.; Wickleder, M.S.; Wontcheu, J.; Schleid, Th. The Unique Crystal Structure of the Triclinic Samarium(III) Oxoselenate(IV) Sm2[SeO3]3. Z. Anorg. Allg. Chem. 2006, 632, 901–904. [Google Scholar] [CrossRef]
- Krügermann, I.; Wickleder, M.S. Kristallstruktur und Phasenumwandlung von Nd2(SeO3)3. Z. Anorg. Allg. Chem. 2002, 628, 2197. [Google Scholar] [CrossRef]
- Krügermann, I. Oxoselenate(IV/VI) der Selten-Erd-Elemente und ihre Derivate. Ph.D. Thesis, Universität Köln, Cologne, Germany, 2002. [Google Scholar]
- Wontcheu, J. Oxoselenates(IV) of the Trivalent Rare-Earth Elements and Some Derivatives. Ph.D. Thesis, Universität Stuttgart, Stuttgart, Germany, 2004. [Google Scholar]
- Chou, S.-C. Rare-Earth Metal(III) Oxoselenates(IV) and Oxotellurates(IV) and Investigation of Their Luminescent Properties. Ph.D. Thesis, Universität Stuttgart, Stuttgart, Germany, 2015. [Google Scholar]
- Wickleder, M.S. Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Jr., Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Elsevier Scinece Publishers: New York, NY, USA, 2005; Volume 35. [Google Scholar]
- Greiner, S. Untersuchungen zum Antennen-Effekt bei lanthanoidischen Leuchtstoffen auf Oxoselenat(IV)-Basis. Bachelor Thesis, Universität Stuttgart, Stuttgart, Germany, 2013. [Google Scholar]
- Wontcheu, J.; Zitzer, S.; Schleid, Th. Die Lanthanoid(III)-Oxid-Oxoselenate(IV) Ln2O[SeO3]2 (Ln = Sm − Tm). Z. Naturforsch. 2016, 71b, 1279–1285. [Google Scholar] [CrossRef]
- Zitzer, S.; Su, S.-H.; Greiner, S.; Schleid, Th. Sm2Se2O7 and Sm3O2Cl[SeO3]2: A New Monoclinic Modification of Samarium(III) Oxide Oxoselenate(IV) and a Samarium(III) Oxide Chloride Oxoselenate(IV) Both Containing Oxygen-Centered Samarium Tetrahedra. Z. Anorg. Allg. Chem. 2018, in press. [Google Scholar]
- Wontcheu, J.; Schleid, Th. Tb2Se2O7: Terbium(III) Oxide Oxoselenate(IV) according to Tb2O[SeO3]2 with a “Lone-Pair” Channel Structure. Z. Anorg. Allg. Chem. 2002, 628, 1941–1945. [Google Scholar] [CrossRef]
