Structural Basis of DEAH/RHA Helicase Activity
Abstract
:1. Introduction
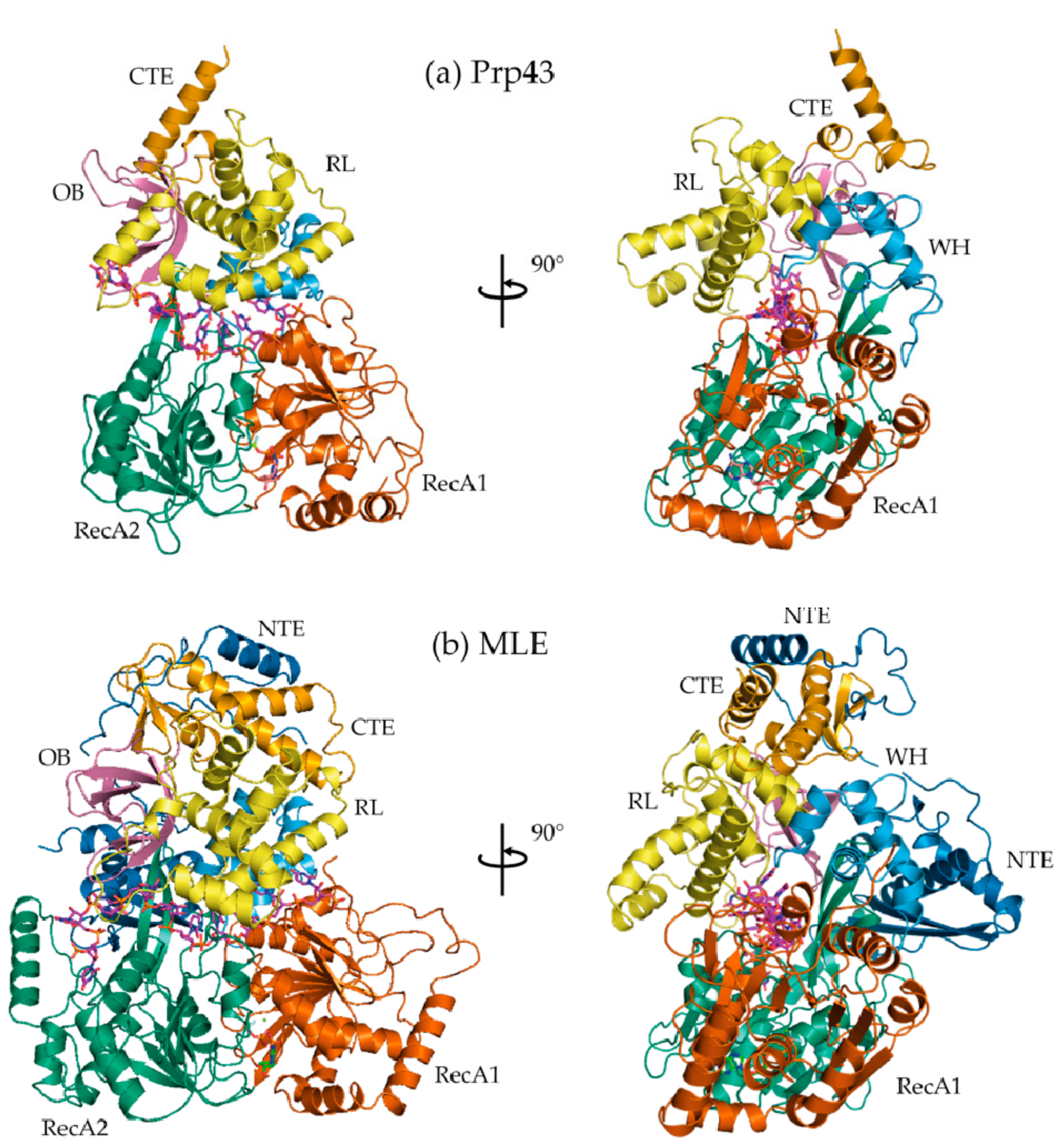
2. Crystallographic Snapshots of DEAH/RHA Helicases
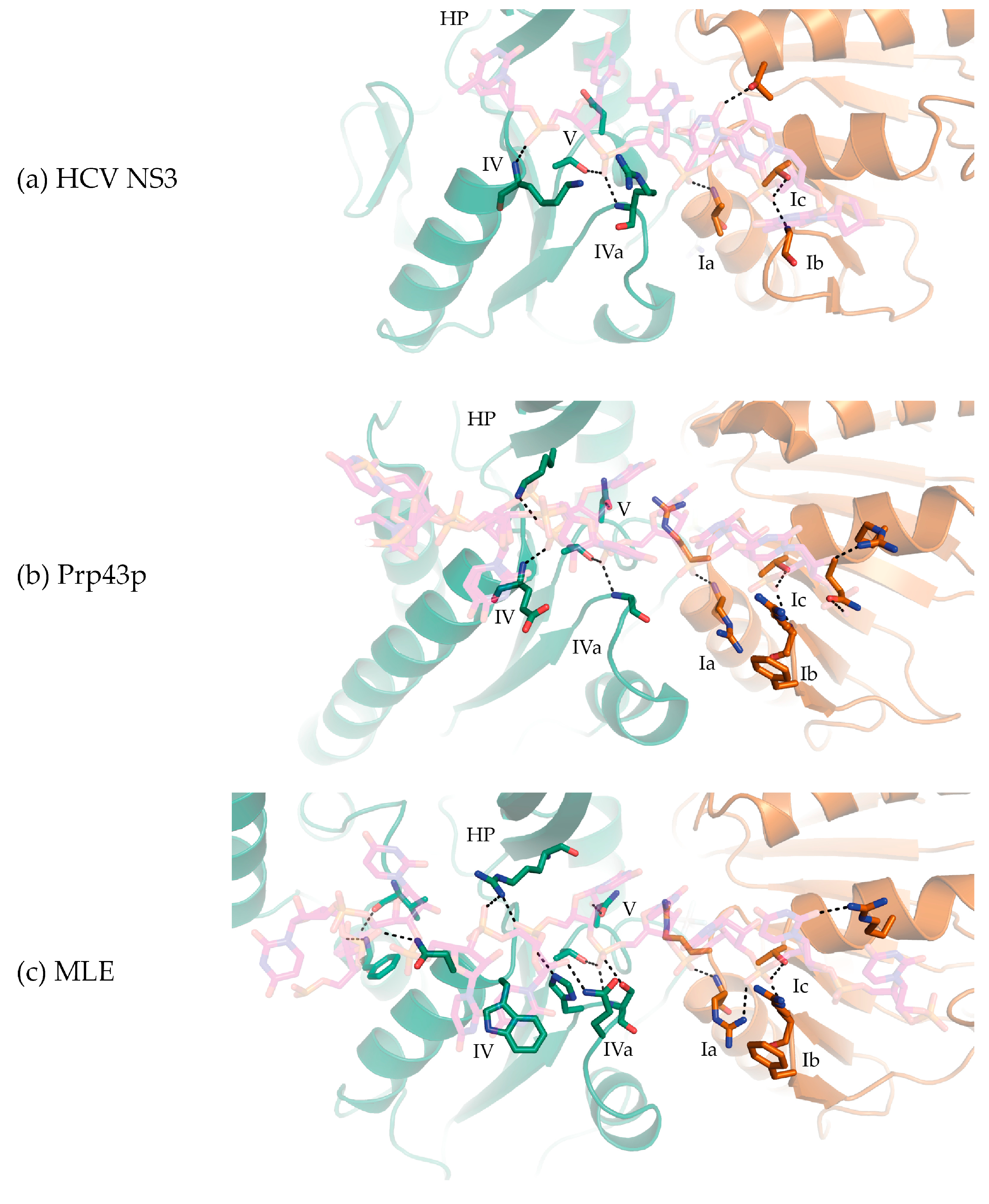
3. DEAH/RHA RecA1 Interactions
4. DEAH/RHA RecA2 Interactions
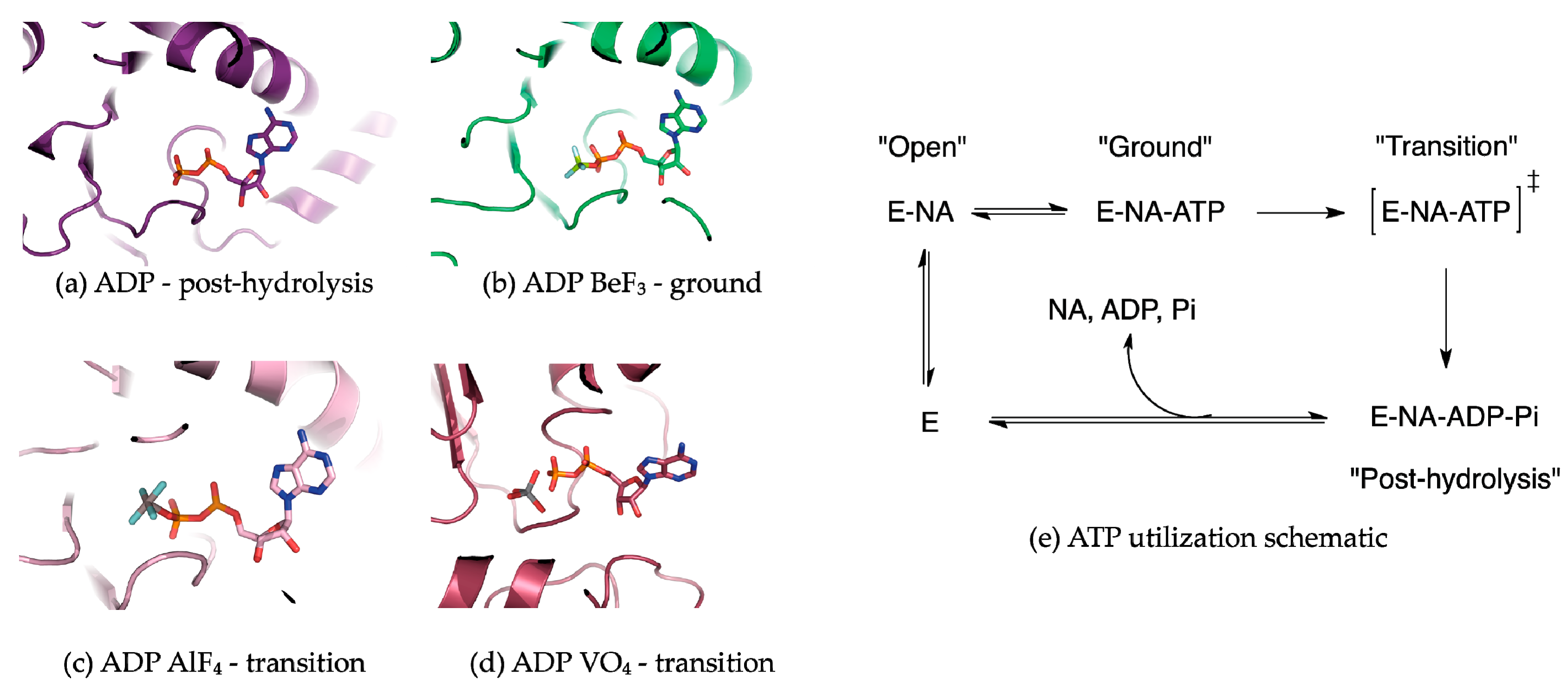
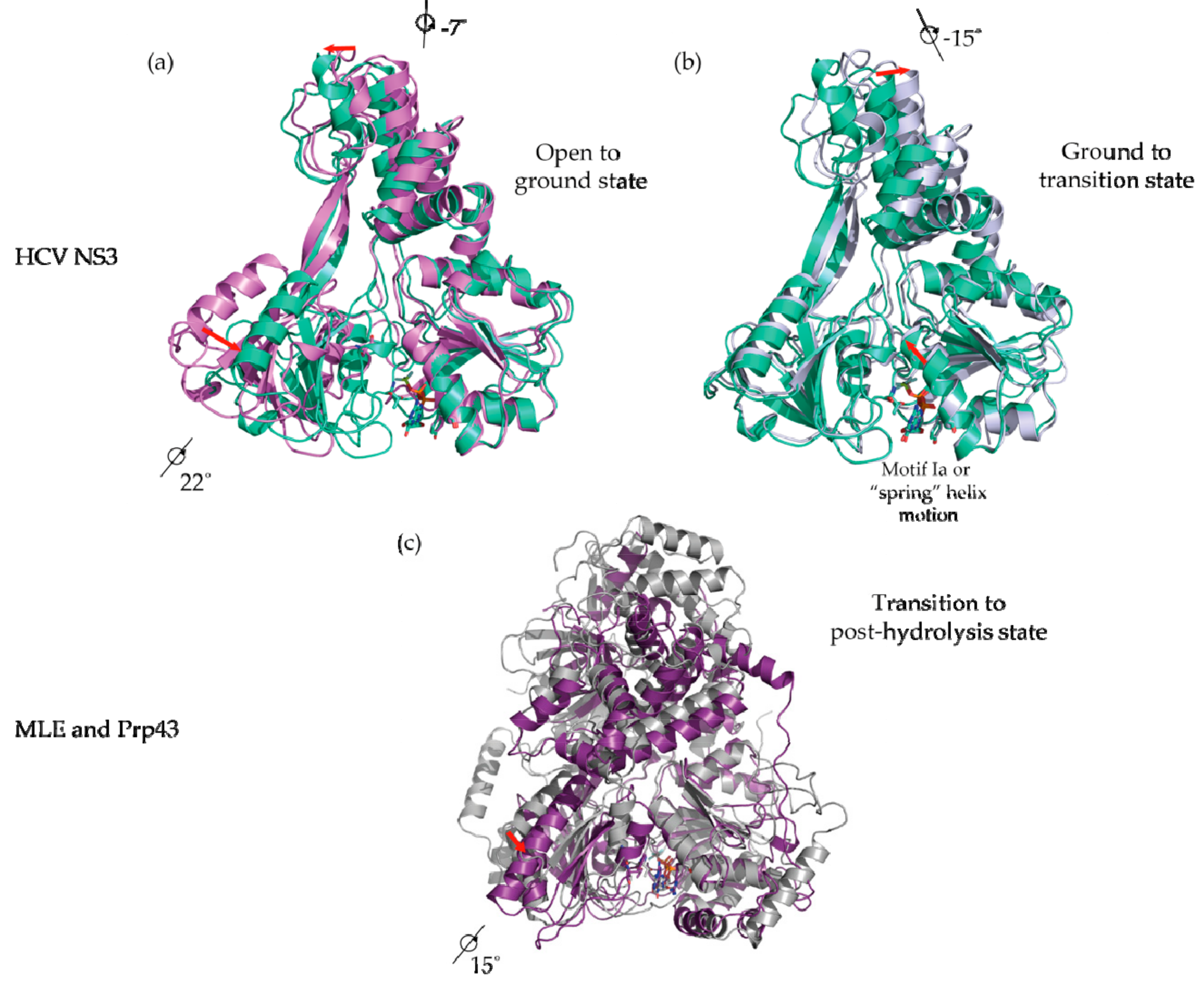
5. Conformational Snapshots of HCV NS3 Through the Use of ATP Analogs
6. DEAH/RHA C-Terminal Domain Interactions
7. Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdelhaleem, M.; Maltais, L.; Wain, H. The human DDX and DHX gene families of putative RNA helicases. Genomics 2003, 81, 618–622. [Google Scholar] [CrossRef]
- Jankowsky, E.; Jankowsky, A. The DExH/D protein family database. Nucleic Acids Res. 2000, 28, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Fairman-Williams, M.E.; Guenther, U.-P.; Jankowsky, E. SF1 and SF2 helicases: Family matters. Curr. Opin. Struct. Biol. 2010, 20, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Fullam, A.; Schröder, M. DExD/H-box RNA helicases as mediators of anti-viral innate immunity and essential host factors for viral replication. Biochim. Biophys. Acta 2013, 1829, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Singleton, M.R.; Dillingham, M.S.; Wigley, D.B. Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 2007, 76, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Straub, A.U.; Doebele, C.; Bohnsack, M.T. DExD/H-box RNA helicases in ribosome biogenesis. RNA Biol. 2013, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Pace, F.V. DExD/H box RNA helicases: Multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Res. 2006, 34, 4206–4215. [Google Scholar] [CrossRef] [PubMed]
- Rocak, S.; Linder, P. DEAD-box proteins: The driving forces behind RNA metabolism. Nat. Rev. Mol. Cell Biol. 2004, 5, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.K.; Linder, P. DExD/H box RNA helicases: From generic motors to specific dissociation functions. Mol. Cell 2001, 8, 251–262. [Google Scholar] [CrossRef]
