Reactive Hydride Composite of Mg2NiH4 with Borohydrides Eutectic Mixtures
Abstract
:1. Introduction
2. Materials and Methods
3. Results
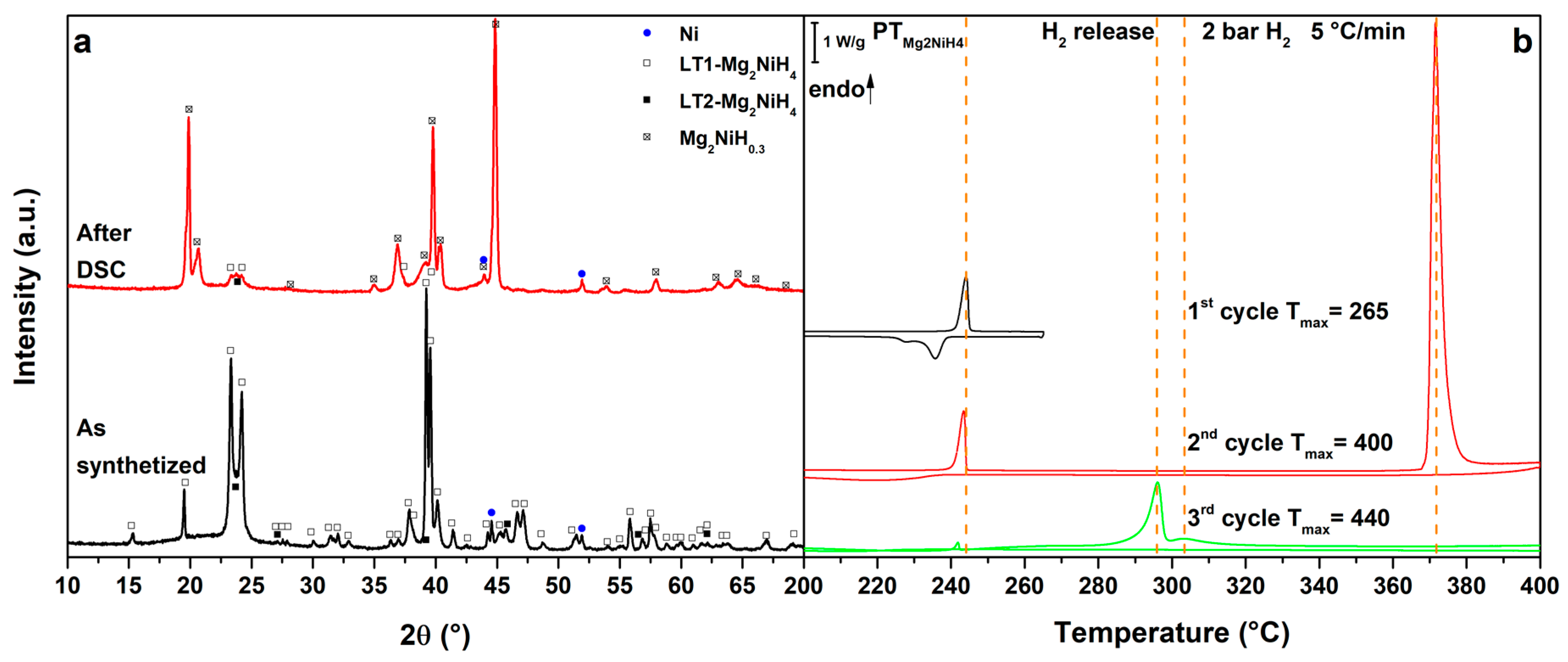
3.1. Mg2NiH4
3.2. RHC Mixtures
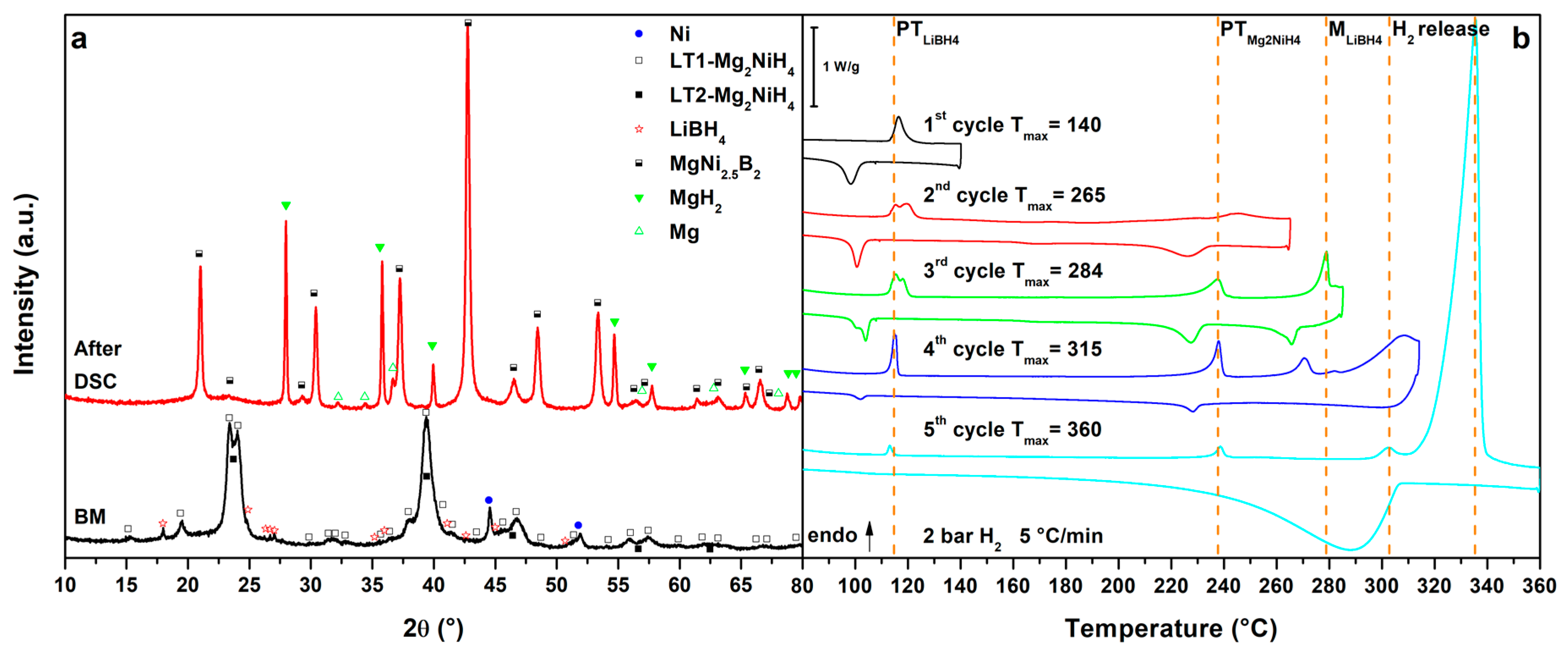
3.3. Mg2NiH4–LiBH4
3.4. Mg2NiH4–LiBH4–KBH4 Eutectic Composition
3.5. Mg2NiH4–LiBH4–NaBH4 Eutectic Composition
3.6. Mg2NiH4–LiBH4–Mg(BH4)2 Eutectic Composition
3.7. Mg2NiH4–LiBH4–Ca(BH4)2 Eutectic Composition
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Callini, E.; Atakli, Z.Ö.K.; Hauback, B.C.; Orimo, S.; Jensen, C.; Dornheim, M.; Grant, D.; Cho, Y.W.; Chen, P.; Hjörvarsson, B.; et al. Complex and liquid hydrides for energy storage. Appl. Phys. A 2016, 122, 353. [Google Scholar] [CrossRef]
- Dornheim, M. Thermodynamics of metal hydrides: Tailoring reaction enthalpies of hydrogen storage materials. In Thermodynamics—Interaction Studies—Solids, Liquids and Gases; InTech: Rijeka, Croatia, 2011; pp. 891–918. [Google Scholar]
- Martínez-Coronado, R.; Retuerto, M.; Torres, B.; Martínez-Lope, M.J.; Fernández-Díaz, M.T.; Alonso, J.A. High-pressure synthesis, crystal structure and cyclability of the Mg2NiH4 hydride. Int. J. Hydrogen Energy 2013, 38, 5738–5745. [Google Scholar] [CrossRef]
- Noréus, D. Properties of formal low-valence transition metal—hydrogen complexes in Mg2NiH4 and Na2PdH2. Z. Phys. Chem. 1989, 163, 575–578. [Google Scholar] [CrossRef]
- Čermák, J.; Král, L.; David, B. Hydrogen diffusion in Mg2NiH4 intermetallic compound. Intermetallics 2008, 16, 508–517. [Google Scholar] [CrossRef]
- Zeng, K.; Klassen, T.; Oelerich, W.; Bormann, R. Thermodynamic analysis of the hydriding process of Mg-Ni alloys. J. Alloys Compd. 1999, 283, 213–224. [Google Scholar] [CrossRef]
- Révész, Á.; Gajdics, M.; Schafler, E.; Calizzi, M.; Pasquini, L. Dehydrogenation-hydrogenation characteristics of nanocrystalline Mg2Ni powders compacted by high-pressure torsion. J. Alloys Compd. 2017, 702, 84–91. [Google Scholar] [CrossRef]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Černý, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal borohydrides and derivatives—Synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef] [PubMed]
