Induced Mesocrystal-Formation, Hydrothermal Growth and Magnetic Properties of α-Fe2O3 Nanoparticles in Salt-Rich Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
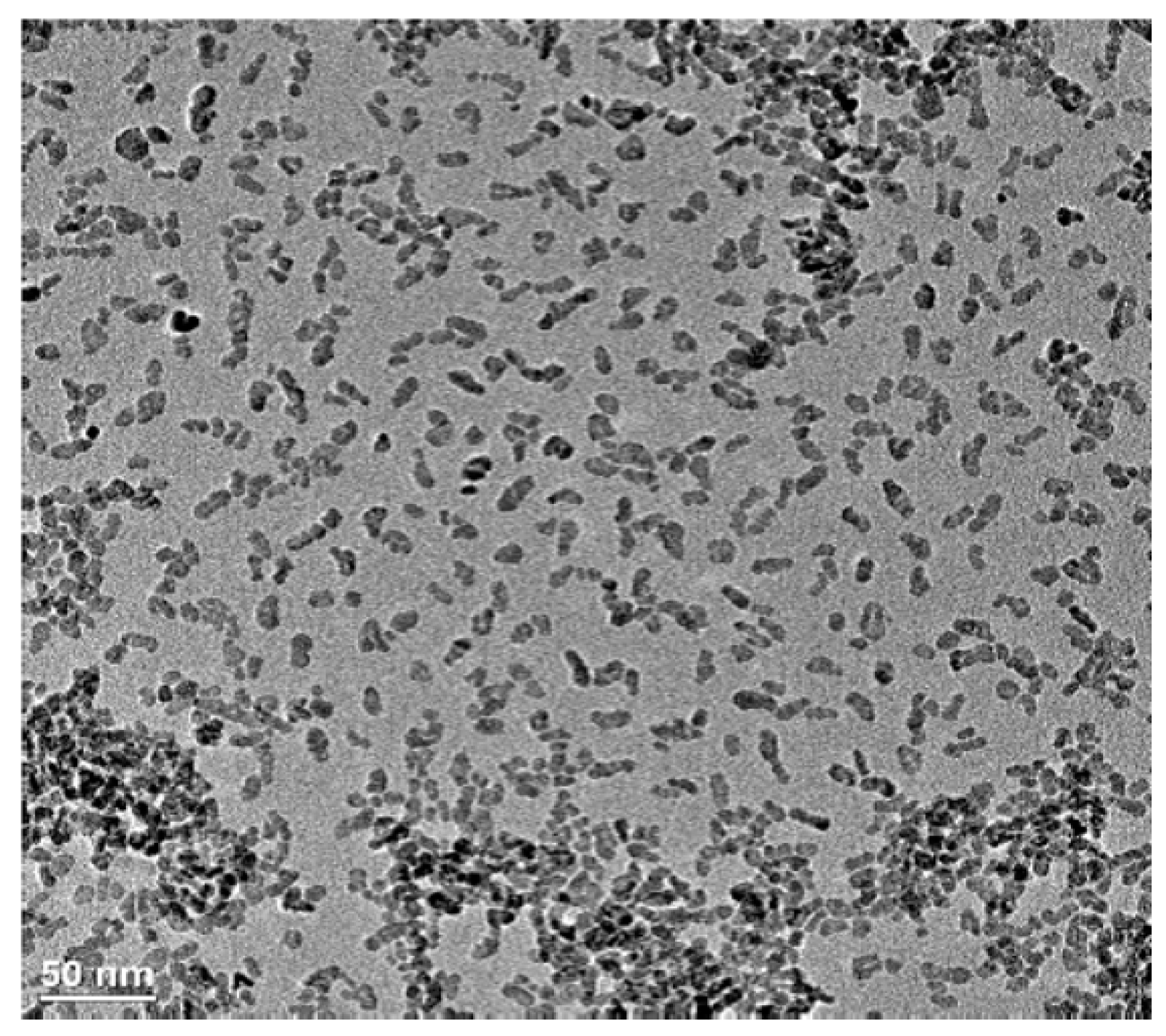
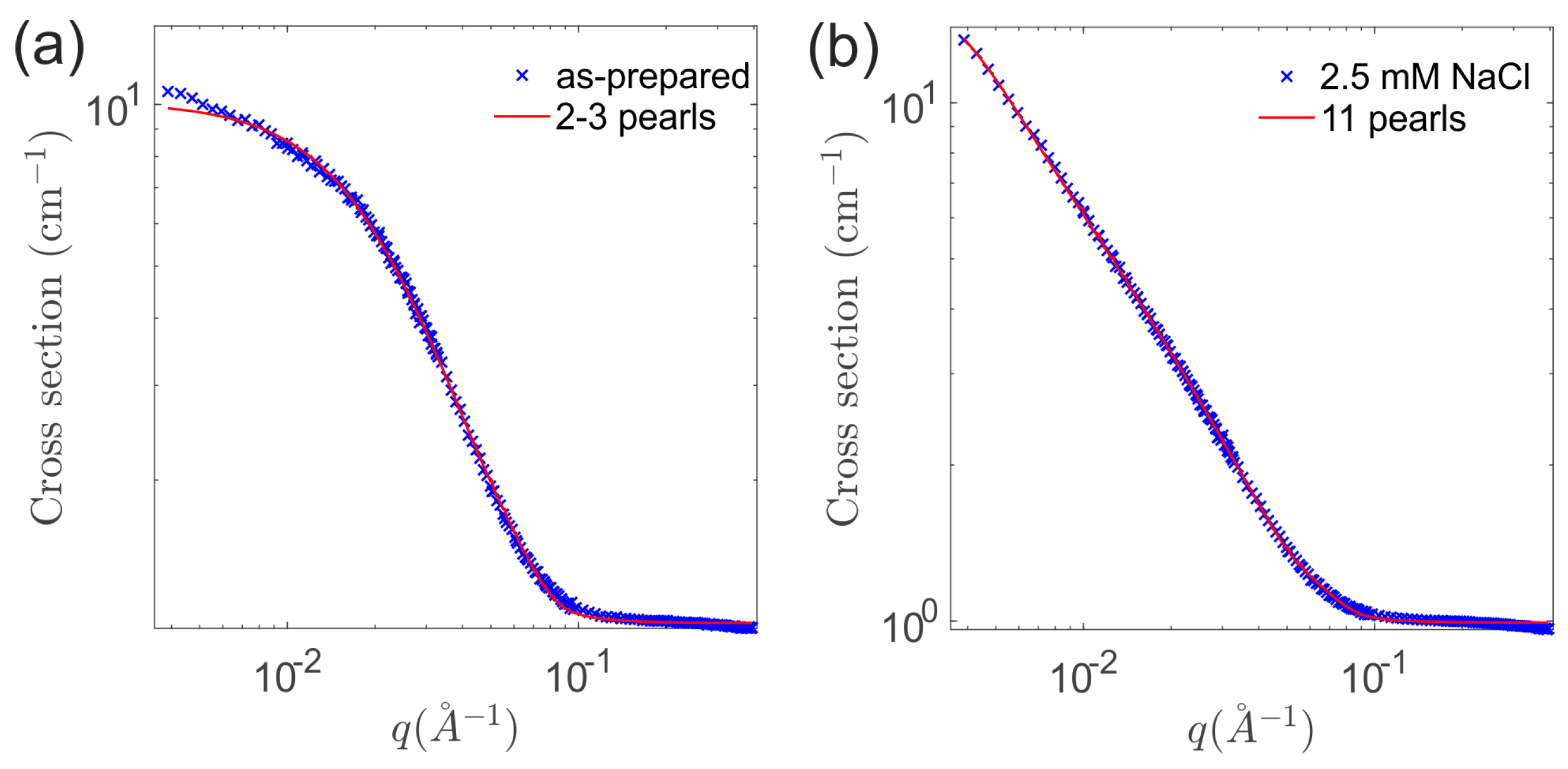
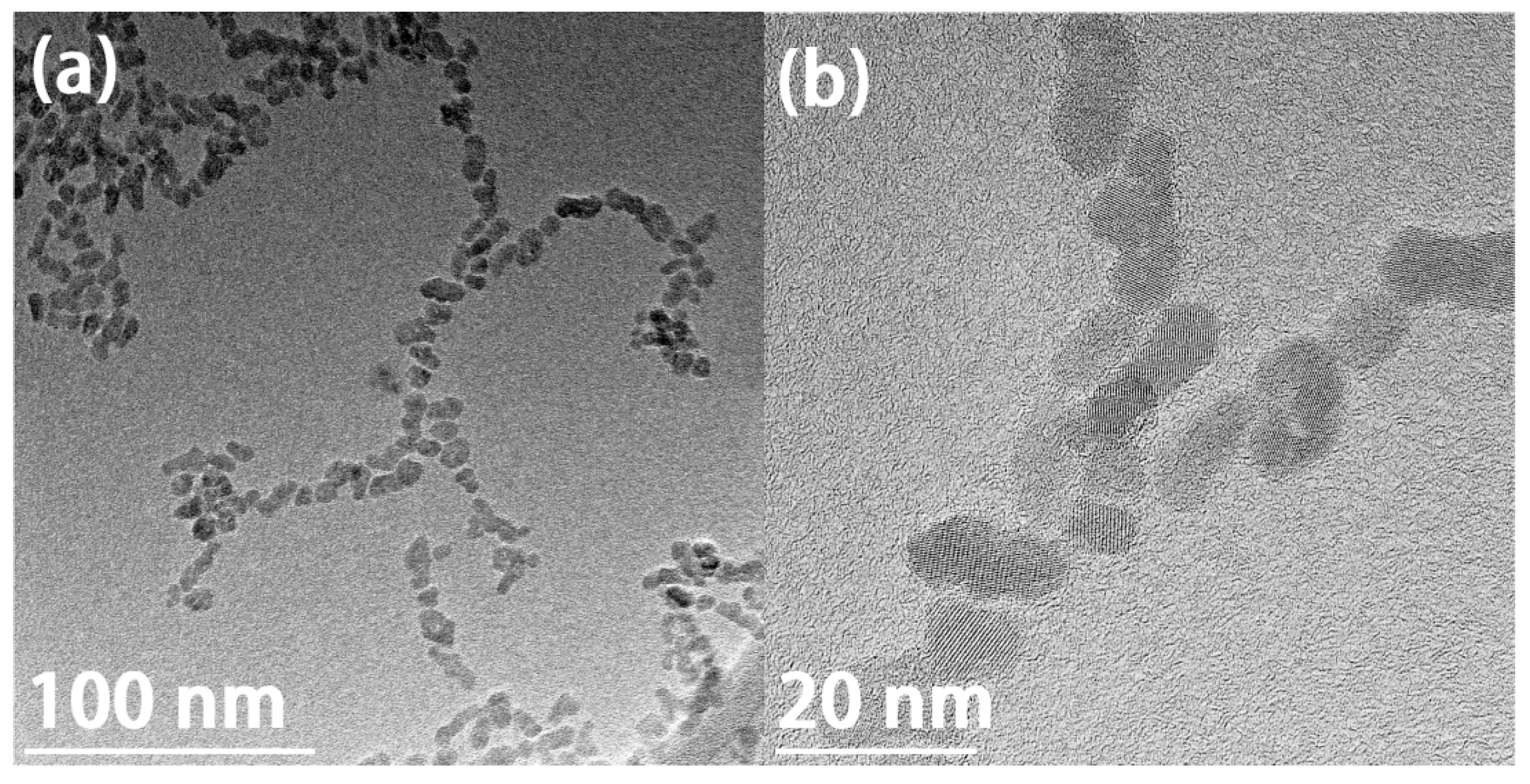
3.1. The As-Prepared Sample
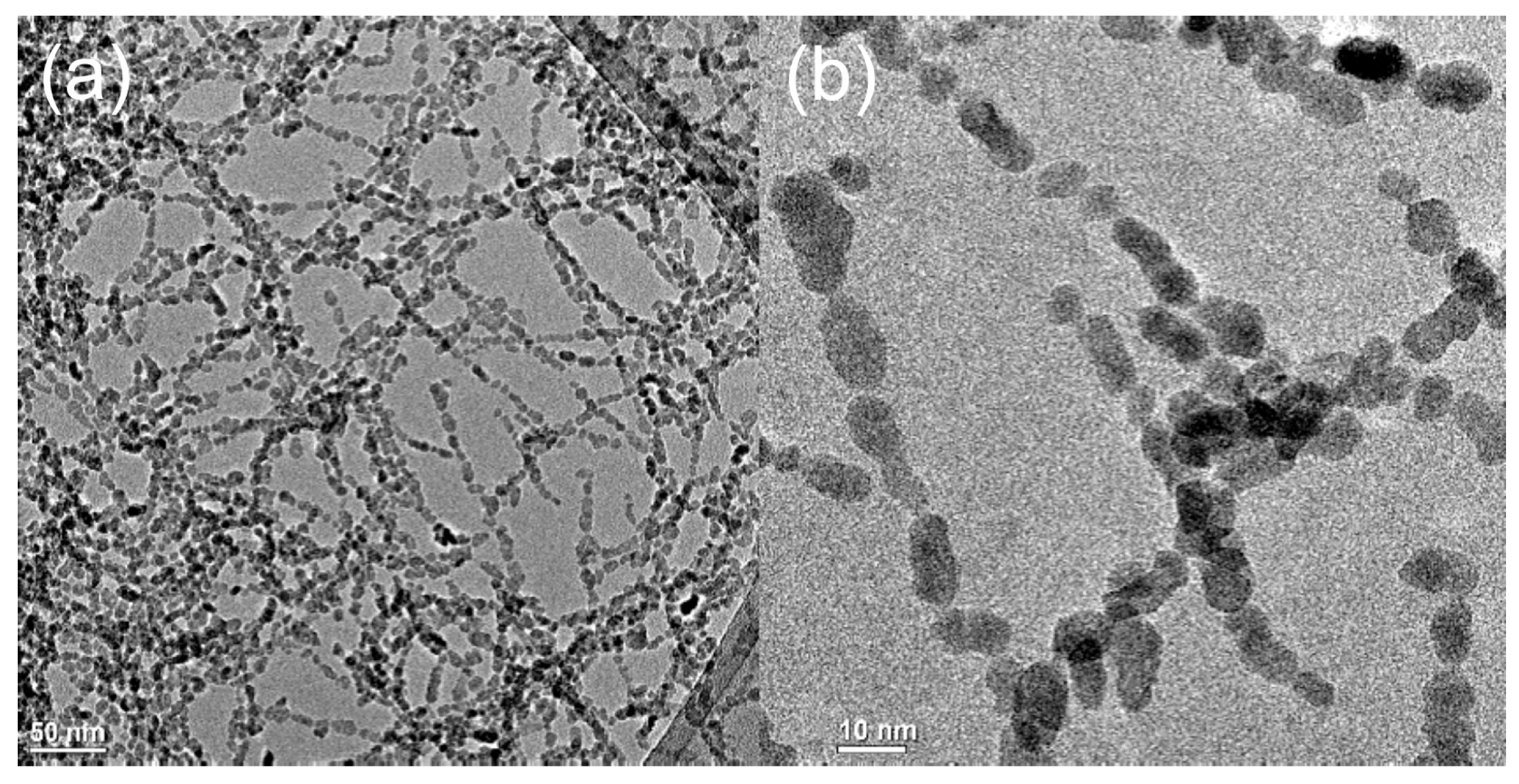
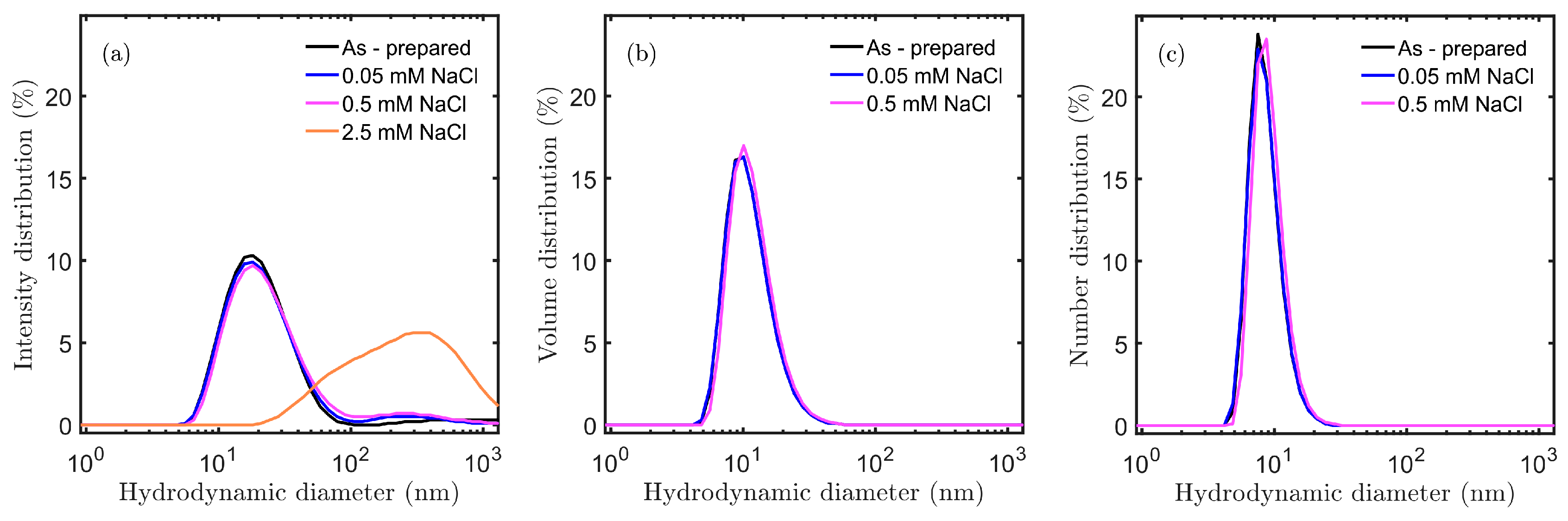
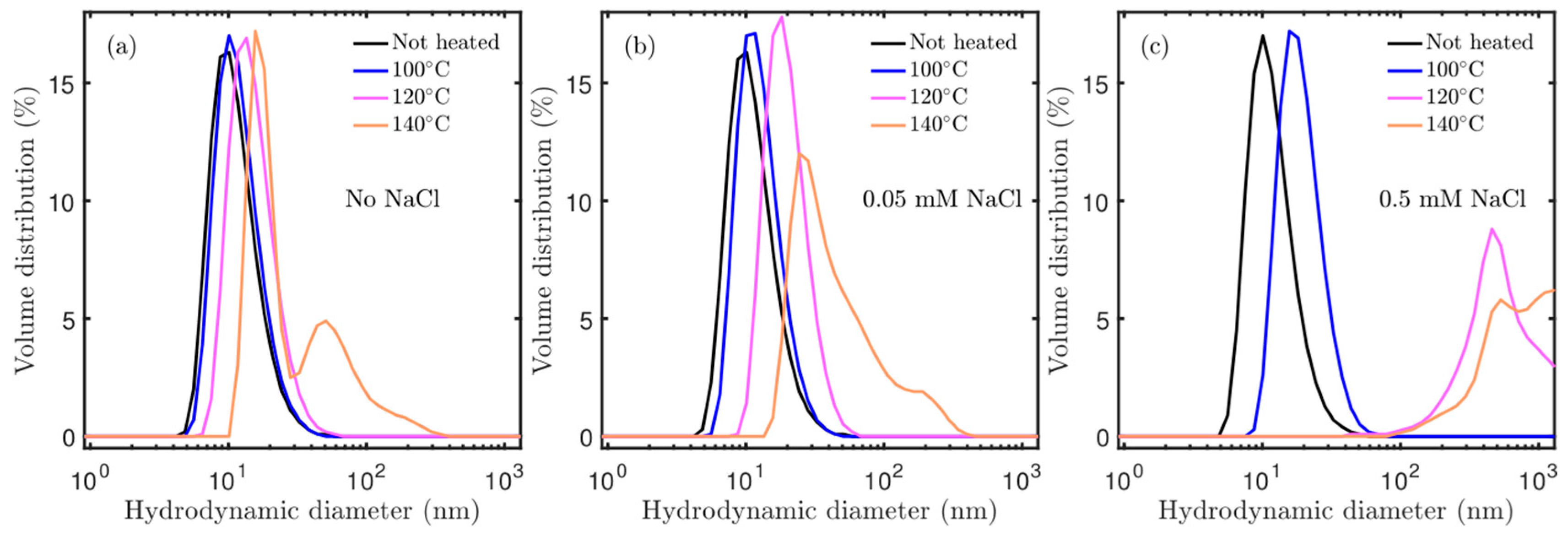
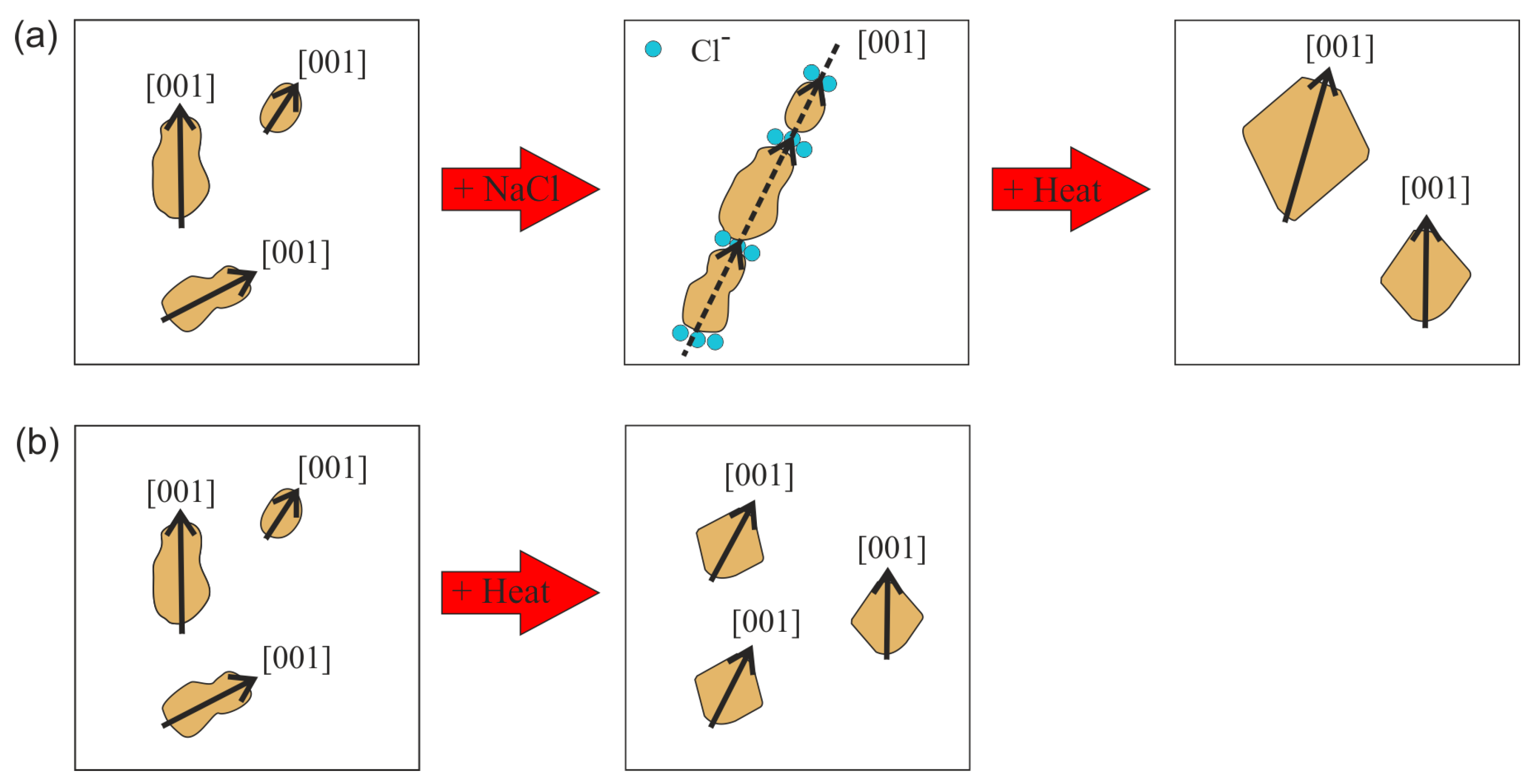
3.2. Effect of Salt Concentration: Aggregation
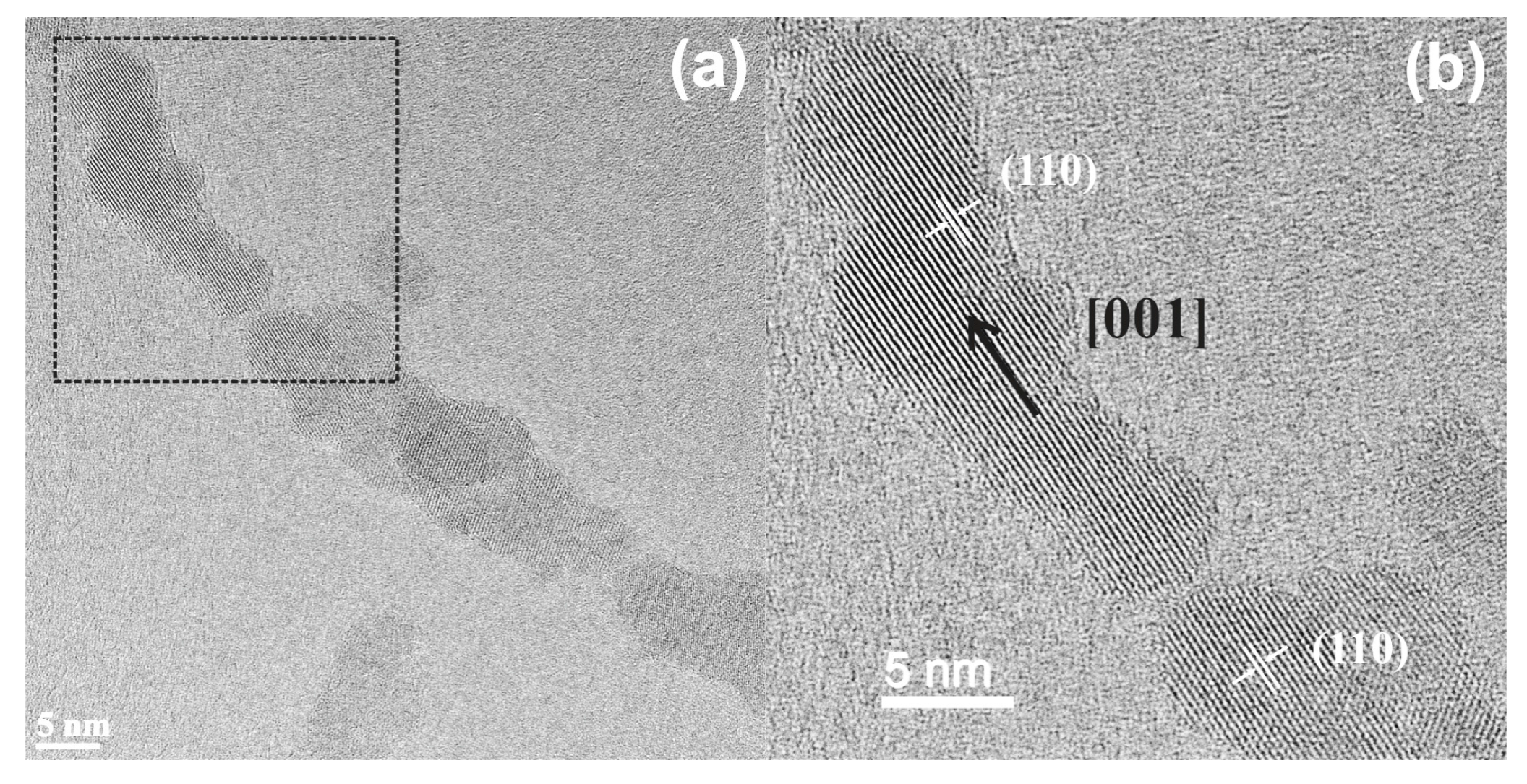
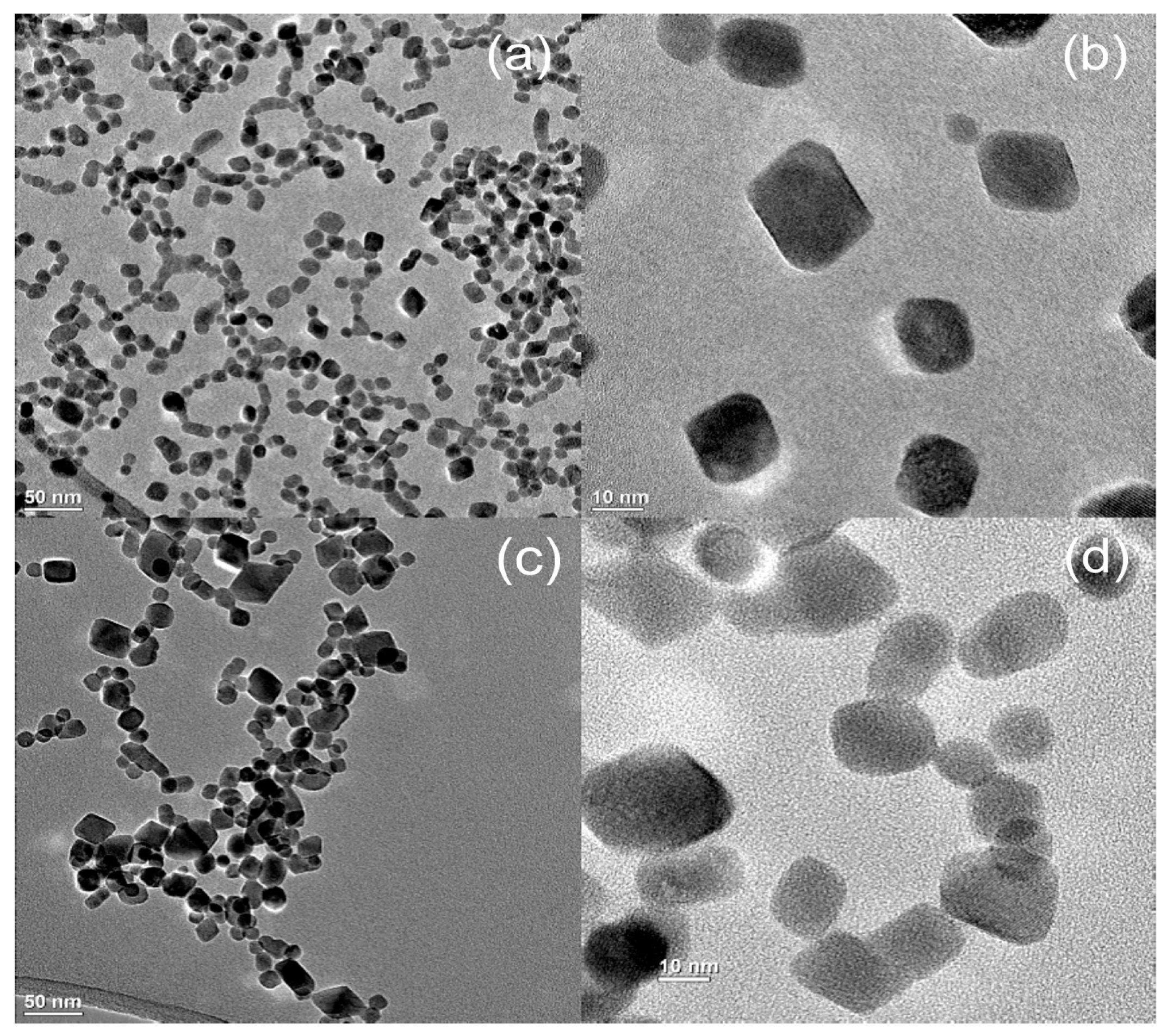
3.3. Effect or Hydrothermal Treatment: Crystal Growth from Different Aggregation States
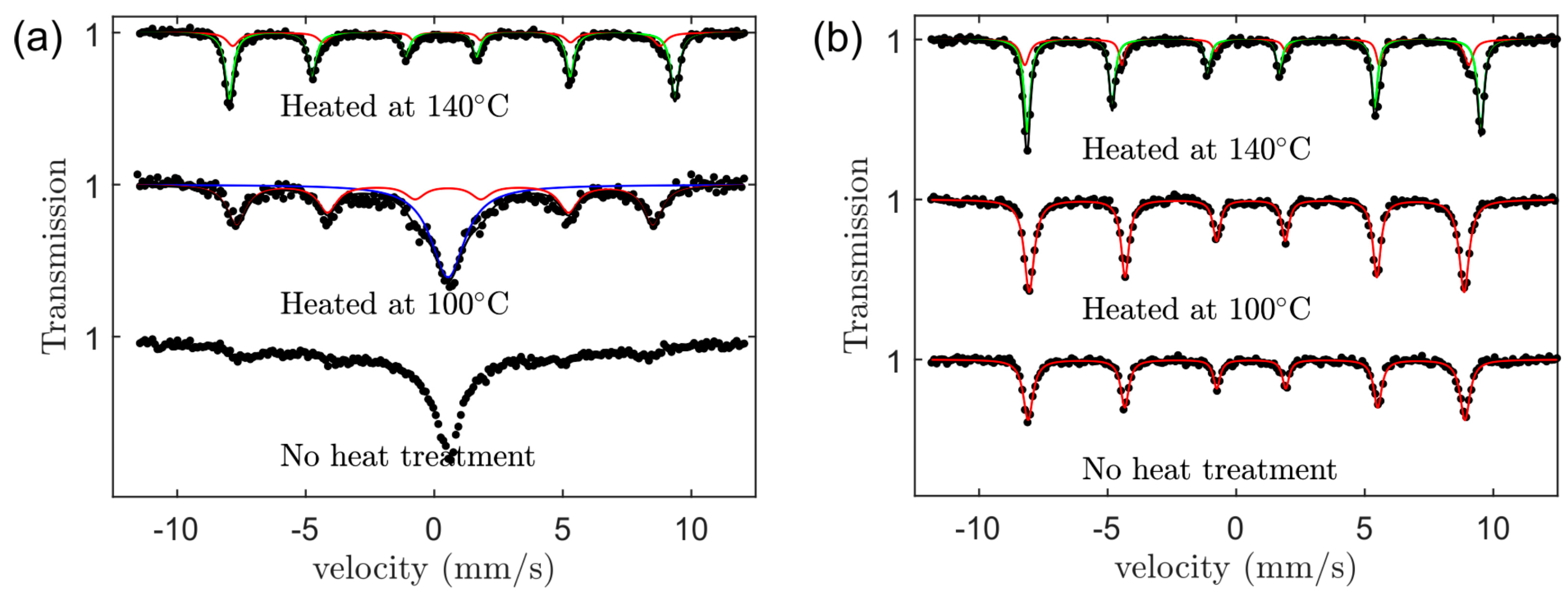
3.4. Magnetic Properties
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Penn, R.L.; Banfield, J.F. Morphology development and crystal growth in nanocrystalline aggregates under hydrothermal conditions: Insights from titania. Geochim. Cosmochim. Acta 1999, 63, 1549–1557. [Google Scholar] [CrossRef]
