The Effects of a Mixed Precipitant on the Morphology and Electrochemical Performance of LiNi0.5Mn1.5O4 Cathode Materials
Abstract
:1. Introduction
2. Experimental
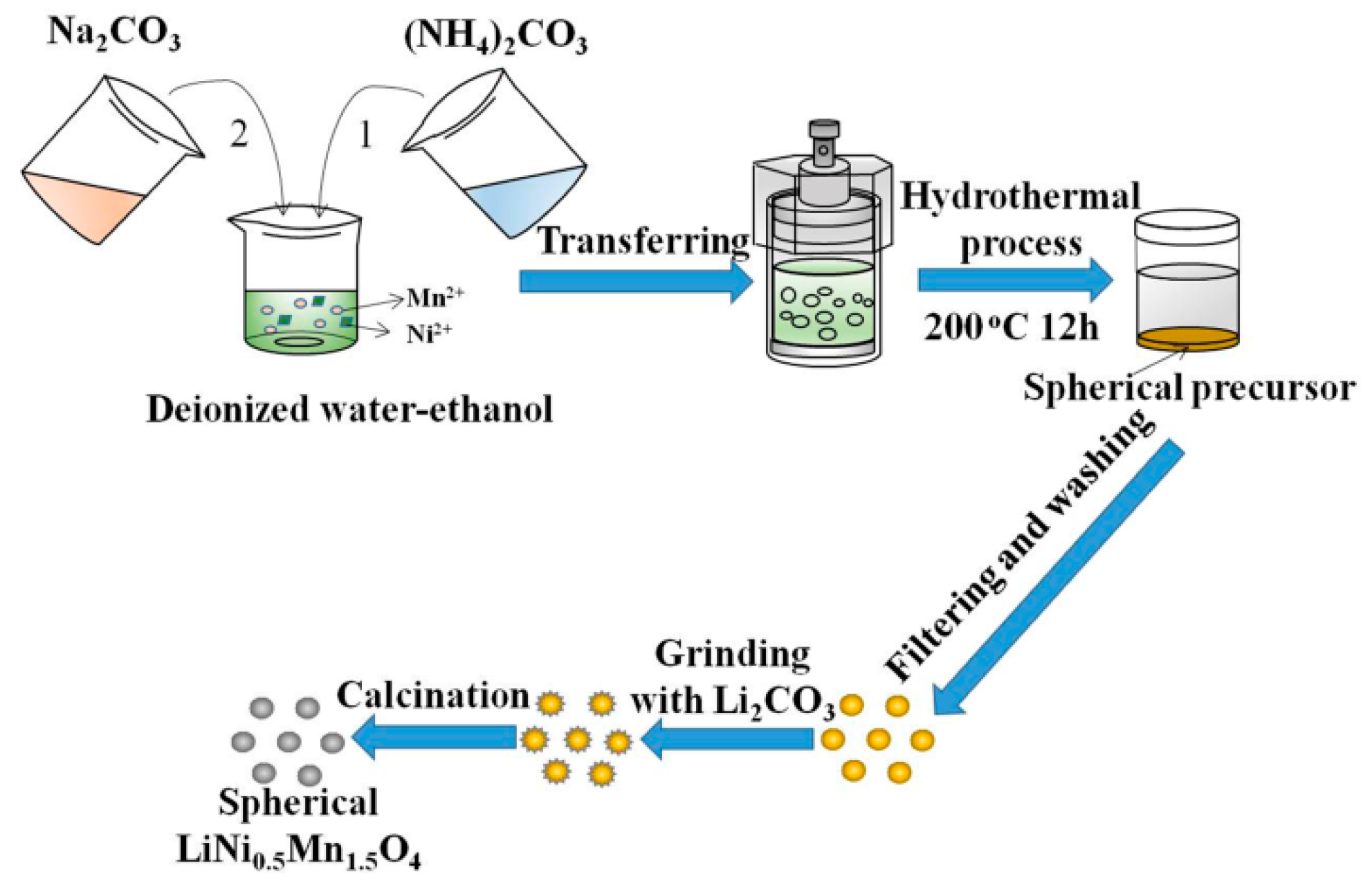
2.1. The Synthesis of Ni0.25Mn0.75CO3 Precursors
2.2. The Synthesis of LNMO Cathode Materials
2.3. Materials Characterizations
2.4. Electrochemical Measurements
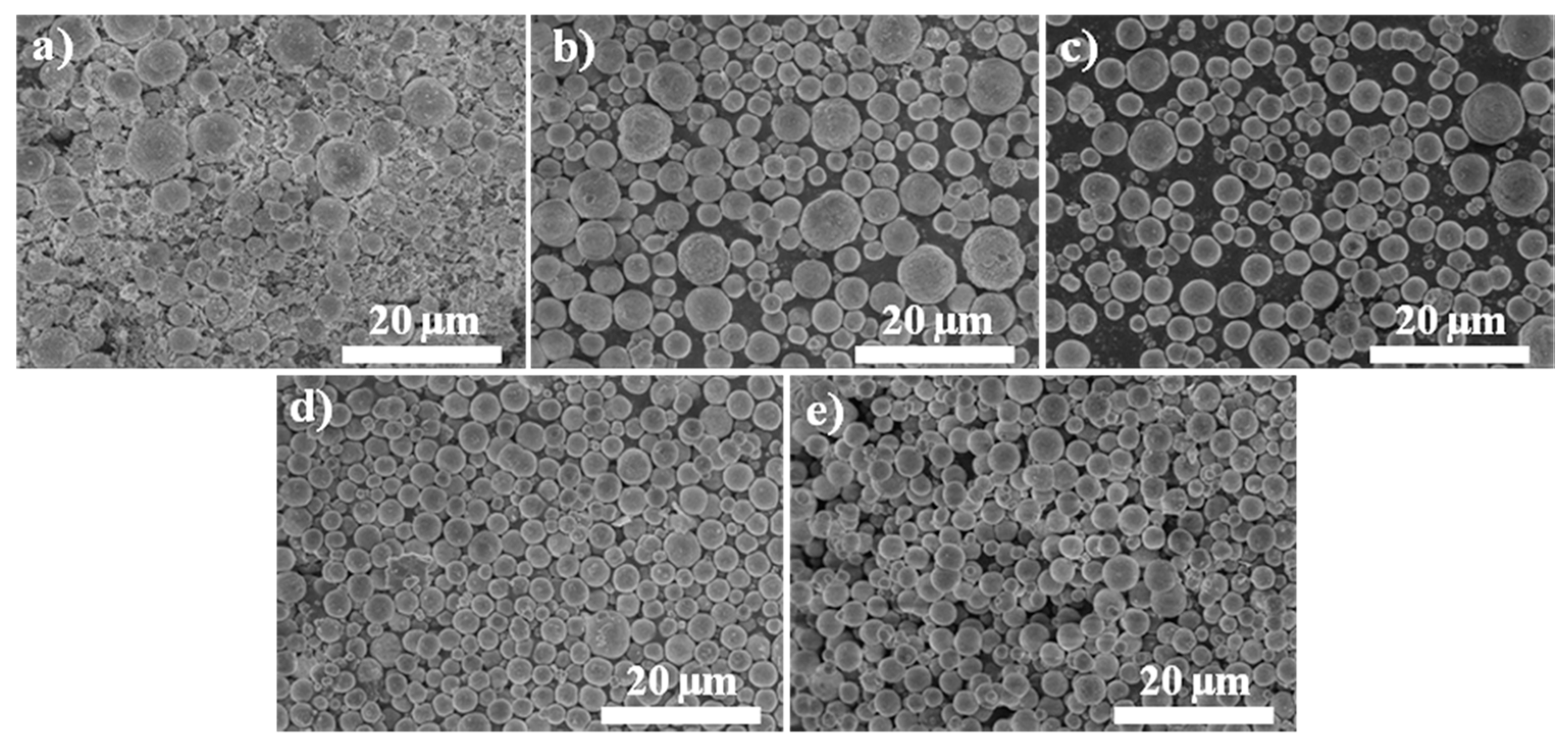
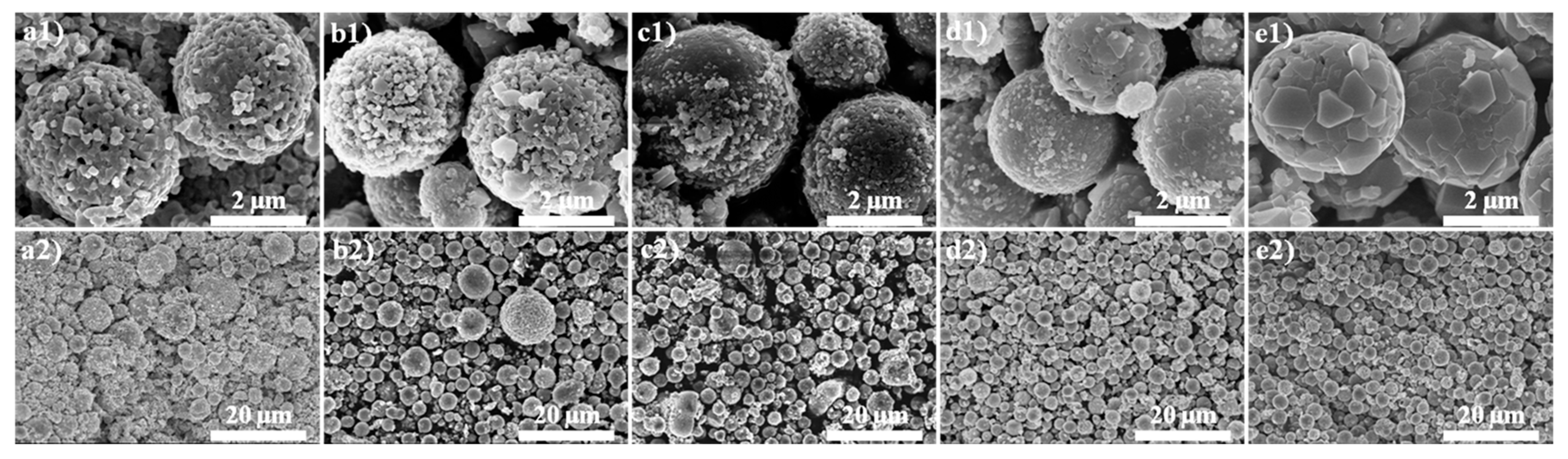
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, Y.; Lu, T.L.; Zhang, Y.X.; Yan, L.Q.; Mao, S.S.; Xie, J.Y. Surface-segregated, high-voltage spinel lithium-ion battery cathode material LiNi0.5Mn1.5O4 cathodes by aluminium doping with improved high-rate cyclability. J. Alloys Compd. 2017, 703, 289–297. [Google Scholar] [CrossRef]
- Sun, P.; Ma, Y.; Zhai, T.Y.; Li, H.Q. High performance LiNi0.5Mn1.5O4 cathode by Al-coating and Al3+-doping through a physical vapor deposition method. Electrochim. Acta 2016, 191, 237–246. [Google Scholar] [CrossRef]
- Alva, G.; Kim, C.J.; Yi, T.H.; Cook, J.B.; Xu, L.P.; Nolis, G.M.; Cabana, J. Surface Chemistry Consequences of Mg-Based Coatings on LiNi0.5Mn1.5O4 Electrode Materials upon Operation at High Voltage. J. Phys. Chem. C 2014, 118, 10596–10605. [Google Scholar] [CrossRef]