- Paskevicius, M.; Ley, M.B.; Sheppard, D.A.; Jensen, T.R.; Buckley, C.E. Eutectic melting in metal borohydrides. Phys. Chem. Chem. Phys. 2013, 15, 19774. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.K.; Besenbacher, F.; Jensen, T.R. Nanoconfined hydrides for energy storage. Nanoscale 2011, 3, 2086. [Google Scholar] [CrossRef] [PubMed]
- Rude, L.H.; Nielsen, T.K.; Ravnsbæk, D.B.; Bösenberg, U.; Ley, M.B.; Richter, B.; Arnbjerg, L.M.; Dornheim, M.; Filinchuk, Y.; Besenbacher, F.; et al. Tailoring properties of borohydrides for hydrogen storage: A review. Phys. Status Solidi (a) 2011, 208, 1754–1773. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Pinatel, E.R.; Corno, M.; Jensen, T.R.; Baricco, M. Phase diagrams of the LiBH4–NaBH4–KBH4 system. Phys. Chem. Chem. Phys. 2017, 19, 25071–25079. [Google Scholar] [CrossRef] [PubMed]
- Roedern, E.; Hansen, B.R.S.; Ley, M.B.; Jensen, T.R. Effect of Eutectic Melting, Reactive Hydride Composites and Nanoconfinement on Decomposition and Reversibility of LiBH4–KBH4. J. Phys. Chem. C 2015, 119, 25818–25825. [Google Scholar] [CrossRef]
- Liu, Y.; Reed, D.; Paterakis, C.; Contreras Vasquez, L.; Baricco, M.; Book, D. Study of the decomposition of a 0.62LiBH4–0.38NaBH4 mixture. Int. J. Hydrogen Energy 2017, 42, 22480–22488. [Google Scholar] [CrossRef]
- Javadian, P.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Hydrogen storage properties of nanoconfined LiBH4–NaBH4. Int. J. Hydrogen Energy 2015, 40, 14916–14924. [Google Scholar] [CrossRef]
- Liu, X.; Peaslee, D.; Sheehan, T.P.; Majzoub, E.H. Decomposition behaviour of eutectic LiBH4–Mg(BH4)2 and its confinement effects in ordered nanoporous carbon. J. Phys. Chem. C 2014, 118, 27265–27271. [Google Scholar] [CrossRef]
- Fang, Z.-Z.; Kang, X.-D.; Wang, P.; Li, H.-W.; Orimo, S.-I. Unexpected dehydrogenation behaviour of LiBH4/Mg(BH4)2 mixture associated with the in situ formation of dual-cation borohydride. J. Alloys Compd. 2010, 491, L1–L4. [Google Scholar] [CrossRef]
- Bardají, E.G.; Zhao-Karger, Z.; Boucharat, N.; Nale, A.; van Setten, M.J.; Lohstroh, W.; Röhm, E.; Catti, M.; Fichtner, M. LiBH4—Mg(BH4)2: A physical mixture of metal borohydrides as hydrogen storage material. J. Phys. Chem. C 2011, 115, 6095–6101. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Witter, R.; Bardaji, E.G.; Wang, D.; Cossement, D.; Fichtner, M. Altered reaction pathways of eutectic LiBH4-Mg(BH4)2 by nanoconfinement. J. Mater. Chem. A 2013, 1, 3379. [Google Scholar] [CrossRef]