- Penn, R.L.; Banfield, J.F. Imperfect oriented attachment: Dislocation generation in defect-free nanocrystals. Science 1998, 281, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; De Yoreo, J.J.; Banfield, J.F. A Unified description of attachment-based crystal growth. ACS Nano 2014, 8, 6526–6530. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, M.; Cölfen, H. Oriented attachment and mesocrystals: Non-classical crystallization mechanisms based on nanoparticle assembly. Phys. Chem. Chem. Phys. 2006, 8, 3271–3287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, F.; Lin, Z. Progress of nanocrystalline growth kinetics based on oriented attachment. Nanoscale 2010, 2, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Nielsen, M.H.; Lee, J.R.I.; Frandsen, C.; Banfield, J.F.; De Yoreo, J.J. Direction-specific interactions control crystal growth by oriented attachment. Science 2012, 336, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.; Rehbock, C.; Hühn, D.; Carrillo-Carrion, C.; de Aberasturi, D.J.; Merk, V.; Barcikowski, S.; Parak, W.J. Interaction of colloidal nanoparticles with their local environment: The (ionic) nanoenvironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles. J. R. Soc. Interface 2014, 11, 20130931. [Google Scholar] [CrossRef] [PubMed]
- Oncsik, T.; Trefalt, G.; Borkovec, M.; Szilagyi, I. Specific ion effects on particle aggregation induced by monovalent salts within the Hofmeister series. Langmuir 2015, 31, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Wang, Y.; Itoh, H.; Muramatsu, A. Systematic control of size, shape and internal structure of monodisperse α-Fe2O3 particles. Colloids Surf. A Physicochem. Eng. Asp. 1998, 134, 265–279. [Google Scholar] [CrossRef]