- Tao, S.; Kong, F.J.; Wu, C.Q.; Su, X.Z.; Xiang, T.; Chen, S.M.; Hou, H.H.; Zhang, L.; Fang, Y.; Wang, Z.C.; et al. Nanoscale TiO2 membrane coating spinel LiNi0.5Mn1.5O4 cathode material for advanced lithium-ion batteries. J. Alloys Compd. 2017, 705, 413–419. [Google Scholar] [CrossRef]
- Ma, J.; Hu, P.; Cui, G.L.; Chen, L.Q. Surface and Interface Issues in Spinel LiNi0.5Mn1.5O4: Insights into a Potential Cathode Material for High Energy Density Lithium Ion Batteries. Chem. Mater. 2016, 28, 3578–3606. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Yang, Y.F.; Zhao, X.C.; Shao, H.X. Synthesis and electrochemical characterization of LiNi0.5Mn1.5O4 by one-step precipitation method with ammonium carbonate as precipitating agent. Electrochim. Acta 2011, 56, 5934–5939. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, D.; Yu, H.Y. Preparation of spherical hierarchical LiNi0.5Mn1.5O4 with high electrochemical performances by a novel composite co-precipitation method for 5V lithium ion secondary batteries. Electrochim. Acta 2014, 115, 290–296. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zhang, M.H.; Xia, Y.G.; Qiu, B.; Liu, Z.P.; Li, X. One-step hydrothermal method synthesis of core–shell LiNi0.5Mn1.5O4 spinel cathodes for Li-ion batteries. J. Power Sources 2014, 256, 66–71. [Google Scholar] [CrossRef]
- Fang, H.S.; Wang, Z.X.; Li, X.H.; Guo, H.J.; Peng, W.J. Low temperature synthesis of LiNi0.5Mn1.5O4 spinel. Mater. Lett. 2006, 60, 1273–1275. [Google Scholar] [CrossRef]
