Figure 1.
Experimental (top) and calculated (bottom) Guinier powder pattern (CuKα1 radiation) of YPt2Al3.
Figure 1.
Experimental (top) and calculated (bottom) Guinier powder pattern (CuKα1 radiation) of YPt2Al3.
Figure 2.
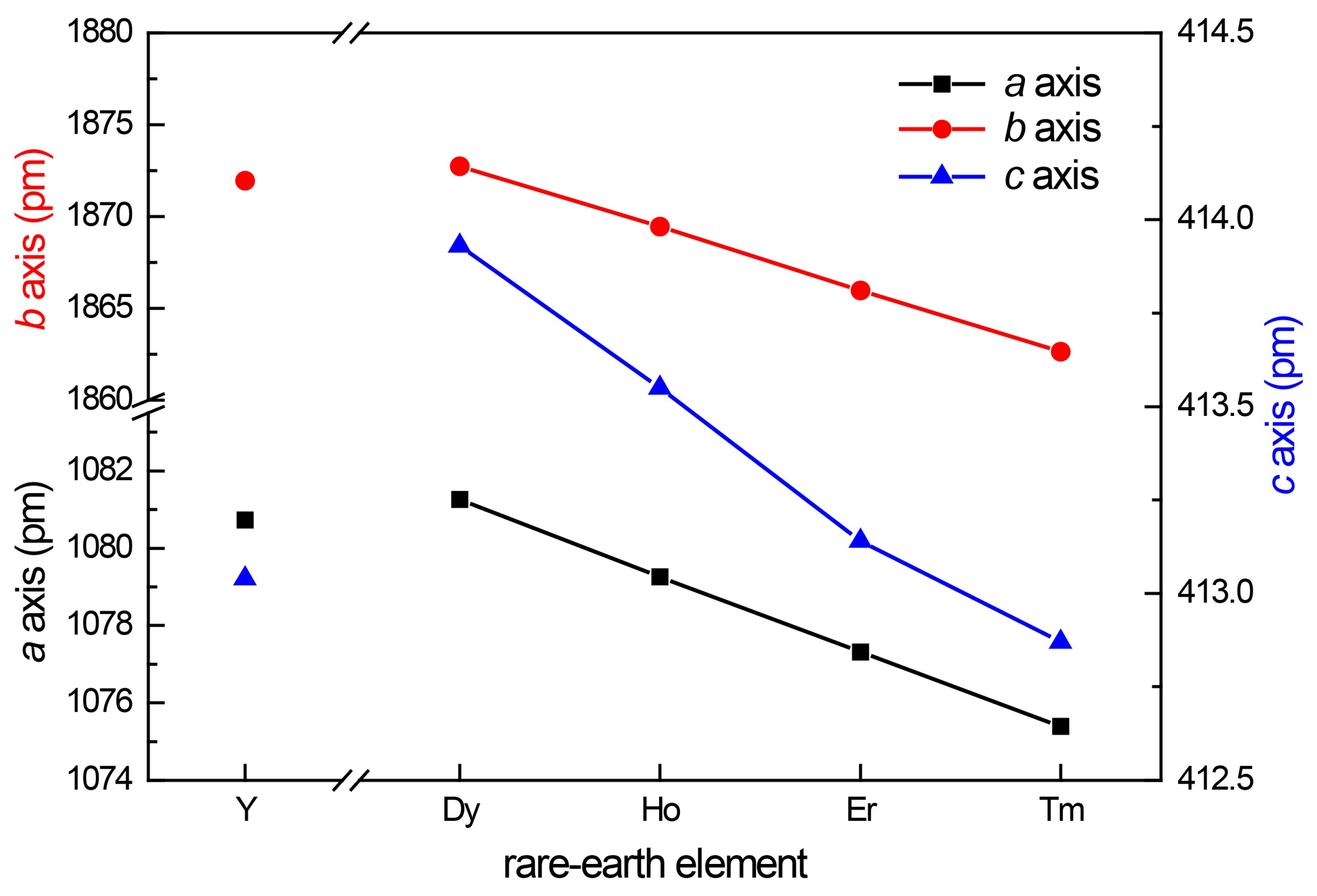
Plot of the unit cell parameters of the REPt2Al3 phases as a function of the rare-earth element.
Figure 2.
Plot of the unit cell parameters of the REPt2Al3 phases as a function of the rare-earth element.
Figure 3.
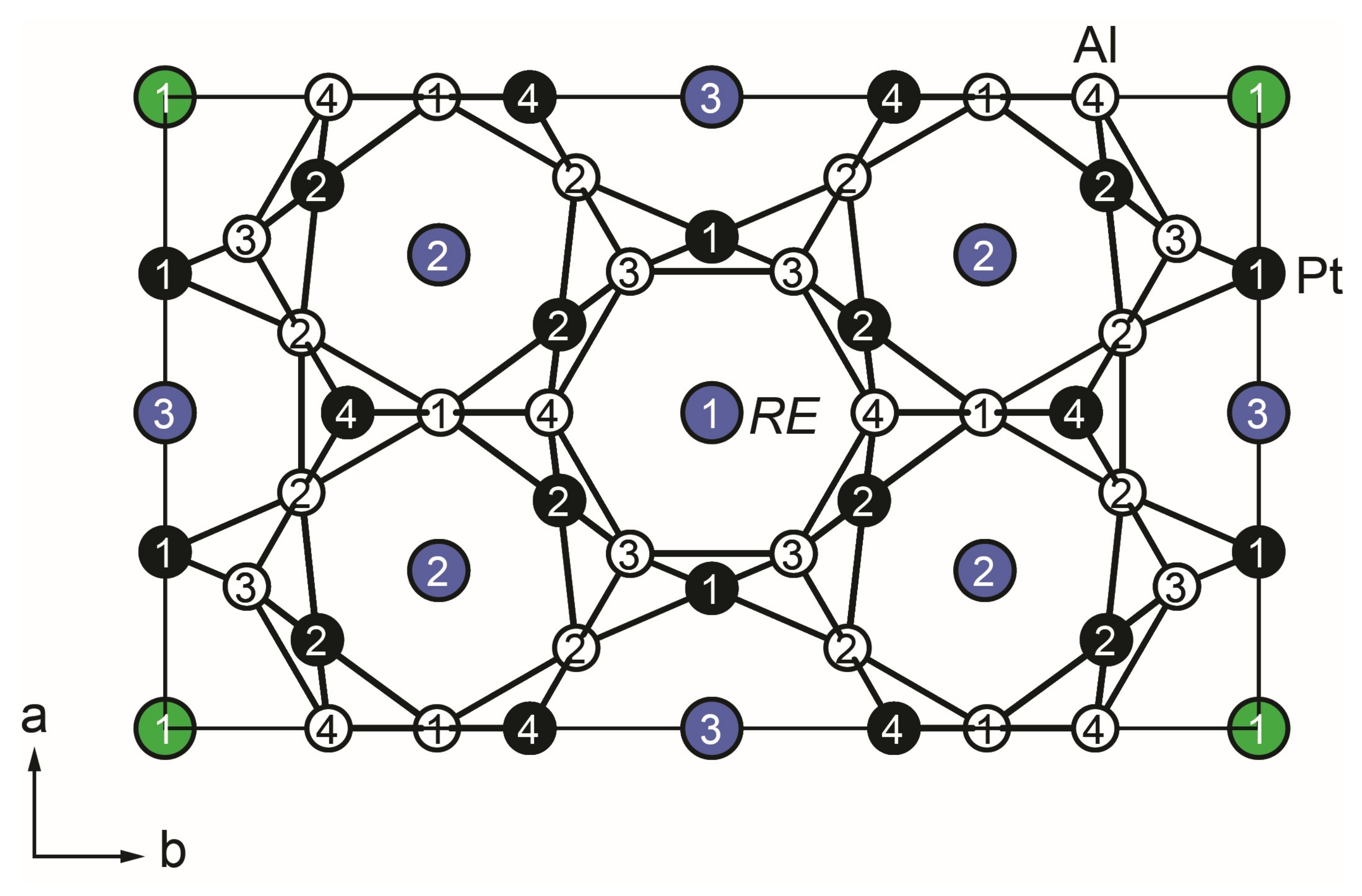
The crystal structure of YPt2Al3. Yttrium, platinum, and aluminum atoms are drawn as green/blue, black-filled, and open circles, respectively. The polyanionic [Pt2Al3]δ– network is highlighted.
Figure 3.
The crystal structure of YPt2Al3. Yttrium, platinum, and aluminum atoms are drawn as green/blue, black-filled, and open circles, respectively. The polyanionic [Pt2Al3]δ– network is highlighted.
Figure 4.
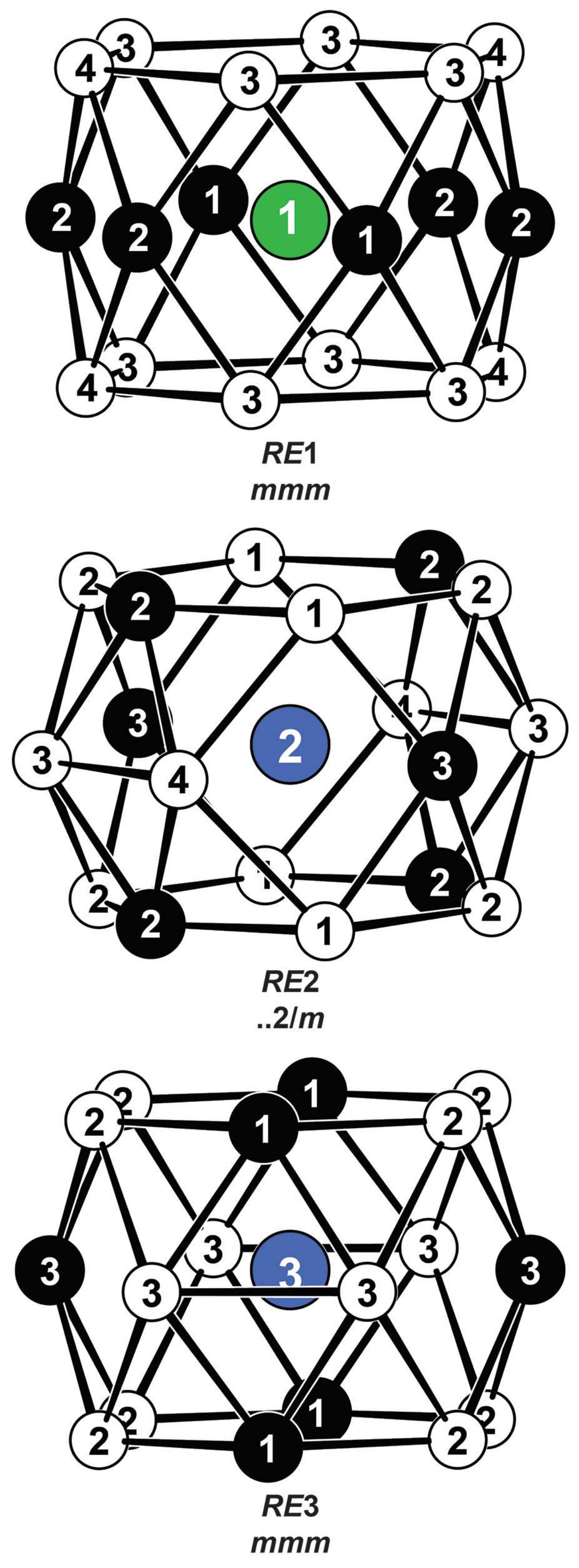
Coordination polyhedra surrounding the three crystallographically independent yttrium sites in YPt2Al3. Yttrium, platinum, and aluminum atoms are drawn as green/blue, black-filled, and open circles, respectively. The local site symmetries are given.
Figure 4.
Coordination polyhedra surrounding the three crystallographically independent yttrium sites in YPt2Al3. Yttrium, platinum, and aluminum atoms are drawn as green/blue, black-filled, and open circles, respectively. The local site symmetries are given.
Figure 5.
Group-subgroup scheme in the Bärnighausen formalism [
36,
37,
38,
39] for the structures of PrNi
2Al
3 and YPt
2Al
3. The index for the isomorphic (i) and
translationengleiche (t) symmetry reduction, the unit cell transformation, and the evolution of the atomic parameters are given.
Figure 5.
Group-subgroup scheme in the Bärnighausen formalism [
36,
37,
38,
39] for the structures of PrNi
2Al
3 and YPt
2Al
3. The index for the isomorphic (i) and
translationengleiche (t) symmetry reduction, the unit cell transformation, and the evolution of the atomic parameters are given.
Figure 6.
Extended crystal structure of Lu2Pt3Al4 along [010]. Lutetium, platinum, and aluminum atoms are drawn as green/blue, black-filled, and open circles, respectively. The polyanionic [Pt3Al4]δ– network and the two different coordination environments for the lutetium atoms are highlighted.