- Silverman, E.; Edwalds-Gilbert, G.; Lin, R.-J. DExD/H-box proteins and their partners: Helping RNA helicases unwind. Gene 2003, 312, 1–16. [Google Scholar] [CrossRef]
- Cordin, O.; Banroques, J.; Tanner, N.K.; Linder, P. The DEAD-box protein family of RNA helicases. Gene 2006, 367, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Linder, P.P. Dead-box proteins: A family affair—Active and passive players in RNP-remodeling. Nucleic Acids Res. 2006, 34, 4168–4180. [Google Scholar] [CrossRef] [PubMed]
- Linder, P.; Jankowsky, E. From unwinding to clamping—The DEAD box RNA helicase family. Nat. Rev. Mol. Cell Biol. 2011, 12, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, E. RNA helicases at work: Binding and rearranging. Trends Biochem. Sci. 2011, 36, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Pace, F.V. DEAD box RNA helicase functions in cancer. RNA Biol. 2013, 10, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Abdelhaleem, M. Do human RNA helicases have a role in cancer? Biochim. Biophys. Acta 2004, 1704, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Abdelhaleem, M. Over-expression of RNA helicases in cancer. Anticancer Res. 2004, 24, 3951–3953. [Google Scholar] [PubMed]
- Robert, F.; Pelletier, J. Perturbations of RNA helicases in cancer. Wiley Interdiscip. Rev. 2013, 4, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.L.; Singh, K.; Zhong, Y.; Drewe, P.; Rajasekhar, V.K.; Sanghvi, V.R.; Mavrakis, K.J.; Jiang, M.; Roderick, J.E.; Van der Meulen, J.; et al. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature 2014, 513, 65–70. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Andersen, G.R.; Nielsen, K.H. The function and architecture of DEAH/RHA helicases. Biomol. Concepts 2011, 2, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Walbott, H.; Mouffok, S.; Capeyrou, R.; Lebaron, S.; Humbert, O.; van Tilbeurgh, H.; Henry, Y.; Leulliot, N. Prp43p contains a processive helicase structural