- Agostini, M.; Matic, A.; Panero, S.; Croce, F.; Gunnella, R.; Reale, P.; Brutti, S. A mixed mechanochemical-ceramic solid-state synthesis as simple and cost effective route to high-performance LiNi0.5Mn1.5O4 spinels. Electrochim. Acta 2017, 235, 262–269. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Yang, Y.F.; Zhan, H.; Shao, H.X.; Zhou, Y.H. Synthesis of high power type LiMn1.5Ni0.5O4 by optimizing its preparation conditions. J. Power Sources 2010, 195, 4322–4326. [Google Scholar] [CrossRef]
- Pang, Q.; Fu, Q.; Wang, Y.H.; Zhang, Y.Q.; Zou, B.; Du, F.; Chen, G.; Wei, Y.J. Improved Electrochemical Properties of Spinel LiNi0.5Mn1.5O4 Cathode Materials by Surface Modification with RuO2 Nanoparticles. Electrochim. Acta 2015, 152, 240–248. [Google Scholar] [CrossRef]
- Kiziltas-Yavuz, N.; Bhaskar, A.; Dixon, D.; Yavuz, M.; Nikolowski, K.; Lu, L.; Eichel, R.-A.; Ehrenberg, H. Improving the rate capability of high voltage lithium-ion battery cathode material LiNi0.5Mn1.5O4 by ruthenium doping. J. Power Sources 2014, 267, 533–541. [Google Scholar] [CrossRef]
- Zhong, G.B.; Wang, Y.Y.; Zhang, Z.C.; Chen, C.H. Effects of Al substitution for Ni and Mn on the electrochemical properties of LiNi0.5Mn1.5O4. Electrochim. Acta 2011, 56, 6554–6561. [Google Scholar] [CrossRef]