Figure 6.
Extended crystal structure of Lu2Pt3Al4 along [010]. Lutetium, platinum, and aluminum atoms are drawn as green/blue, black-filled, and open circles, respectively. The polyanionic [Pt3Al4]δ– network and the two different coordination environments for the lutetium atoms are highlighted.
Figure 7.
The Al arrangement in the crystal structure of Lu2Pt3Al4 (top). The Pt atoms capping the layers are depicted in the bottom image. Platinum and aluminum atoms are drawn as black-filled and open circles, respectively. The Pt–Al bonds in the polyanionic [Pt3Al4]δ– network are highlighted.
Figure 7.
The Al arrangement in the crystal structure of Lu2Pt3Al4 (top). The Pt atoms capping the layers are depicted in the bottom image. Platinum and aluminum atoms are drawn as black-filled and open circles, respectively. The Pt–Al bonds in the polyanionic [Pt3Al4]δ– network are highlighted.
Figure 8.
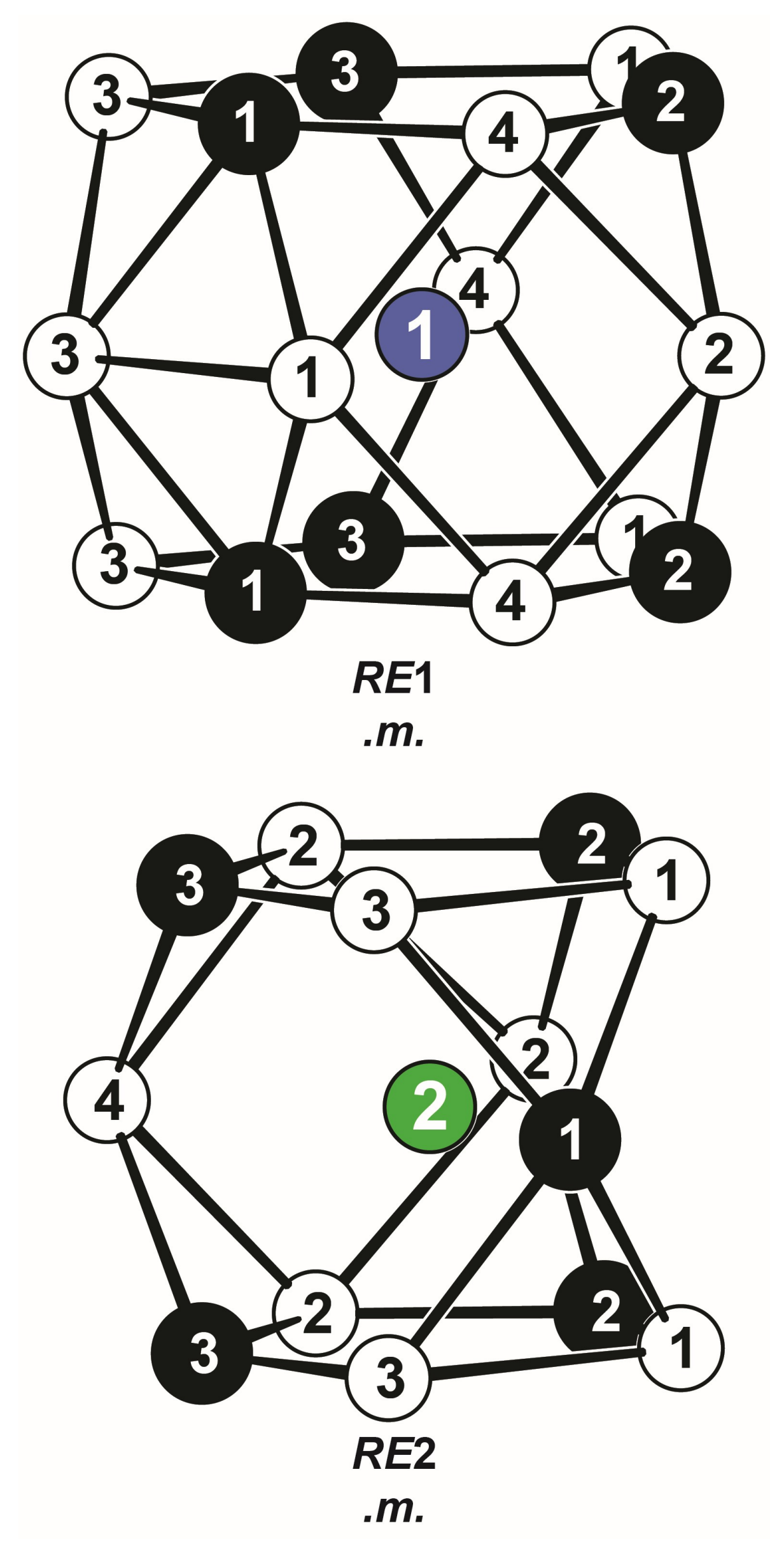
Coordination polyhedra surrounding the two crystallographically independent lutetium sites in Lu2Pt3Al4. Lutetium, platinum, and aluminum atoms are drawn as green/blue, black-filled, and open circles, respectively. The local site symmetries are given.
Figure 8.
Coordination polyhedra surrounding the two crystallographically independent lutetium sites in Lu2Pt3Al4. Lutetium, platinum, and aluminum atoms are drawn as green/blue, black-filled, and open circles, respectively. The local site symmetries are given.