architecture with a specific regulatory domain. EMBO J. 2010, 29, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Andersen, G.R.; Nielsen, K.H. Structural basis for the function of DEAH helicases. EMBO Rep. 2010, 11, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Prabu, J.R.; Müller, M.; Thomae, A.W.; Schüssler, S.; Bonneau, F.; Becker, P.B.; Conti, E. Structure of the RNA Helicase MLE Reveals the Molecular Mechanisms for Uridine Specificity and RNA-ATP Coupling. Mol. Cell 2015, 60, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Lattmann, S.; Giri, B.; Vaughn, J.P.; Nagamine, Y.; Akman, S.A. Role of the amino terminal RHAU-specific motif in the recognition and resolution of guanine quadruplex-RNA by the DEAH-box RNA helicase RHAU. Nucleic Acids Res. 2010, 38, 6219–6233. [Google Scholar] [CrossRef] [PubMed]
- Murat, P.; Balasubramanian, S. Existence and consequences of G-quadruplex structures in DNA. Curr. Opin. Genet. Dev. 2014, 25, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Heddi, B.; Cheong, V.V.; Martadinata, H.; Phan, A.T. Insights into G-quadruplex specific recognition by the DEAH-box helicase RHAU: Solution structure of a peptide-quadruplex complex. Proc. Natl. Acad. Sci. USA 2015, 112, 9608–9613. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.; Patel, T.R.; Booy, E.P.; Marushchak, O.; Okun, N.; Deo, S.; Howard, R.; McEleney, K.; Harding, S.E.; Stetefeld, J.; et al. Binding of G-quadruplexes to the N-terminal recognition domain of the RNA helicase associated with AU-rich element (RHAU). J. Biol. Chem. 2013, 288, 35014–35027. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.K.; Cordin, O.; Banroques, J.; Doère, M.; Linder, P. The Q motif: A newly identified motif in DEAD box helicases may regulate ATP binding and hydrolysis. Mol. Cell 2003, 11, 127–138. [Google Scholar] [CrossRef]