- Sugimoto, T.; Wang, Y. Mechanism of the Shape and Structure Control of Monodispersed α-Fe2O3 Particles by Sulfate Ions. J. Colloid Interface Sci. 1998, 207, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Muramatsu, A. Formation Mechanism of monodispersed α-Fe2O3 particles in dilute FeCl3 solutions. J. Colloid Interface Sci. 1996, 184, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Reeves, N.J.; Mann, S.; Heller, W.; Docherty, R.; Bils, R.; Saltman, P. Influence of inorganic and organic additives on the tailored synthesis of iron oxides. J. Chem. Soc. Faraday Trans. 1991, 87, 3875. [Google Scholar] [CrossRef]
- Livage, J.; Henry, M.; Sanchez, C. Sol-gel chemistry of transition metal oxides. Prog. Solid State Chem. 1988, 18, 259–341. [Google Scholar] [CrossRef]
- He, Y.T.; Wan, J.; Tokunaga, T. Kinetic stability of hematite nanoparticles: The effect of particle sizes. J. Nanopart. Res. 2008, 10, 321–332. [Google Scholar] [CrossRef]
- Morrish, A.H. Canted Antiferromagnetism: Hematite; World Scientific: Singapore, 1994; ISBN 9810220073. [Google Scholar]
- Besser, P.J.; Morrish, A.H.; Searle, C.W. Magnetocrystalline anisotropy of pure and doped hematite. Phys. Rev. 1967, 153, 632–640. [Google Scholar] [CrossRef]
- Yamamoto, N. The Shift of the spin flip temperature of α-Fe2O3 fine particles. J. Phys. Soc. Jpn. 1968, 24, 23–28. [Google Scholar] [CrossRef]
- Varón, M.; Beleggia, M.; Kasama, T.; Harrison, R.J.; Dunin-Borkowski, R.E.; Puntes, V.F.; Frandsen, C. Dipolar magnetism in ordered and disordered low-dimensional nanoparticle assemblies. Sci. Rep. 2013, 3, 1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, M.F.; Koch, C.B.; Mørup, S. Magnetic dynamics of weakly and strongly interacting hematite nanoparticles. Phys. Rev. B 2000, 62, 1124–1135. [Google Scholar] [CrossRef] [Green Version]