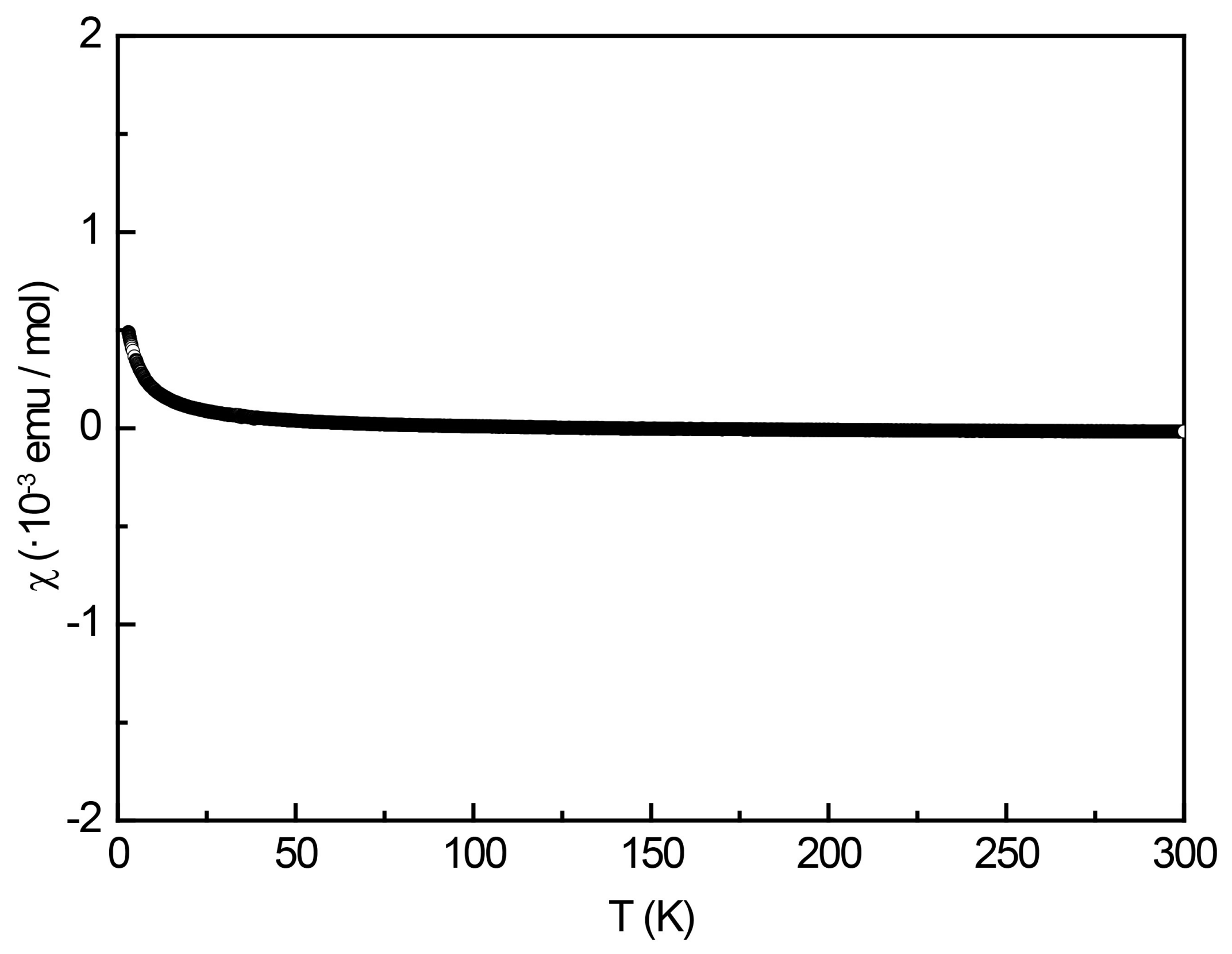
Figure 9.
Temperature dependence of the magnetic susceptibility (data) of YPt2Al3 measured at 10 kOe.
Figure 9.
Temperature dependence of the magnetic susceptibility (data) of YPt2Al3 measured at 10 kOe.
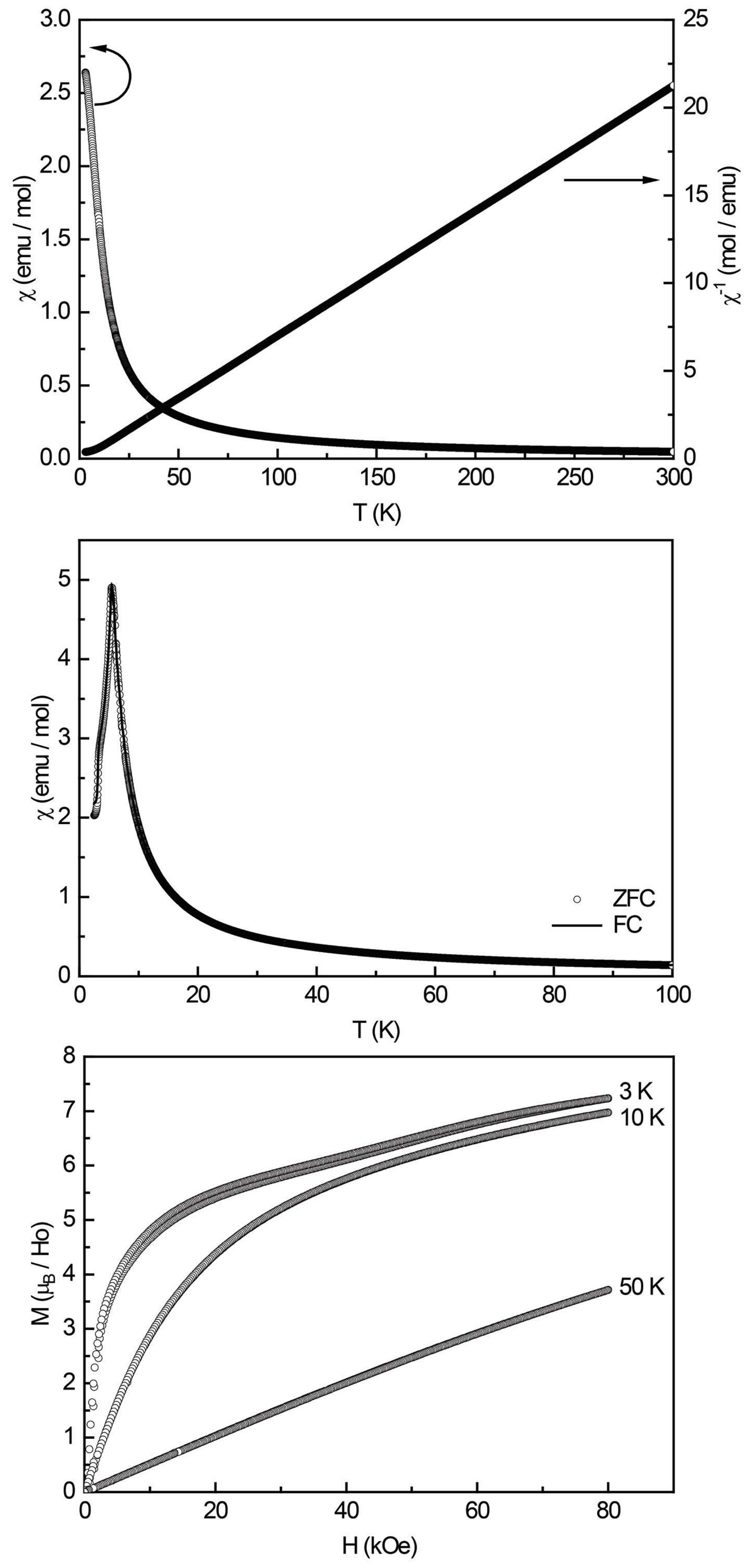
Figure 10.