- Robert-Paganin, J.; Halladjian, M.; Blaud, M.; Lebaron, S.; Delbos, L.; Chardon, F.; Capeyrou, R.; Humbert, O.; Henry, Y.; Henras, A.K.; et al. Functional link between DEAH/RHA helicase Prp43 activation and ATP base binding. Nucleic Acids Res. 2017, 45, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.L.; Morgenstern, K.A.; Griffith, J.P.; Dwyer, M.D.; Thomson, J.A.; Murcko, M.A.; Lin, C.; Caron, P.R. Hepatitis C virus NS3 RNA helicase domain with a bound oligonucleotide: The crystal structure provides insights into the mode of unwinding. Structure 1998, 6, 89–100. [Google Scholar] [CrossRef]
- Luo, D.; Xu, T.; Watson, R.P.; Becker, D.S.; Sampath, A.; Jahnke, W.; Yeong, S.S.; Wang, C.H.; Lim, S.P.; Strongin, A.; et al. Insights into RNA unwinding and ATP hydrolysis by the flavivirus NS3 protein. EMBO J. 2008, 27, 3209–3219. [Google Scholar] [CrossRef] [PubMed]
- Büttner, K.; Nehring, S.; Hopfner, K.-P. Structural basis for DNA duplex separation by a superfamily-2 helicase. Nat. Struct. Mol. Biol. 2007, 14, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Del Campo, M.; Lambowitz, A.M.; Jankowsky, E. DEAD-Box Proteins Unwind Duplexes by Local Strand Separation. Mol. Cell 2007, 28, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Murat, P.; Abecassis, K.; Ferré-D’Amaré, A.R.; Balasubramanian, S. Insights into the mechanism of a G-quadruplex-unwinding DEAH-box helicase. Nucleic Acids Res. 2015, 43, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Pyle, A.M.; Jankowsky, E.; Gross, C.H.; Shuman, S. The DExH protein NPH-II is a processive and directional motor for unwinding RNA. Nature 2000, 403, 447–451. [Google Scholar]
- Myong, S.; Bruno, M.M.; Pyle, A.M.; Ha, T. Spring-loaded mechanism of DNA unwinding by hepatitis C virus NS3 helicase. Science 2007, 317, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Myong, S.; Ha, T. Stepwise translocation of nucleic acid motors. Curr. Opin. Struct. Biol. 2010, 20, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Tippana, R.; Hwang, H.; Opresko, P.L.; Bohr, V.A.; Myong, S. Single-molecule imaging reveals a common mechanism shared by G-quadruplex-resolving helicases. Proc. Natl. Acad. Sci. USA 2016, 113, 8448–8453. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.R.; Xing, L.; Kleiman, L.; Myong, S. Repetitive RNA unwinding by RNA helicase A facilitates RNA annealing. Nucleic Acids Res. 2014, 42, 8556–8564. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Arunajadai, S.G.; Moffitt, J.R.; Tinoco, I.; Bustamante, C. Single-base pair unwinding and asynchronous RNA release by the hepatitis C virus NS3 helicase. Science 2011, 333, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Pyle, A.M. Translocation and unwinding mechanisms of RNA and DNA helicases. Annu. Rev. Biophys. 2008, 37, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Dumont, S.; Cheng, W.; Serebrov, V.; Beran, R.K.; Tinoco, I.; Pyle, A.M.; Bustamante, C. RNA translocation and unwinding mechanism of HCV NS3 helicase and its coordination by ATP. Nature 2006, 439, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Tritschler, F.; Lee, K.S.; Gu, M.; Rice, C.M.; Ha, T. Single-molecule imaging reveals the translocation and DNA looping dynamics of hepatitis C virus NS3 helicase. Protein Sci. 2017, 26, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Tauchert, M.J.; Fourmann, J.-B.; Lührmann, R.; Ficner, R. Structural insights into the mechanism of the DEAH-box RNA helicase Prp43. eLife 2017, 6, e21510. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Staley, J.P.; Andersen, G.R.; Nielsen, K.H. Structure of the DEAH/RHA ATPase Prp43p bound to RNA implicates a pair of hairpins and motif Va in translocation along RNA. RNA 2017, 23, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Bigay, J.; Deterre, P.; Pfister, C.; Chabre, M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985, 191, 181–185. [Google Scholar] [CrossRef]
- Chabre, M. Aluminofluoride and beryllofluoride complexes: A new phosphate analogs in enzymology. Trends Biochem. Sci. 1990, 15, 6–10. [Google Scholar] [CrossRef]