- Zhong, G.B.; Wang, Y.Y.; Zhao, X.J.; Wang, Q.S.; Yu, Y.; Chen, C.H. Structural, electrochemical and thermal stability investigations on LiNi0.5−xAl2xMn1.5−xO4 (0 ≤ 2x ≤ 1.0) as 5 V cathode materials. J. Power Sources 2012, 216, 368–375. [Google Scholar] [CrossRef]
- Choi, H.W.; Kim, S.J.; Rim, Y.H.; Yang, Y.S. Effect of Lithium Deficiency on Lithium-Ion Battery Cathode LixNi0.5Mn1.5O4. J. Phys. Chem. C 2015, 119, 27192–27199. [Google Scholar] [CrossRef]
- Gao, J.; Li, J.J.; Song, F.; Lin, J.X.; He, X.M.; Jiang, C.Y. Strategy for synthesizing spherical LiNi0.5Mn1.5O4 cathode material for lithium ion batteries. Mater. Chem. Phys. 2015, 152, 177–182. [Google Scholar] [CrossRef]
- Cheng, J.L.; Li, X.H.; Wang, Z.X.; Guo, H.J. Hydrothermal synthesis of LiNi0.5Mn1.5O4 sphere and its performance as high-voltage cathode material for lithium ion batteries. Ceram. Int. 2016, 42, 3715–3719. [Google Scholar] [CrossRef]
- Hoshina, K.; Yoshima, K.; Kotobuki, M.; Kanamura, K. Fabrication of LiNi0.5Mn1.5O4 thin film cathode by PVP sol–gel process and its application of all-solid-state lithium ion batteries using Li1+xAlxTi2−x(PO4)3 solid electrolyte. Solid State Ion. 2012, 209–210, 30–35. [Google Scholar] [CrossRef]
- Fang, J.C.; Xu, Y.F.; Xu, G.L.; Shen, S.Y.; Li, J.T.; Huang, L.; Sun, S.G. Fabrication of densely packed LiNi0.5Mn1.5O4 cathode material with excellent long-term cycleability for high-voltage lithium ion batteries. J. Power Sources 2016, 304, 15–23. [Google Scholar] [CrossRef]