Magnetic properties of DyPt2Al3: (top) temperature dependence of the magnetic susceptibility χ and its inverse χ–1 measured at 10 kOe; (middle) zero-field-cooled/field-cooled (ZFC/FC) data (100 Oe) and the dχ/dT derivative (red curve) of the FC curve; and (bottom) magnetization isotherms recorded at 3, 10, and 50 K.
Figure 10.
Magnetic properties of DyPt2Al3: (top) temperature dependence of the magnetic susceptibility χ and its inverse χ–1 measured at 10 kOe; (middle) zero-field-cooled/field-cooled (ZFC/FC) data (100 Oe) and the dχ/dT derivative (red curve) of the FC curve; and (bottom) magnetization isotherms recorded at 3, 10, and 50 K.
Figure 11.
Magnetic properties of HoPt2Al3: (top) temperature dependence of the magnetic susceptibility χ and its inverse χ–1 measured at 10 kOe; (middle) zero-field-cooled/field-cooled (ZFC/FC) data (100 Oe); and (bottom) magnetization isotherms recorded at 3, 10, and 50 K.
Figure 11.
Magnetic properties of HoPt2Al3: (top) temperature dependence of the magnetic susceptibility χ and its inverse χ–1 measured at 10 kOe; (middle) zero-field-cooled/field-cooled (ZFC/FC) data (100 Oe); and (bottom) magnetization isotherms recorded at 3, 10, and 50 K.
Figure 12.
Magnetic properties of ErPt2Al3: (top) temperature dependence of the magnetic susceptibility χ and its inverse χ–1 measured at 10 kOe; (middle) zero-field-cooled/field-cooled (ZFC/FC) data (100 Oe); and (bottom) magnetization isotherms recorded at 3, 10, and 50 K.
Figure 12.
Magnetic properties of ErPt2Al3: (top) temperature dependence of the magnetic susceptibility χ and its inverse χ–1 measured at 10 kOe; (middle) zero-field-cooled/field-cooled (ZFC/FC) data (100 Oe); and (bottom) magnetization isotherms recorded at 3, 10, and 50 K.
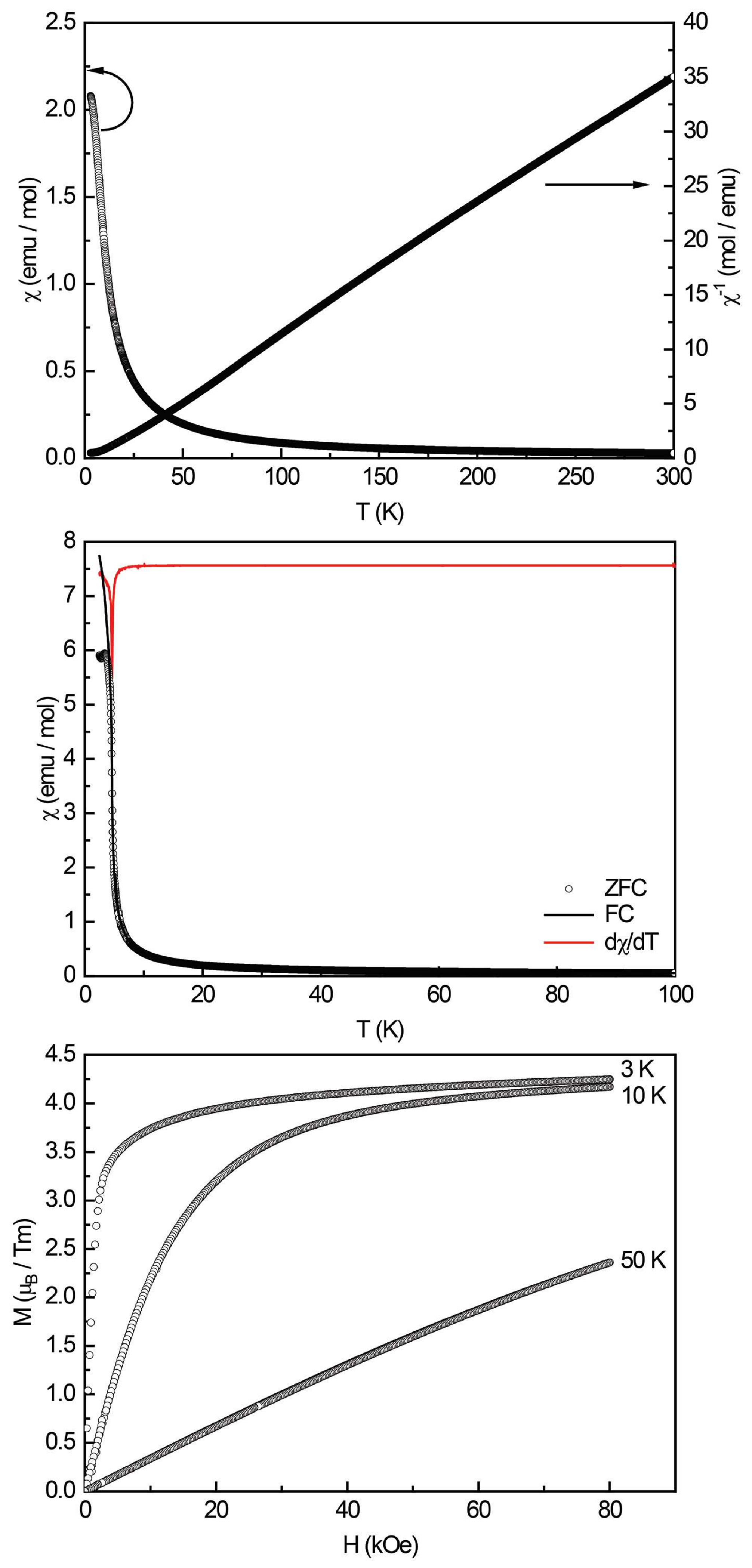
Figure 13.
Magnetic properties of TmPt2Al3: (top) temperature dependence of the magnetic susceptibility χ and its inverse χ–1 measured at 10 kOe; (middle) zero-field-cooled/field-cooled (ZFC/FC) data (100 Oe) and the dχ/dT derivative (red curve) of the FC curve; and (bottom) magnetization isotherms recorded at 3, 10, and 50 K.