- Greiner, S. Scandium(III)-Oxoselenate(IV) als kompakte Wirtsgitter für Lanthanoid-Dotierungen. Master’s Thesis, Universität Stuttgart, Stuttgart, Germany, 2015. [Google Scholar]
- Greiner, S.; Chou, S.-C.; Schleid, Th. Two anionically derivatized scandium oxoselenates(IV): ScF[SeO3] and Sc2O2[SeO3]. J. Solid State Chem. 2017, 246, 160–166. [Google Scholar] [CrossRef]
- Zhang-Preße, M.; Oppermann, H. Thermochemical Investigation of RE2O3–SeO2 Systems, III. Yttrium selenium oxides in the pseudo-binary system. J. Therm. Anal. Calorim. 2002, 69, 301–316. [Google Scholar] [CrossRef]
- Zhang-Preße, M.; Oppermann, H. Thermochemische Untersuchungen zu den Systemen SE2O3–SeO2, IV. Lösungskalorimetrie der SE2SexO3+2x-Phasen (SE = Nd, Sm, Y). Z. Naturforsch 2002, 57b, 661–667. [Google Scholar]
- Oppermann, H.; Zhang-Preße, M.; Schmidt, P. Thermochemische Untersuchungen zu den Systemen SE2O3–SeO2, V. Ytterbiumselenoxide auf dem Schnitt Yb2O3–SeO2. Z. Naturforsch. 2002, 57b, 868–876. [Google Scholar]
- Oppermann, H.; Zhang-Preße, M.; Weck, S.; Liebig, S. Thermochemische Untersuchungen zu den Systemen SE2O3/SeO2, I. Neodymselenoxide auf dem Schnitt Nd2O3–SeO2. Z. Anorg. Allg. Chem. 2002, 628, 81–90. [Google Scholar] [CrossRef]
- Oppermann, H.; Zhang-Preße, M. Thermochemische Untersuchungen zu den Systemen SE2O3–SeO2, II. Samariumselenoxide auf dem Schnitt Sm2O3–SeO2. Z. Naturforsch 2001, 56b, 917–926. [Google Scholar]
- Wickleder, M.S. Sm2Se5O13: A Selenite–Diselenite according to Sm2(SeO3)(Se2O5)2. Z. Anorg. Allg. Chem. 2006, 632, 2377–2379. [Google Scholar] [CrossRef]
- Song, S.Y.; Ok, K.M. Modulation of Framework and Centricity: Cation Size Effect in New Quaternary Selenites, ASc(SeO3)2 (A = Na, K, Rb, and Cs). Inorg. Chem. 2015, 54, 5032–5038. [Google Scholar] [CrossRef] [PubMed]