- Yao, Y.L.; Liu, H.C.; Li, G.C.; Peng, H.R.; Chen, K.Z. Multi-shelled porous LiNi0.5Mn1.5O4 microspheres as a 5 V cathode material for lithium-ion batteries. Mater. Chem. Phys. 2014, 143, 867–872. [Google Scholar] [CrossRef]
- Zhu, X.B.; Li, X.N.; Zhu, Y.C.; Jin, S.S.; Wang, Y.; Qian, Y.T. Porous LiNi0.5Mn1.5O4 microspheres with different pore conditions: Preparation and application as cathode materials for lithium-ion batteries. J. Power Sources 2014, 261, 93–100. [Google Scholar] [CrossRef]
- Zhao, E.Q.; Liu, W.; Guo, Y.D.; Xu, Y.J.; Yan, W.C.; Sun, D.Y.; Jin, Y.C. Rapid hydrothermal and post-calcination synthesis of well-shaped LiNi0.5Mn1.5O4 cathode materials for lithium ion batteries. J. Alloys Compd. 2017, 695, 3393–3401. [Google Scholar] [CrossRef]
- Qian, Y.X.; Deng, Y.F.; Shi, Z.C.; Zhou, Y.B.; Zhuang, Q.C.; Chen, G.H. Sub-micrometer-sized LiMn1.5Ni0.5O4 spheres as high rate cathode materials for long-life lithium ion batteries. Electrochem. Commun. 2013, 27, 92–95. [Google Scholar] [CrossRef]
- Nageswaran, S.; Keppeler, M.; Kim, S.J.; Srinivasan, M. Morphology controlled Si-modified LiNi0.5Mn1.5O4 microspheres as high performance high voltage cathode materials in lithium ion batteries. J. Power Sources 2017, 346, 89–96. [Google Scholar] [CrossRef]