Figure 13.
Magnetic properties of TmPt2Al3: (top) temperature dependence of the magnetic susceptibility χ and its inverse χ–1 measured at 10 kOe; (middle) zero-field-cooled/field-cooled (ZFC/FC) data (100 Oe) and the dχ/dT derivative (red curve) of the FC curve; and (bottom) magnetization isotherms recorded at 3, 10, and 50 K.
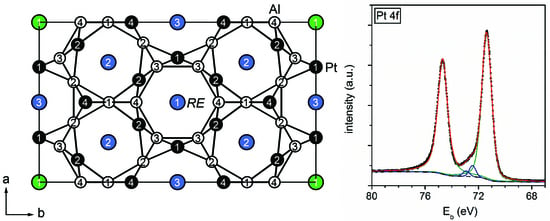
Figure 14.
Fitted X-ray photoemission spectrum of Pt 4f in YPt2Al3. The experimental data is shown as black squares, the Pt 4f components are depicted in green, the Al 2p lines in blue, and the envelope function in red. The background is depicted as a dashed line.
Figure 14.
Fitted X-ray photoemission spectrum of Pt 4f in YPt2Al3. The experimental data is shown as black squares, the Pt 4f components are depicted in green, the Al 2p lines in blue, and the envelope function in red. The background is depicted as a dashed line.
Table 1.
Lattice parameters of the orthorhombic REPt2Al3 series (YPt2Al3-type, rare-earth (RE) = Y, Dy–Tm), space group Cmmm, and RE2Pt3Al4 series (Ce2Ir3Sb4-type, RE = Y, Dy–Tm), space group Pnma.
Table 1.
Lattice parameters of the orthorhombic REPt2Al3 series (YPt2Al3-type, rare-earth (RE) = Y, Dy–Tm), space group Cmmm, and RE2Pt3Al4 series (Ce2Ir3Sb4-type, RE = Y, Dy–Tm), space group Pnma.
| Compound | a (pm) | b (pm) | c (pm) | V (nm³) |
|---|
| YPt2Al3 | 1080.73(6) | 1871.96(9) | 413.04(2) | 0.8356 |
| DyPt2Al3 | 1081.3(1) | 1872.7(2) | 413.93(5) | 0.8382 |
| HoPt2Al3 | 1079.26(4) | 1869.46(6) | 413.55(2) | 0.8344 |
| ErPt2Al3 | 1077.31(6) | 1866.0(1) | 413.14(4) | 0.8305 |
| TmPt2Al3 | 1075.38(9) | 1862.6(1) | 412.87(4) | 0.8270 |
| Tm2Pt3Al4 | 1349.9(3) | 418.22(8) | 1143.7(2) | 0.6429 |
| Lu2Pt3Al4 | 1343.4(2) | 416.41(8) | 1141.1(2) | 0.6383 |
Table 2.
Crystallographic data and structure refinement for YPt2Al3, space group Cmmm, Z = 8, own type and Lu2Pt3Al4, space group Pnma, Z = 4, Ce2Ir3Sb4-type.
Table 2.
Crystallographic data and structure refinement for YPt2Al3, space group Cmmm, Z = 8, own type and Lu2Pt3Al4, space group Pnma, Z = 4, Ce2Ir3Sb4-type.
| Compound | YPt2Al3 | Lu2Pt3Al4 |
|---|
| Molar mass, g mol–1 | 560.0 | 1043.1 |
| Density calc., g cm–3 | 8.93 | 10.91 |
| Crystal size, µm | 25 × 40 × 55 | 30 × 30 × 40 |
| Detector distance, mm | 40 | 40 |
| Exposure time, s | 25 | 50 |
| Integr. param. A, B, EMS | 6.2; −5.2; 0.017 | 5.0; −4.1; 0.012 |
| Range in hkl | ±16; ±28, ±6 | ±21; ±6, ±18 |
| θmin, θmax, deg | 2.2–32.9 | 2.3–35.5 |
| Linear absorption coeff., mm–1 | 81.2 | 97.0 |
| No. of reflections | 11,714 | 21,601 |
| Rint/Rσ | 0.1124/0.0178 | 0.1411/0.1152 |
| No. of independent reflections | 942 | 1605 |
| Reflections used [I ≥ 3σ(I)] | 795 | 679 |
| F(000), e | 1872 | 1712 |
| R1/wR2 for I ≥ 3σ(I) | 0.0341/0.0770 | 0.0415/0.0798 |
| R1/wR2 for all data | 0.0422/0.0780 | 0.1095/0.0940 |
| Data/parameters | 942/46 | 1605/56 |
| Goodness-of-fit on F2 | 2.22 | 1.23 |
| Extinction coefficient | 161(17) | 73(6) |
| Diff. Fourier residues/e– Å–3 | −4.15/3.97 | −4.98/4.51 |
Table 3.
Atom positions and equivalent isotropic displacement parameters (pm2) for YPt2Al3. Ueq is defined as one-third of the trace of the orthogonalized Uij tensor.
Table 3.
Atom positions and equivalent isotropic displacement parameters (pm2) for YPt2Al3. Ueq is defined as one-third of the trace of the orthogonalized Uij tensor.
| Atom | Wyckoff | x | y | z | Ueq |
|---|
| | Position | | | | |
|---|
| Y1 | 2d | 0 | 0 | 1/2 | 151(7) |
| Y2 | 4e | 1/4 | 1/4 | 0 | 137(4) |
| Y3 | 2b | 1/2 | 0 | 0 | 137(6) |
| Pt1 | 4h | 0.27855(6) | 0 | 1/2 | 120(2) |
| Pt2 | 8q | 0.13928(4) | 0.13927(3) | 1/2 | 120(1) |
| Pt3 | 4i | 0 | 0.33333(4) | 0 | 136(2) |
| Al1 | 4j | 0 | 0.2483(3) | 1/2 | 128(14) |
| Al2 | 8q | 0.3729(4) | 0.1244(2) | 1/2 | 138(10) |
| Al3 | 8p | 0.2244(4) | 0.0748(2) | 0 | 160(11) |
| Al4 | 4i | 0 | 0.1494(4) | 0 | 157(16) |
Table 4.
Atom positions and equivalent isotropic displacement parameters (pm2) for Lu2Pt3Al4. Ueq is defined as one-third of the trace of the orthogonalized Uij tensor. y = 1/4 all 4c.