- Herrendorf, W.; Bärnighausen, H. HABITUS: Programm zur Optimierung der Kristallgestalt für die Numerische Absorptionskorrektur als Version X-SHAPE, version 1.06; Universität Karlsruhe 1993, Universität Gießen 1996; Fa. Stoe: Darmstadt, Germany, 1999. [Google Scholar]
- Sheldrick, G.M. SHELX-97: Program Suite for the Solution and Refinement of Crystal Structures; Universität Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- McCullough, J.D. The Crystal Structure of Selenium Dioxide. J. Am. Chem. Soc. 1937, 59, 789–794. [Google Scholar] [CrossRef]
- Siebert, H. Anwendung der Schwinungsspektroskopie in der Anorganischen Chemie; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1966. [Google Scholar]
- Zitzer, S. Selten-Erd-Metall(III)-Oxoselenate(IV) und -Oxotellurate(IV): Synthese, Kristallstrukturaufklärung und fluoreszenzspektroskopische Untersuchungen ausgewählter Leuchtstoffe. Ph.D. Thesis, Universität Stuttgart, Stuttgart, Germany, 2011. [Google Scholar]
- Ok, K.M.; Halasyamani, P.S. New Selenites: Syntheses, Structures, and Characterization of Centrosymmetric Al2(Se2O5)3 and Ga2(Se2O5)3 and Non-centrosymmetric In2(Se2O5)3. Chem. Mater. 2002, 14, 2360–2364. [Google Scholar] [CrossRef]
- Verma, V.P. A review of synthetic, thermoanalytical, IR, Raman and X-ray studies on metal selenites. Thermochim. Acta 1999, 327, 63–102. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Chou, S.-C.; Greiner, S.; Magdysyuk, O.V.; Dinnebier, R.E.; Schleid, Th. Theoretical and Experimental Analysis of Structural Phase Transitions for ScF[SeO3] and YF[SeO3]. Z. Anorg. Allg. Chem. 2014, 640, 3203–3211. [Google Scholar] [CrossRef]
- Greiner, S.; Schleid, T.H. ScCl[SeO3]: The Chloride Oxoselenate(IV) of the Smallest Rare-Earth Metal. Z. Anorg. Allg. Chem. 2016, 642, 1076. [Google Scholar]
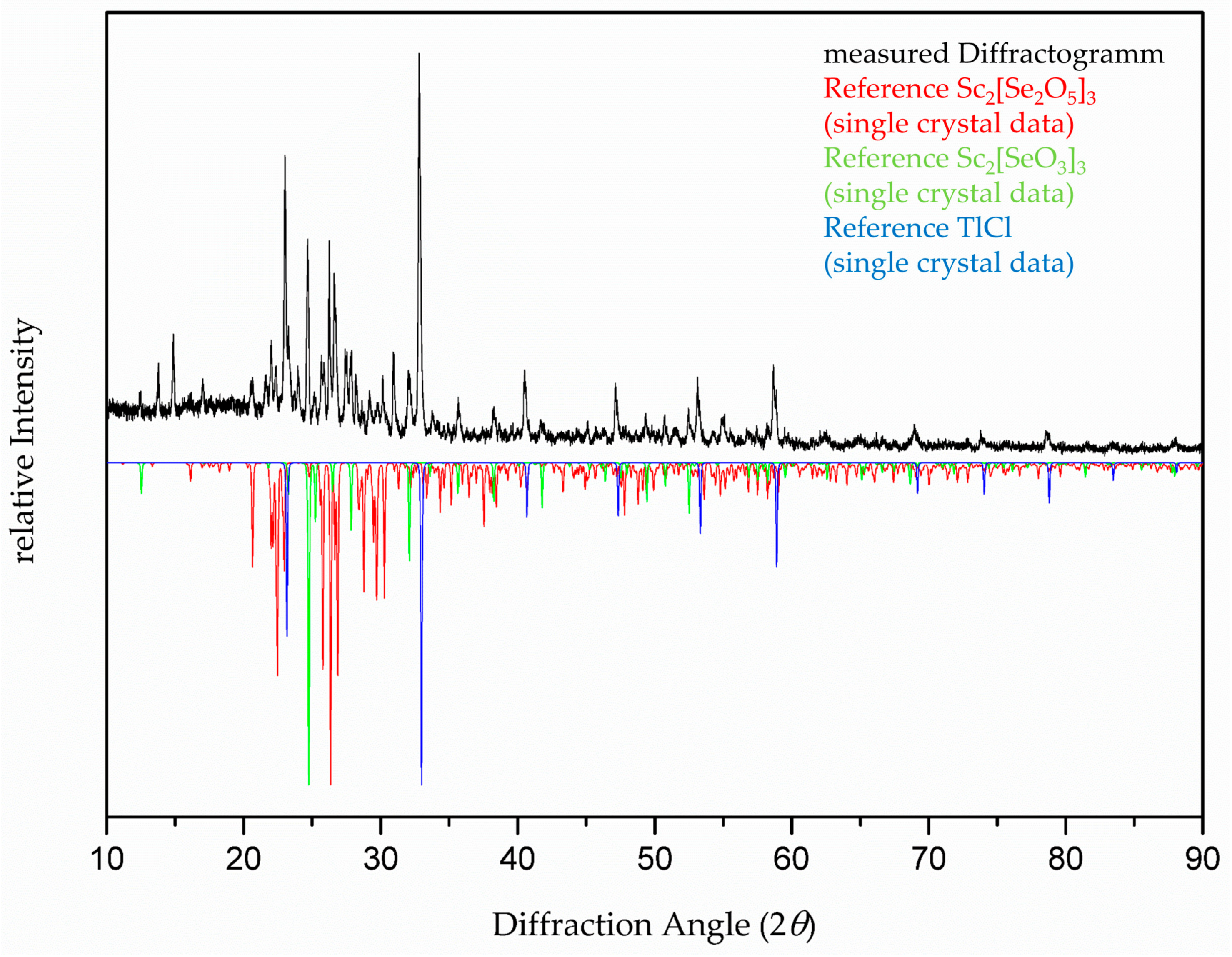

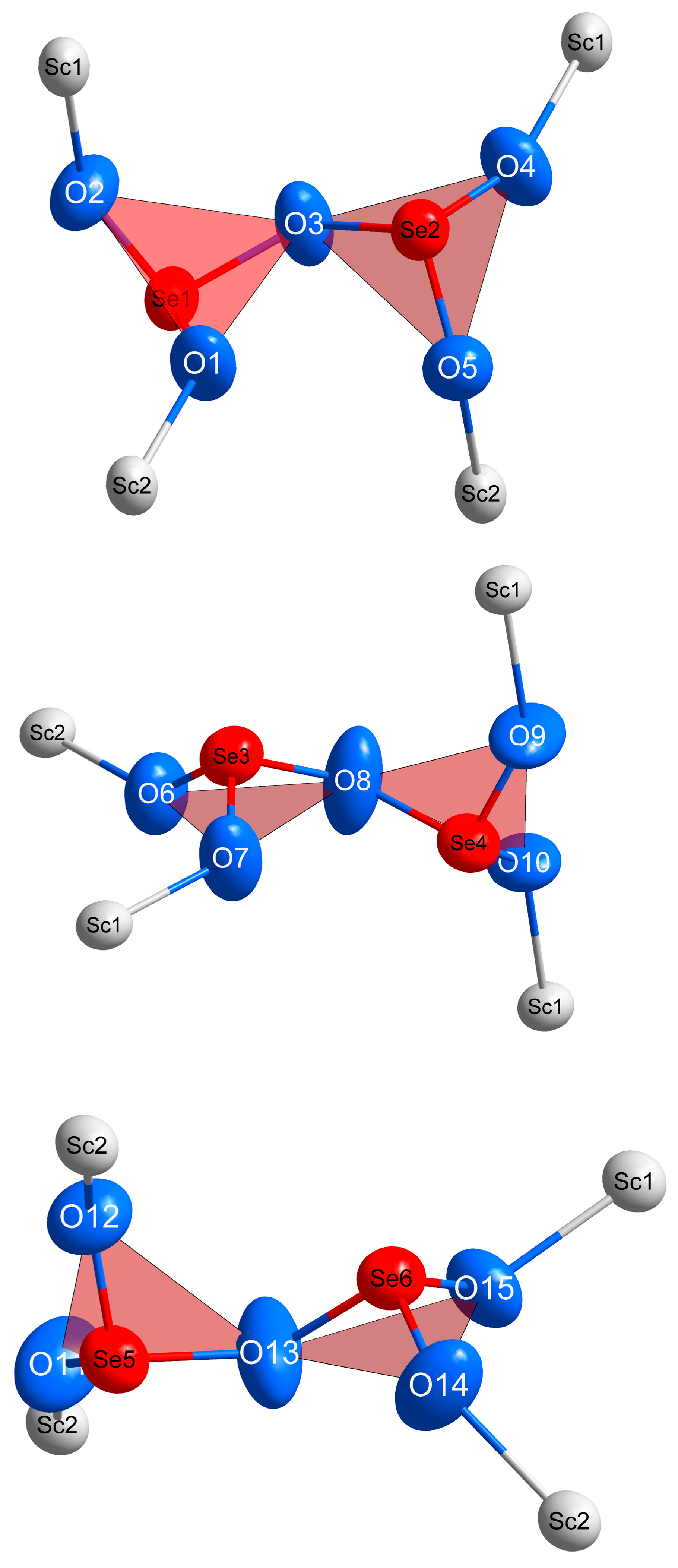


| Empirical Formula | Sc2[Se2O5]3 (≡ Sc2Se6O15) |
|---|---|
| Crystal system | triclinic |
| Space group | P (no. 2) |
| Lattice parameters | |
| a/pm | 663.71(5) |