- Frandsen, C.; Mørup, S. Inter-particle interactions in composites of antiferromagnetic nanoparticles. J. Magn. Magn. Mater. 2003, 266, 36–48. [Google Scholar] [CrossRef]
- Frandsen, C.; Bahl, C.R.H.; Lebech, B.; Lefmann, K.; Kuhn, L.T.; Keller, L.; Andersen, N.H.; Zimmermann, M.V.; Johnson, E.; Klausen, S.N.; et al. Oriented attachment and exchange coupling of α-Fe2O3 nanoparticles. Phys. Rev. B 2005, 72, 214406. [Google Scholar] [CrossRef]
- Gilbert, B.; Frandsen, C.; Maxey, E.R.; Sherman, D.M. Band-gap measurements of bulk and nanoscale hematite by soft X-ray spectroscopy. Phys. Rev. B 2009, 79, 35108. [Google Scholar] [CrossRef]
- Kline, S.R. Reduction and analysis of SANS and USANS data using IGOR Pro. J. Appl. Crystallogr. 2006, 39, 895–900. [Google Scholar] [CrossRef]
- SasView for Small Angle Scattering Analysis. Available online: http://www.sasview.org (accessed on 15 June 2017).
- Howard, C.J.; Hill, R.J. AAEC (now ANSTO) Report M112; Lucas Heights Research Laboratory: Lucas Heights, Australia, 1986. [Google Scholar]
- Dobrynin, A.V.; Rubinstein, M.; Obukhov, S.P. Cascade of transitions of polyelectrolytes in poor solvents. Macromolecules 1996, 29, 2974–2979. [Google Scholar] [CrossRef]
- Frandsen, C.; Lefmann, K.; Lebech, B.; Bahl, C.R.H.; Brok, E.; Ancoña, S.N.; Theil Kuhn, L.; Keller, L.; Kasama, T.; Gontard, L.C.; et al. Spin reorientation in α-Fe2O3 nanoparticles induced by interparticle exchange interactions in α-Fe2O3/NiO nanocomposites. Phys. Rev. B 2011, 84, 214435. [Google Scholar] [CrossRef]
- Brok, E.; Frandsen, C.; Madsen, D.E.; Jacobsen, H.; Birk, J.O.; Lefmann, K.; Bendix, J.; Pedersen, K.S.; Boothroyd, C.B.; Berhe, A.A.; et al. Magnetic properties of ultra-small goethite nanoparticles. J. Phys. D Appl. Phys. 2014, 47, 365003. [Google Scholar] [CrossRef]