Table 4.
Atom positions and equivalent isotropic displacement parameters (pm2) for Lu2Pt3Al4. Ueq is defined as one-third of the trace of the orthogonalized Uij tensor. y = 1/4 all 4c.
| Atom | x | z | Ueq |
|---|
| Lu1 | 0.01840(10) | 0.71349(12) | 199(3) |
| Lu2 | 0.29143(10) | 0.57858(14) | 218(3) |
| Pt1 | 0.13365(9) | 0.24522(11) | 196(3) |
| Pt2 | 0.38024(9) | 0.06876(11) | 201(3) |
| Pt3 | 0.62220(9) | 0.58482(11) | 189(3) |
| Al1 | 0.0017(7) | 0.0827(9) | 210(2) |
| Al2 | 0.0714(8) | 0.4553(8) | 180(20) |
| Al3 | 0.3017(7) | 0.8651(9) | 190(30) |
| Al4 | 0.3174(7) | 0.2828(9) | 170(20) |
Table 5.
Anisotropic displacement parameters (pm2) for YPt2Al3. Coefficients Uij of the anisotropic displacement factor tensor of the atoms are defined by: −2π2[(ha*)2U11+ ... + 2hka*b*U12]. U13 = U23 = 0.
Table 5.
Anisotropic displacement parameters (pm2) for YPt2Al3. Coefficients Uij of the anisotropic displacement factor tensor of the atoms are defined by: −2π2[(ha*)2U11+ ... + 2hka*b*U12]. U13 = U23 = 0.
| Atom | U11 | U22 | U33 | U12 |
|---|
| Y1 | 144(10) | 139(11) | 169(14) | 0 |
| Y2 | 137(7) | 137(7) | 136(9) | −1(6) |
| Y3 | 135(10) | 140(10) | 136(12) | 0 |
| Pt1 | 126(3) | 122(3) | 112(3) | 0 |
| Pt2 | 117(2) | 131(2) | 112(3) | 7(1) |
| Pt3 | 147(3) | 153(3) | 107(4) | 0 |
| Al1 | 160(2) | 120(20) | 110(30) | 0 |
| Al2 | 138(16) | 145(17) | 130(20) | −18(14) |
| Al3 | 210(20) | 156(18) | 110(20) | 40(15) |
| Al4 | 110(20) | 220(30) | 140(30) | 0 |
Table 6.
Anisotropic displacement parameters (pm2) for Lu2Pt3Al4. Coefficients Uij of the anisotropic displacement factor tensor of the atoms are defined by: −2π2[(ha*)2U11 + ... + 2hka*b*U12]. U13 = U23 = 0.
Table 6.
Anisotropic displacement parameters (pm2) for Lu2Pt3Al4. Coefficients Uij of the anisotropic displacement factor tensor of the atoms are defined by: −2π2[(ha*)2U11 + ... + 2hka*b*U12]. U13 = U23 = 0.
| Atom | U11 | U22 | U33 | U12 |
|---|
| Lu1 | 194(5) | 209(6) | 194(5) | –4(4) |
| Lu2 | 234(6) | 228(6) | 191(5) | –5(5) |
| Pt1 | 192(5) | 206(6) | 190(5) | –14(4) |
| Pt2 | 215(5) | 203(5) | 183(5) | –15(4) |
| Pt3 | 179(5) | 204(5) | 184(4) | –9(4) |
| Al1 | 200(40) | 220(40) | 210(40) | 10(4) |
| Al2 | 280(50) | 130(40) | 120(30) | 30(3) |
| Al3 | 120(40) | 210(50) | 240(50) | 0 |
| Al4 | 200(40) | 130(40) | 170(40) | 20(3) |
Table 7.
Interatomic distances (pm) for YPt2Al3. All distances of the first coordination spheres are listed. All standard uncertainties were less than 0.2 pm.
Table 7.
Interatomic distances (pm) for YPt2Al3. All distances of the first coordination spheres are listed. All standard uncertainties were less than 0.2 pm.
| Y1: | 2 | Pt1 | 300.7 | Pt2: | 1 | Al1 | 253.3 | Al2: | 1 | Pt2 | 253.7 |
| | 4 | Pt2 | 300.7 | | 1 | Al2 | 253.7 | | 1 | Pt1 | 253.7 |
| | 4 | Al4 | 347.3 | | 2 | Al4 | 256.0 | | 2 | Pt3 | 260.1 |
| | 8 | Al3 | 347.6 | | 2 | Al3 | 256.0 | | 1 | Al2 | 274.4 |
| | | | | | 1 | Pt1 | 300.7 | | 1 | Al1 | 274.8 |
| Y2: | 2 | Pt3 | 311.7 | | 1 | Y1 | 300.7 | | 2 | Al3 | 277.3 |
| | 4 | Pt2 | 315.8 | | 1 | Pt2 | 300.7 | | 2 | Y2 | 339.6 |
| | 2 | Al3 | 328.8 | | 1 | Y2 | 315.8 | | 2 | Y3 | 339.9 |
| | 2 | Al4 | 329.0 | | | | | | | | |
| | 4 | Al2 | 339.6 | Pt3: | 4 | Al2 | 260.1 | Al3: | 2 | Pt2 | 256.0 |
| | 4 | Al1 | 339.7 | | 2 | Al1 | 260.5 | | 2 | Pt1 | 256.0 |
| | | | | | 2 | Y2 | 311.7 | | 2 | Al2 | 277.3 |
| Y3: | 2 | Pt3 | 311.7 | | 1 | Y3 | 311.7 | | 1 | Al4 | 279.6 |
| | 4 | Pt2 | 315.8 | | 2 | Al3 | 343.6 | | 1 | Al3 | 279.7 |
| | 2 | Al3 | 328.8 | | 1 | Al4 | 343.9 | | 1 | Y3 | 328.8 |
| | 2 | Al4 | 329.0 | | | | | | 1 | Y2 | 328.8 |
| | 4 | Al2 | 339.6 | Al1: | 2 | Pt2 | 253.3 | | 1 | Pt3 | 343.6 |
| | 4 | Al1 | 339.7 | | 2 | Pt3 | 260.5 | | 2 | Y1 | 347.6 |
| | | | | | 2 | Al2 | 274.8 | | | | |
| Pt1: | 2 | Al2 | 253.9 | | 2 | Al4 | 277.1 | Al4: | 4 | Pt2 | 256.0 |
| | 4 | Al3 | 256.0 | | 4 | Y2 | 339.7 | | 2 | Al1 | 277.1 |
| | 2 | Pt2 | 300.7 | | | | | | 2 | Al3 | 279.6 |
| | 1 | Y1 | 300.7 | | | | | | 2 | Y2 | 329.0 |
| | 2 | Y3 | 315.8 | | | | | | 1 | Pt3 | 343.9 |
| | | | | | | | | | 2 | Y1 | 347.6 |
Table 8.