| b/pm | 1024.32(7) |
| c/pm | 1057.49(8) |
| α/deg | 81.034(2) |
| β/deg | 87.468(2) |
| γ/deg | 89.237(2) |
| Number of formula units (Z) | 2 |
| Calculated density (Dx in g/cm3) | 3.762 |
| Molar volume (Vm in cm3/mol) | 188.58(9) |
| Diffractometer | κ-CCD (Bruker Nonius) |
| Wavelength (λ in pm) | 71.07 (Mo Kα) |
| Index range (±hmax, ±kmax, ±lmax) | 8/13/13 |
| Number of e− per unit cell (F(000)) | 732 |
| Absorption coefficient (μ in mm−1) | 16.426 |
| Number of collected vs. unique reflections | 31217/3256 |
| Data-set residuals (Rint/Rσ) | 0.084/0.036 |
| Structure residuals (R1/wR2) | 0.034/0.074 |
| Goodness of fit (GooF) | 1.058 |
| Extinction coefficient (g) | 0.0101(5) |
| Residual electron density (max./min. in e− 10−6 pm−3) | 1.318/−1.267 |
| CSD-number | 433,355 |
| Atom | x/a | y/b | z/c | Ueq1 |
|---|---|---|---|---|
| Sc1 | 0.16448(9) | 0.58715(7) | 0.26677(7) | 145(2) |
| Sc2 | 0.31102(9) | 0.06071(7) | 0.28974(7) | 143(2) |
| Se1 | 0.22321(5) | 0.77750(4) | 0.51356(4) | 173(1) |
| Se2 | 0.31368(5) | 0.30185(4) | 0.49159(4) | 156(1) |
| Se3 | 0.14680(5) | 0.33785(4) | 0.08075(4) | 197(1) |
| Se4 | 0.34062(5) | 0.46713(4) | 0.80898(4) | 189(1) |
| Se5 | 0.20134(5) | 0.01376(4) | 0.77299(4) | 171(1) |
| Se6 | 0.32216(5) | 0.85034(4) | 0.04488(4) | 173(1) |
| O1 | 0.3565(4) | 0.8834(3) | 0.4083(3) | 257(6) |
| O2 | 0.1123(4) | 0.6849(3) | 0.4203(3) | 241(6) |
| O3 | 0.4252(4) | 0.6720(3) | 0.5744(3) | 229(6) |
| O4 | 0.2120(4) | 0.4093(3) | 0.3801(3) | 245(6) |
| O5 | 0.2599(4) | 0.1567(3) | 0.4489(3) | 210(6) |
| O6 | 0.3013(4) | 0.2435(3) | 0.1727(3) | 288(7) |
| O7 | 0.1988(4) | 0.4927(3) | 0.1010(3) | 289(7) |
| O8 | 0.2873(4) | 0.3302(3) | 0.9362(3) | 354(8) |
| O9 | 0.1421(4) | 0.4542(3) | 0.7240(3) | 308(7) |
| O10 | 0.4805(4) | 0.6157(3) | 0.2594(3) | 241(6) |
| O11 | 0.0055(4) | 0.0288(3) | 0.2913(3) | 289(7) |
| O12 | 0.3733(4) | 0.9148(3) | 0.7186(3) | 227(6) |
| O13 | 0.1454(4) | 0.9259(3) | 0.9300(3) | 346(8) |