- Frandsen, C.; Mørup, S. Reversible aggregation and magnetic coupling of α-Fe2O3 nanoparticles. J. Phys. C Condens. Matter 2006, 18, 7079. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brok, E.; Larsen, J.; Varón, M.; Hansen, T.W.; Frandsen, C. Induced Mesocrystal-Formation, Hydrothermal Growth and Magnetic Properties of α-Fe2O3 Nanoparticles in Salt-Rich Aqueous Solutions. Crystals 2017, 7, 248. https://doi.org/10.3390/cryst7080248
Brok E, Larsen J, Varón M, Hansen TW, Frandsen C. Induced Mesocrystal-Formation, Hydrothermal Growth and Magnetic Properties of α-Fe2O3 Nanoparticles in Salt-Rich Aqueous Solutions. Crystals. 2017; 7(8):248. https://doi.org/10.3390/cryst7080248
Chicago/Turabian StyleBrok, Erik, Jacob Larsen, Miriam Varón, Thomas W. Hansen, and Cathrine Frandsen. 2017. "Induced Mesocrystal-Formation, Hydrothermal Growth and Magnetic Properties of α-Fe2O3 Nanoparticles in Salt-Rich Aqueous Solutions" Crystals 7, no. 8: 248. https://doi.org/10.3390/cryst7080248