- Luo, W.B. Effect of morphology on the physical and electrochemical properties of the high-voltage spinel cathode LiMn1.5Ni0.5O4. J. Alloys Compd. 2015, 636, 24–28. [Google Scholar] [CrossRef]
- Zhang, M.H.; Wang, J.; Xia, Y.G.; Liu, Z.P. Microwave synthesis of spherical spinel LiNi0.5Mn1.5O4 as cathode material for lithium-ion batteries. J. Alloys Compd. 2012, 518, 68–73. [Google Scholar] [CrossRef]
- Yan, W.C.; Jiang, J.C.; Liu, W.; Sun, D.Y.; Zhao, E.Q.; Jin, Y.C.; Kanamura, K. Effect of precipitators on the morphologies and electrochemical properties of Li1.2Mn0.54Ni0.13Co0.13O2 via rapid nucleation and post-solvothermal method. Electrochim. Acta 2017, 224, 161–170. [Google Scholar] [CrossRef]
- Keppeler, M.; Nageswaran, S.; Kim, S.J.; Srinivasan, M. Silicon Doping of High Voltage Spinel LiNi0.5Mn1.5O4 towards Superior Electrochemical Performance of Lithium Ion Batteries. Electrochim. Acta 2016, 213, 904–910. [Google Scholar] [CrossRef]
- Liu, G.Q.; Du, Y.L.; Liu, W.B.; Wen, L. Study on the action mechanism of doping transitional elements in spinel LiNi0.5Mn1.5O4. Electrochim. Acta 2016, 209, 308–314. [Google Scholar] [CrossRef]
- Deng, J.C.; Xu, Y.L.; Xiong, L.L.; Li, L.; Sun, X.F.; Zhang, Y. Improving the fast discharge performance of high-voltage LiNi0.5Mn1.5O4 spinel by Cu2+, Al3+, Ti4+ tri-doping. J. Alloys Compd. 2016, 677, 18–26. [Google Scholar] [CrossRef]