| O14 | 0.3719(4) | 0.9735(3) | 0.1228(3) | 288(7) |
| O15 | 0.1445(4) | 0.7649(3) | 0.1370(3) | 229(6) |
| Sc1–O4 | 204.7(3) | Sc2–O11 | 205.7(3) |
| Sc1–O2 | 205.1(3) | Sc1–O1 | 206.7(3) |
| Sc1–O9 | 207.9(3) | Sc1–O6 | 207.9(3) |
| Sc1–O15 | 210.9(3) | Sc1–O5 | 209.1(3) |
| Sc1–O10 | 211.8(3) | Sc1–O12 | 211.0(3) |
| Sc1–O7 | 213.3(3) | Sc1–O14 | 212.1(3) |
| Se1–O1 | 166.1(3) | Se2–O4 | 164.4(3) |
| Se1–O2 | 167.0(3) | Se1–O5 | 166.7(3) |
| Se1–O3 | 178.9(3) | Se1–O3 | 184.6(3) |
| Se3–O6 | 164.3(3) | Se4–O9 | 164.5(3) |
| Se1–O7 | 167.6(3) | Se1–O10 | 165.6(3) |
| Se1–O8 | 176.7(3) | Se1–O8 | 181.3(3) |
| Se5–O11 | 165.2(3) | Se6–O14 | 165.5(3) |
| Se1–O12 | 166.0(3) | Se1–O15 | 166.3(6) |
| Se1–O13 | 178.7(3) | Se1–O13 | 180.6(3) |
| O1–Se1–O3 | 098.52(13) | O3–Se2–O4 | 095.05(13) |
| O2–Se1–O3 | 101.80(14) | O3–Se2–O5 | 101.58(14) |
| O1–Se1–O2 | 102.67(14) | O4–Se2–O5 | 103.23(14) |
| O6–Se3–O8 | 095.50(15) | O8–Se4–O10 | 093.04(15) |
| O7–Se3–O8 | 099.83(15) | O8–Se4–O9 | 098.24(16) |
| O6–Se3–O7 | 105.18(15) | O9–Se4–O10 | 104.03(15) |
| O11–Se5–O13 | 096.13(15) | O13–Se6–O15 | 093.44(13) |
| O12–Se5–O13 | 100.96(15) | O13–Se6–O14 | 102.13(15) |
| O11–Se5–O12 | 102.11(14) | O14–Se6–O15 | 103.96(15) |
| Se1–O3–Se2 | 121.32(14) | Se3–O8–Se4 | 126.31(18) |
| Se5–O13–Se6 | 127.48(17) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greiner, S.; Schleid, T. Sc2[Se2O5]3: The First Rare-Earth Metal Oxoselenate(IV) with Exclusively [Se2O5]2− Anions. Crystals 2018, 8, 187. https://doi.org/10.3390/cryst8050187
Greiner S, Schleid T. Sc2[Se2O5]3: The First Rare-Earth Metal Oxoselenate(IV) with Exclusively [Se2O5]2− Anions. Crystals. 2018; 8(5):187. https://doi.org/10.3390/cryst8050187
Chicago/Turabian StyleGreiner, Stefan, and Thomas Schleid. 2018. "Sc2[Se2O5]3: The First Rare-Earth Metal Oxoselenate(IV) with Exclusively [Se2O5]2− Anions" Crystals 8, no. 5: 187. https://doi.org/10.3390/cryst8050187