- Javadian, P.; Jensen, T.R. Enhanced hydrogen reversibility of nanoconfined LiBH4–Mg(BH4)2. Int. J. Hydrogen Energy 2014, 39, 9871–9876. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ravnsbæk, D.B.; Lee, Y.S.; Kim, Y.; Cerenius, Y.; Shim, J.; Jensen, T.R.; Hur, N.H.; Cho, Y.W. Decomposition reactions and reversibility of the LiBH4-Ca(BH4)2 composite. J. Phys. Chem. C 2009, 113, 15080–15086. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Filinchuk, Y.; Lee, H.S.; Suh, J.-Y.; Kim, J.W.; Yu, J.-S.; Cho, Y.W. On the Formation and the Structure of the First Bimetallic Borohydride Borate, LiCa 3 (BH 4)(BO 3) 2. J. Phys. Chem. C 2011, 115, 10298–10304. [Google Scholar] [CrossRef]
- Ampoumogli, A.; Charalambopoulou, G.; Javadian, P.; Richter, B.; Jensen, T.R.; Steriotis, T. Hydrogen desorption and cycling properties of composites based on mesoporous carbons and a LiBH4–Ca(BH4)2 eutectic mixture. J. Alloys Compd. 2015, 645, S480–S484. [Google Scholar] [CrossRef]
- Javadian, P.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Hydrogen storage properties of nanoconfined LiBH4-Ca(BH4)2. Int. J. Hydrogen Energy 2015, 11, 96–103. [Google Scholar] [CrossRef]
- Lee, H.S.; Hwang, S.-J.; Kim, H.K.; Lee, Y.-S.; Park, J.; Yu, J.-S.; Cho, Y.W. In Situ NMR Study on the Interaction between LiBH4–Ca(BH4)2 and Mesoporous Scaffolds. J. Phys. Chem. Lett. 2012, 3, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.-L.; Li, G.; Matsuo, M.; Orimo, S.; Deledda, S.; Sørby, M.H.; Hauback, B.C.; Pistidda, C.; Klassen, T.; Dornheim, M. Simultaneous desorption behaviour of M borohydrides and Mg2FeH6 reactive hydride composites (M = Mg, then Li, Na, K, Ca). Appl. Phys. Lett. 2015, 107, 073905. [Google Scholar] [CrossRef]
- Vajo, J.J.; Li, W.; Liu, P. Thermodynamic and kinetic destabilization in LiBH4/Mg2NiH4: Promise for borohydride-based hydrogen storage. Chem. Commun. 2010, 46, 6687–6689. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Vajo, J.J.; Cumberland, R.W.; Liu, P.; Hwang, S.-J.; Kim, C.; Bowman, R.C. Hydrogenation of magnesium nickel boride for reversible hydrogen storage. J. Phys. Chem. Lett. 2010, 1, 69–72. [Google Scholar] [CrossRef]