- Davies, D.R.; Hol, W.G.J. The power of vanadate in crystallographic investigations of phosphoryl transfer enzymes. FEBS Lett. 2004, 577, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.W.; Moréra, S.; Janin, J.; Cherfils, J. AlF3 mimics the transition state of protein phosphorylation in the crystal structure of nucleoside diphosphate kinase and MgADP. Proc. Natl. Acad. Sci. USA 1997, 94, 3579–3583. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Putnam, A.; Jankowsky, E. ATP hydrolysis is required for DEAD-box protein recycling but not for duplex unwinding. Proc. Natl. Acad. Sci. USA 2008, 105, 20209–20214. [Google Scholar] [CrossRef] [PubMed]
- Mallam, A.L.; Sidote, D.J.; Lambowitz, A.M. Molecular insights into RNA and DNA helicase evolution from the determinants of specificity for a DEAD-box RNA helicase. eLife 2014, 3, e04630. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Rice, C.M. Three conformational snapshots of the hepatitis C virus NS3 helicase reveal a ratchet translocation mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Schwer, B. Mutations in PRP43 that uncouple RNA-dependent NTPase activity and pre-mRNA splicing function. Biochemistry 2006, 45, 6510–6521. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Grosse, F. Human DHX9 helicase preferentially unwinds RNA-containing displacement loops (R-loops) and G-quadruplexes. DNA Repair 2011, 10, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Creacy, S.D.; Routh, E.D.; Iwamoto, F.; Nagamine, Y.; Akman, S.A.; Vaughn, J.P. G4 Resolvase 1 Binds Both DNA and RNA Tetramolecular Quadruplex with High Affinity and Is the Major Source of Tetramolecular Quadruplex G4-DNA and G4-RNA Resolving Activity in HeLa Cell Lysates. J. Biol. Chem. 2008, 283, 34626–34634. [Google Scholar] [CrossRef] [PubMed]
- Appleby, T.C.; Anderson, R.; Fedorova, O.; Pyle, A.M.; Wang, R.; Liu, X.; Brendza, K.M.; Somoza, J.R. Visualizing ATP-dependent RNA translocation by the NS3 helicase from HCV. J. Mol. Biol. 2011, 405, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Silverman, E.J.; Maeda, A.; Wei, J.; Smith, P.; Beggs, J.D.; Lin, R.-J. Interaction between a G-patch protein and a spliceosomal DEXD/H-box ATPase that is critical for splicing. Mol. Cell. Biol. 2004, 24, 10101–10110. [Google Scholar] [CrossRef] [PubMed]