Interatomic distances (pm) for Lu2Pt3Al4. All distances of the first coordination spheres are listed. All standard uncertainties were less than 0.2 pm.
Table 8.
Interatomic distances (pm) for Lu2Pt3Al4. All distances of the first coordination spheres are listed. All standard uncertainties were less than 0.2 pm.
| Lu1: | 2 | Pt3 | 298.8 | Pt2: | 2 | Al2 | 253.1 | Al2: | 2 | Pt2 | 253.1 |
| | 2 | Pt1 | 302.0 | | 1 | Al4 | 254.9 | | 1 | Pt3 | 253.6 |
| | 2 | Pt2 | 310.4 | | 1 | Al2 | 258.1 | | 1 | Pt2 | 258.1 |
| | 1 | Al4 | 326.8 | | 1 | Al1 | 258.1 | | 2 | Al4 | 287.6 |
| | 1 | Al2 | 327.1 | | 2 | Lu2 | 298.0 | | 1 | Lu2 | 302.7 |
| | 1 | Al3 | 336.8 | | 2 | Lu1 | 310.4 | | 2 | Lu2 | 307.6 |
| | 1 | Al1 | 338.9 | | | | | | 1 | Lu1 | 327.1 |
| | 2 | Al4 | 343.4 | Pt3: | 1 | Al1 | 250.3 | | | | |
| | 2 | Al1 | 344.4 | | 1 | Al2 | 253.5 | Al3: | 1 | Pt1 | 250.2 |
| | 2 | Al3 | 346.8 | | 1 | Al3 | 256.3 | | 1 | Pt3 | 256.3 |
| | | | | | 2 | Al4 | 266.3 | | 2 | Pt1 | 266.0 |
| Lu2: | 1 | Pt1 | 268.8 | | 2 | Lu2 | 294.9 | | 2 | Al3 | 280.5 |
| | 2 | Pt3 | 294.9 | | 2 | Lu1 | 298.8 | | 1 | Al1 | 291.0 |
| | 2 | Pt2 | 298.0 | | | | | | 2 | Lu2 | 312.7 |
| | 1 | Al2 | 302.7 | Al1: | 1 | Pt3 | 250.3 | | 1 | Lu1 | 336.8 |
| | 1 | Al4 | 304.4 | | 1 | Pt2 | 258.1 | | 2 | Lu1 | 346.8 |
| | 2 | Al2 | 307.6 | | 2 | Pt1 | 269.1 | | | | |
| | 2 | Al3 | 312.7 | | 1 | Al4 | 278.3 | Al4: | 1 | Pt1 | 247.6 |
| | 2 | Al1 | 312.9 | | 1 | Al3 | 291.0 | | 1 | Pt2 | 254.9 |
| | | | | | 2 | Lu2 | 312.9 | | 2 | Pt3 | 263.3 |
| Pt1: | 1 | Al4 | 247.6 | | 1 | Lu1 | 338.9 | | 2 | Al1 | 278.3 |
| | 1 | Al3 | 250.2 | | 2 | Lu1 | 344.4 | | 2 | Al2 | 287.6 |
| | 2 | Al3 | 266.0 | | | | | | 1 | Lu2 | 304.4 |
| | 1 | Lu2 | 268.8 | | | | | | 2 | Lu1 | 326.8 |
| | 2 | Al1 | 269.1 | | | | | | 1 | Lu1 | 343.4 |
| | 2 | Lu1 | 302.0 | | | | | | | | |
Table 9.
Magnetic properties of the YPt2Al3-type compounds. TN, Néel temperature; TC, Curie temperature; μeff, effective magnetic moment; μcalc, calculated magnetic moment; θp, paramagnetic Curie temperature; μsat, saturation moment; and saturation according to gJ × J. The experimental saturation magnetizations were obtained at 3 K and 80 kOe.
Table 9.
Magnetic properties of the YPt2Al3-type compounds. TN, Néel temperature; TC, Curie temperature; μeff, effective magnetic moment; μcalc, calculated magnetic moment; θp, paramagnetic Curie temperature; μsat, saturation moment; and saturation according to gJ × J. The experimental saturation magnetizations were obtained at 3 K and 80 kOe.
| | TN (K) | TC (K) | μeff (μB) | μcalc (μB) | θp (K) | μsat (μB) | gJ × J (μB) |
|---|
| YPt2Al3 | Pauli-paramagnetic χ(300 K) = 1.85(1) × 10–4 emu mol–1 |
| DyPt2Al3 | – | 10.8(1) | 10.67(1) | 10.65 | +1.0(1) | 5.54(1) | 10 |
| HoPt2Al3 | 5.5(1) | – | 10.59(1) | 10.61 | +2.0(1) | 7.23(1) | 10 |
| ErPt2Al3 | – | – | 9.77(1) | 9.58 | +4.0(1) | 6.26(1) | 9 |
| TmPt2Al3 | – | 4.7(1) | 7.69(1) | 7.56 | +12.8(1) | 4.25(1) | 7 |
Table 10.
Fitted binding energies (in eV) determined by XPS of YPt2Al3, YPt5Al2, YPtAl, PrPtAl, and Pt and data from the literature. The determined uncertainty of binding energies in this work is ±0.1 eV.
Table 10.
Fitted binding energies (in eV) determined by XPS of YPt2Al3, YPt5Al2, YPtAl, PrPtAl, and Pt and data from the literature. The determined uncertainty of binding energies in this work is ±0.1 eV.
| Compound | Pt 4f7/2 | Al 2s | Y 3d5/2 | Lit. |
|---|
| YPt2Al3 | 70.6 | 117.2 | 156.9 | * |
| YPt5Al2 | 70.9 | 117.8 | 157.0 | * |
| YPtAl | 70.4 | 116.7 | 156.6 | * |
| PrPtAl | 70.7 | ** | – | * |
| Pt | 71.4 | – | – | * |
| Pt | 71.2 | – | – | [46] |
| Ba3Pt4Al4 | 70.9 | – | – | [46] |
| PtAl | 71.6 | – | – | [46] |
| PtAl2 | 72.1 | – | – | [46] |