- Chemelewski, K.R.; Lee, E.S.; Li, W.; Manthiram, A. Factors Influencing the Electrochemical Properties of High-Voltage Spinel Cathodes: Relative Impact of Morphology and Cation Ordering. Chem. Mater. 2013, 25, 2890–2897. [Google Scholar] [CrossRef]
- Yan, W.C.; Jiang, J.C.; Liu, W.; Yan, X.; Sun, D.Y.; Jin, Y.C.; Wang, J.; Xiang, L.; Munakata, H.; Kanamura, K. Synthesis and Evaluation of Microspherical Li1.2Mn0.54Co0.13Ni0.13O2 through Carbon Dioxides-assisted Co-precipitation Method for Lithium-ion Battery. Electrochim. Acta 2016, 212, 16–24. [Google Scholar] [CrossRef]
- Liu, H.D.; Wang, J.; Zhang, X.F.; Zhou, D.; Qi, X.; Qiu, B.; Fang, J.H.; Kloepsch, R.; Schumacher, G.; Liu, Z.P.; et al. Morphological Evolution of High-Voltage Spinel LiNi0.5Mn1.5O4 Cathode Materials for Lithium-Ion Batteries: The Critical Effects of Surface Orientations and Particle Size. ACS Appl. Mater. Interfaces 2016, 8, 4661–4675. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Zhao, S.X.; Deng, Y.F.; Deng, H.; Nan, C.W. Improved electrochemical performance of 5 V spinel LiNi0.5Mn1.5O4 microspheres by F-doping and Li4SiO4 coating. J. Materiomics 2016, 2, 265–272. [Google Scholar] [CrossRef]
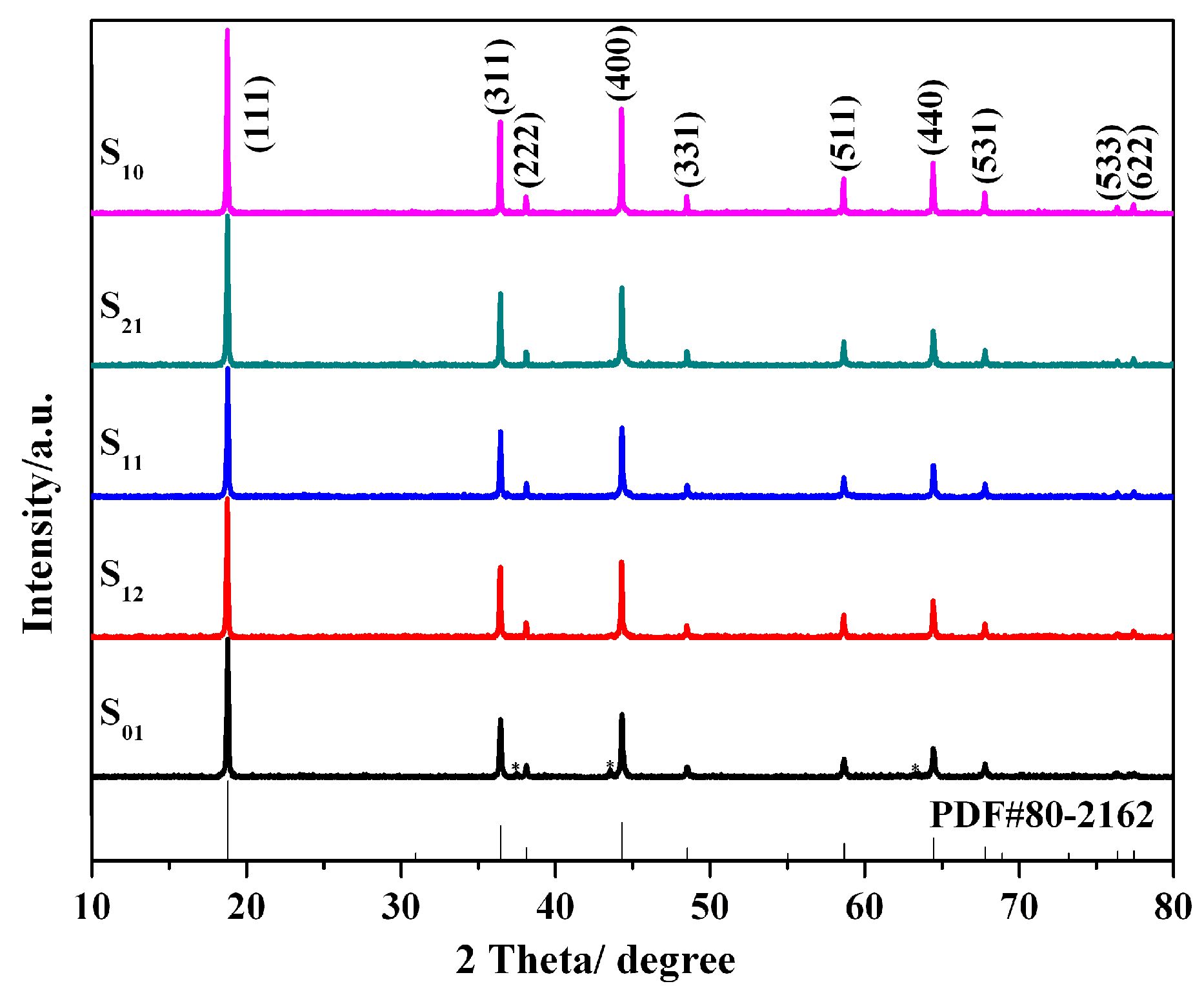
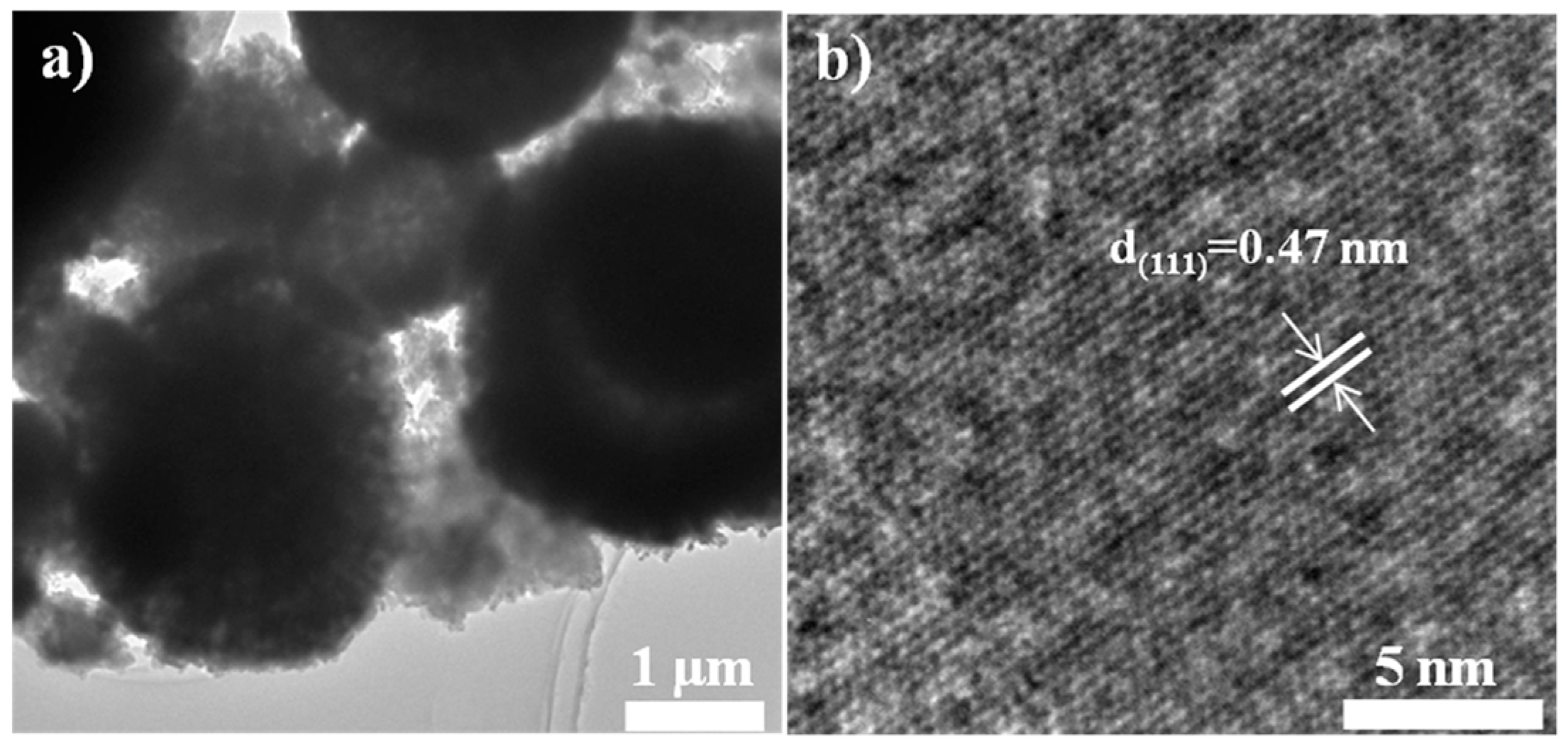
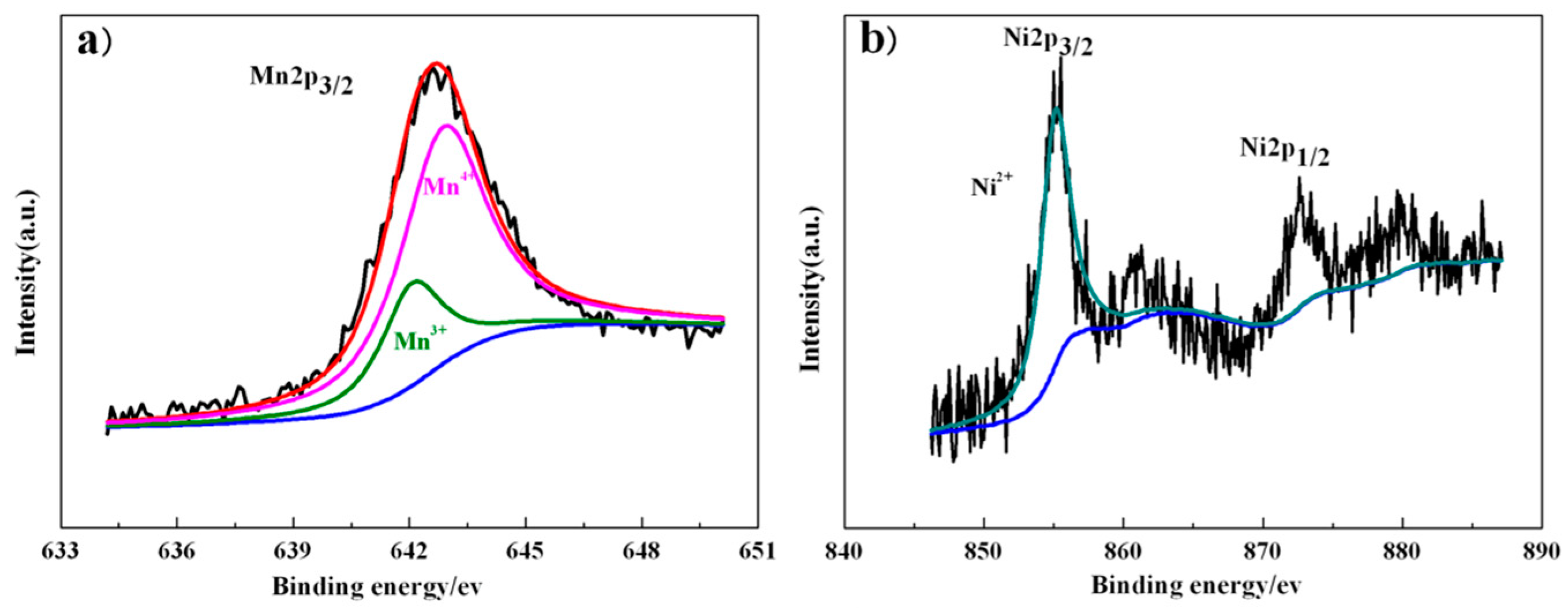
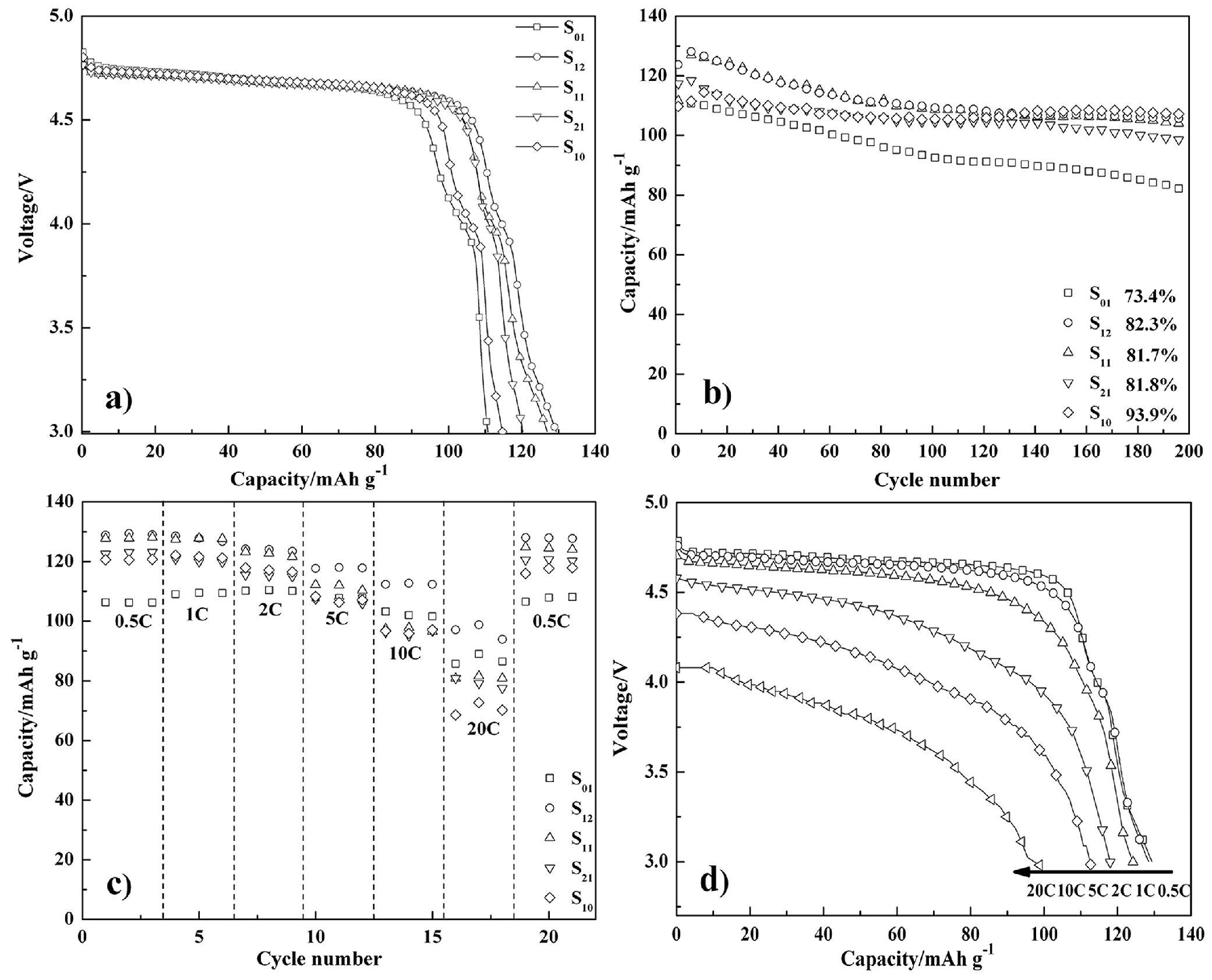
| Samples | Lattice Parameter (Å) | Cell Volume (Å3) | Crystallite Size (nm) |
|---|---|---|---|