- Afonso, G.; Bonakdarpour, A.; Wilkinson, D.P. Hydrogen storage properties of the destabilized 4NaBH4/5Mg2NiH4 composite system. J. Phys. Chem. C 2013, 117, 21105–21111. [Google Scholar] [CrossRef]
- Bergemann, N.; Pistidda, C.; Milanese, C.; Emmler, T.; Karimi, F.; Chaudhary, A.-L.; Chierotti, M.R.; Klassen, T.; Dornheim, M. Ca(BH4)2–Mg2NiH4: On the pathway to a Ca(BH4)2 system with a reversible hydrogen cycle. Chem. Commun. 2016, 52, 4836–4839. [Google Scholar] [CrossRef] [PubMed]
- Javadian, P.; Zlotea, C.; Ghimbeu, C.M.; Latroche, M.; Jensen, T.R. Hydrogen storage properties of nanoconfined LiBH4–Mg2NiH4 Reactive Hydride Composites. J. Phys. Chem. C 2015, 119, 5819–5826. [Google Scholar] [CrossRef]
- Ley, M.B.; Roedern, E.; Jensen, T.R. Eutectic melting of LiBH4–KBH4. Phys. Chem. Chem. Phys. 2014, 16, 24194–24199. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, H.; Noréus, D. Mechanically reversible conductor–insulator transition in Mg2NiH4. J. Appl. Phys. 2002, 91, 5141–5148. [Google Scholar] [CrossRef]
- Polanski, M.; Nielsen, T.K.; Kunce, I.; Norek, M.; Płociński, T.; Jaroszewicz, L.R.; Gundlach, C.; Jensen, T.R.; Bystrzycki, J. Mg2NiH4 synthesis and decomposition reactions. Int. J. Hydrogen Energy 2013, 38, 4003–4010. [Google Scholar] [CrossRef]
- El Kharbachi, A.; Pinatel, E.R.; Nuta, I.; Baricco, M. A thermodynamic assessment of LiBH4. Calphad 2012, 39, 80–90. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Roedern, E.; Pinatel, E.R.; Corno, M.; Jensen, T.R.; Baricco, M. A thermodynamic investigation of the LiBH4–NaBH4 system. RSC Adv. 2016, 6, 60101–60108. [Google Scholar] [CrossRef]
- Javadian, P.; GharibDoust, S.P.; Li, H.-W.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Reversibility of LiBH4 facilitated by the LiBH4–Ca(BH4)2 eutectic. J. Phys. Chem. C 2017, 121, 18439–18449. [Google Scholar] [CrossRef]




| Sample | Borohydrides Composition (Molar Fraction) | RHC Composition (Molar Fraction) |
|---|---|---|
| MgNiLi | LiBH4 | 0.56 Mg2NiH4, 0.44 LiBH4 |
| MgNiLiK | 0.72 LiBH4, 0.28 KBH4 | 0.56 Mg2NiH4, 0.32 LiBH4, 0.12 KBH4 |
| MgNiLiNa | 0.70 LiBH4, 0.30 NaBH4 | 0.56 Mg2NiH4, 0.31 LiBH4, 0.13 NaBH4 |
| MgNiLiMg | 0.55 LiBH4, 0.45 Mg(BH4)2 | 0.64 Mg2NiH4, 0.20 LiBH4, 0.16 Mg(BH4)2 |
| MgNiLiCa | 0.68 LiBH4, 0.32 Ca(BH4)2 | 0.62 Mg2NiH4, 0.27 LiBH4, 0.11 Ca(BH4)2 |