- Lebaron, S.; Papin, C.; Capeyrou, R.; Chen, Y.-L.; Froment, C.; Monsarrat, B.; Caizergues-Ferrer, M.; Grigoriev, M.; Henry, Y. The ATPase and helicase activities of Prp43p are stimulated by the G-patch protein Pfa1p during yeast ribosome biogenesis. EMBO J. 2009, 28, 3808–3819. [Google Scholar] [CrossRef] [PubMed]
- Christian, H.; Hofele, R.V.; Urlaub, H.; Ficner, R. Insights into the activation of the helicase Prp43 by biochemical studies and structural mass spectrometry. Nucleic Acids Res. 2014, 42, 1162–1179. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.R.; Bonneau, F.; Hentschel, J.; Conti, E. Structural analysis reveals the characteristic features of Mtr4, a DExH helicase involved in nuclear RNA processing and surveillance. Proc. Natl. Acad. Sci. USA 2010, 107, 12139–12144. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.L.; Jackson, R.N.; Rexhepaj, M.; King, A.K.; Lott, L.K.; van Hoof, A.; Johnson, S.J. The Mtr4 ratchet helix and arch domain both function to promote RNA unwinding. Nucleic Acids Res. 2014, 42, 13861–13872. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Rice, C.M. The Spring α-Helix Coordinates Multiple Modes of HCV (Hepatitis C Virus) NS3 Helicase Action. J. Biol. Chem. 2016, 291, 14499–14509. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.C.; Ferré-D’Amaré, A.R. Structural Basis of DEAH/RHA Helicase Activity. Crystals 2017, 7, 253. https://doi.org/10.3390/cryst7080253
Chen MC, Ferré-D’Amaré AR. Structural Basis of DEAH/RHA Helicase Activity. Crystals. 2017; 7(8):253. https://doi.org/10.3390/cryst7080253
Chicago/Turabian StyleChen, Michael C., and Adrian R. Ferré-D’Amaré. 2017. "Structural Basis of DEAH/RHA Helicase Activity" Crystals 7, no. 8: 253. https://doi.org/10.3390/cryst7080253