| S01 | 8.1682 | 544.9781 | 263.6 |
| S12 | 8.1723 | 545.7992 | 256.8 |
| S11 | 8.1692 | 545.1783 | 193.4 |
| S21 | 8.1705 | 545.4386 | 287.8 |
| S10 | 8.1732 | 545.9796 | 320.5 |
| Samples | Ni/Mn (Atomic Ratio) | Ni/Mn (fix Mn = 3) |
|---|---|---|
| S01 | 0.5098:1.4902 | 1.03:3 |
| S12 | 0.5046:1.4954 | 1.01:3 |
| S11 | 0.4921:1.5079 | 0.98:3 |
| S21 | 0.4812:1.5189 | 0.95:3 |
| S10 | 0.4616:1.5385 | 0.90:3 |
| Samples | S01 | S12 | S11 | S21 | S10 |
|---|---|---|---|---|---|
| BET/m2 g−1 | 4.3612 | 3.5522 | 3.1217 | 3.0837 | 2.8820 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, Y.; Yan, W.; Wang, H.; Jiang, J.; Sun, D.; Ma, X.; Jin, Y. The Effects of a Mixed Precipitant on the Morphology and Electrochemical Performance of LiNi0.5Mn1.5O4 Cathode Materials. Crystals 2017, 7, 275. https://doi.org/10.3390/cryst7090275
Shu Y, Yan W, Wang H, Jiang J, Sun D, Ma X, Jin Y. The Effects of a Mixed Precipitant on the Morphology and Electrochemical Performance of LiNi0.5Mn1.5O4 Cathode Materials. Crystals. 2017; 7(9):275. https://doi.org/10.3390/cryst7090275
Chicago/Turabian StyleShu, Yang, Wenchao Yan, Haisong Wang, Jicheng Jiang, Deye Sun, Xiaodi Ma, and Yongcheng Jin. 2017. "The Effects of a Mixed Precipitant on the Morphology and Electrochemical Performance of LiNi0.5Mn1.5O4 Cathode Materials" Crystals 7, no. 9: 275. https://doi.org/10.3390/cryst7090275