| System | PT | PT | M/Cr | PT | H2 Release | H2 Uptake | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle | Ramp | LiBH4 | Ca(BH4)2 | MBH4 | Mg2NiH4 | MBH4 | Mg2NiH0.3 | MgH2 | RHC | ||
| MgNi | 1 | H | 244 | ||||||||
| 1 | C | 234 | |||||||||
| 2 | H | 243 | 372 | ||||||||
| 2 | C | <235 | |||||||||
| 3 | H | 242 | 296 | 303 | |||||||
| 3 | C | <235 | |||||||||
| MgNiLi | 1 | H | 117 | ||||||||
| 1 | C | 98 | |||||||||
| 2 | H | 115 | 245 | ||||||||
| 2 | C | 104 | 226 | ||||||||
| 3 | H | 115 | 279 | 238 | |||||||
| 3 | C | 102 | 265 | 227 | |||||||
| 4 | H | 113 | 271 | 238 | 308 | ||||||
| 4 | C | 228 | |||||||||
| 5 | H | 238 | 302 | 335 | |||||||
| 5 | C | <307 | |||||||||
| MgNiLiK | 1 | H | 110 | ||||||||
| 1 | C | 106 | |||||||||
| 2 | H | 110 | 246 | 325 | |||||||
| 2 | C | <300 | |||||||||
| 3 | H | 292 | 329 | ||||||||
| 3 | C | <300 | |||||||||
| MgNiLiNa | 1 | H | 98 | 228 | |||||||
| 1 | C | 90 | 226 | ||||||||
| 2 | H | 102 | 228 | 246 | 327 | ||||||
| 2 | C | <302 | |||||||||
| 3 | H | 101 | 290 | 331 | |||||||
| 3 | C | <302 | |||||||||
| MgNiLiMg | 1 | H | 111 | 176 | |||||||
| 1 | C | 103 | |||||||||
| 2 | H | 114 | 174 | 246 | 243 | 336 | |||||
| 2 | C | <286 | |||||||||
| 3 | H | >231 | |||||||||
| 3 | C | <297 | |||||||||
| 4 | H | 293 | 317 | ||||||||
| 4 | C | <297 | |||||||||
| MgNiLiCa | 1 | H | 115 | 150 | 202 | 243 | 246 | ||||
| 1 | C | 102 | 193 | ||||||||
| 2 | H | 115 | 151 | 200 | 241 | >306 | 332 | ||||
| 2 | C | <304 | |||||||||
| 3 | H | >290 | |||||||||
| 3 | C | <304 | |||||||||
| 4 | H | 335 | |||||||||
| 4 | C | <304 | |||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dematteis, E.M.; Vaunois, S.; Pistidda, C.; Dornheim, M.; Baricco, M. Reactive Hydride Composite of Mg2NiH4 with Borohydrides Eutectic Mixtures. Crystals 2018, 8, 90. https://doi.org/10.3390/cryst8020090
Dematteis EM, Vaunois S, Pistidda C, Dornheim M, Baricco M. Reactive Hydride Composite of Mg2NiH4 with Borohydrides Eutectic Mixtures. Crystals. 2018; 8(2):90. https://doi.org/10.3390/cryst8020090
Chicago/Turabian StyleDematteis, Erika M., Silvère Vaunois, Claudio Pistidda, Martin Dornheim, and Marcello Baricco. 2018. "Reactive Hydride Composite of Mg2NiH4 with Borohydrides Eutectic Mixtures" Crystals 8, no. 2: 90. https://doi.org/10.3390/cryst8020090







